The Relationship between Protein Intake and Source on Factors Associated with Glycemic Control in Individuals with Prediabetes and Type 2 Diabetes
Abstract
:1. Introduction
2. Materials and Methods
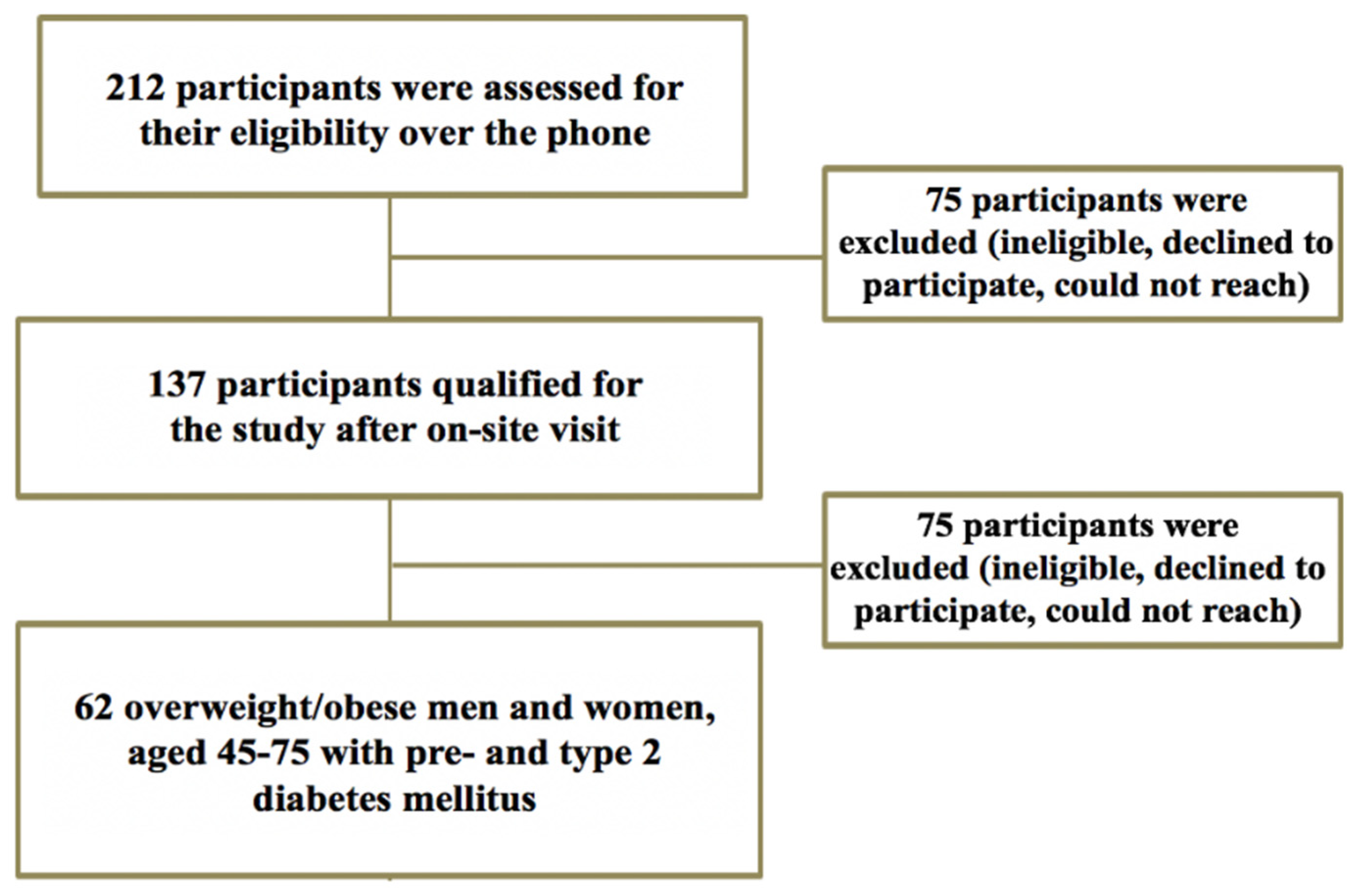
2.1. Participants
2.2. Study Overview
2.3. Dietary Assessment and Determination of Animal-to-Plant Ratio
2.4. Assessment of Anthropometrics and Blood Pressure
2.5. Statistical Analysis
3. Results
3.1. Anthropometrics
3.2. Differences in Nutrient Intake and Body Composition
3.3. Differences in Lipid Profile, Glycemic Control, and Oxidized LDL
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prevention CFDCa. National Diabetes Statistics Report. Centers For Disease Control and Prevention; 2018. Available online: https://www.cdc.gov/diabetes/data/statistics/statistics-report.html (accessed on 6 July 2020).
- Association, A.D. Diagnosis and classification of diabetes mellitus. Diabetes Care 2009, 32 (Suppl. 1), S62–S67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eikenberg, J.D.; Davy, B.M. Prediabetes: A prevalent and treatable, but often unrecognized, clinical condition. J. Acad. Nutr. Diet. 2013, 113, 213–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Report NDS. Estimates of Diabetes and Its Burden in the United States. 2014. Available online: https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.Pdf (accessed on 6 July 2020).
- Olokoba, A.B.; Obateru, O.A.; Olokoba, L.B. Type 2 diabetes mellitus: A review of current trends. Oman Med. J. 2012, 27, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, B.; Gulanick, M.; Lamendola, C. Risk factors for type 2 diabetes mellitus. J. Cardiovasc. Nurs. 2002, 16, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Rivellese, A.A.; Riccardi, G.; Vaccaro, O. Cardiovascular risk in women with diabetes. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 474–480. [Google Scholar] [CrossRef]
- Kodama, S.; Horikawa, C.; Fujihara, K.; Heianza, Y.; Hirasawa, R.; Yachi, Y.; Sugawara, A.; Tanaka, S.; Shimano, H.; Iida, K.T.; et al. Comparisons of the strength of associations with future type 2 diabetes risk among anthropometric obesity indicators, including waist-to-height ratio: A meta-analysis. Am. J. Epidemiol. 2012, 176, 959–969. [Google Scholar] [CrossRef] [Green Version]
- Rabe, K.; Lehrke, M.; Parhofer, K.G.; Broedl, U.C. Adipokines and insulin resistance. Mol. Med. 2008, 14, 741–751. [Google Scholar] [CrossRef]
- American Diebetes Association. Treatment of hypertension in diabetes. Diabetes Care 1993, 16, 1394–1401. Available online: https://care.diabetesjournals.org/content/16/10/1394 (accessed on 6 July 2020). [CrossRef] [Green Version]
- Adiels, M.; Olofsson, S.O.; Taskinen, M.R.; Borén, J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Saydah, S.H.; Fradkin, J.; Cowie, C.C. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA 2004, 291, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Prevention CfDCa. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States. 2011. Available online: http://www.diabetesincontrol.com/wp-content/uploads/PDF/ndep_diabetes_facts_2011.pdf (accessed on 6 July 2020).
- Mooradian, A.D. Dyslipidemia in type 2 diabetes mellitus. Nat. Clin. Pract. Endocrinol. Metab. 2009, 5, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Sami, W.; Ansari, T.; Butt, N.S.; Hamid, M.R.A. Effect of diet on type 2 diabetes mellitus: A review. Int. J. Health Sci. 2017, 11, 65–71. [Google Scholar]
- Ley, S.H.; Pan, A.; Li, Y.; Manson, J.E.; Willett, W.C.; Sun, Q.; Hu, F.B. Changes in Overall Diet Quality and Subsequent Type 2 Diabetes Risk: Three U.S. Prospective Cohorts. Diabetes Care 2016, 39, 2011–2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwingshackl, L.; Hoffmann, G.; Lampousi, A.M.; Knüppel, S.; Iqbal, K.; Schwedhelm, C.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 363–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G. Dietary protein intake and human health. Food Funct. 2016, 7, 1251–1265. [Google Scholar] [CrossRef] [Green Version]
- Pasiakos, S.M.; Agarwal, S.; Lieberman, H.R.; Fulgoni, V.L. Sources and Amounts of Animal, Dairy, and Plant Protein Intake of US Adults in 2007-2010. Nutrients 2015, 7, 7058–7069. [Google Scholar] [CrossRef] [Green Version]
- Paddon-Jones, D.; Westman, E.; Mattes, R.D.; Wolfe, R.R.; Astrup, A.; Westerterp-Plantenga, M. Protein, weight management, and satiety. Am. J. Clin. Nutr. 2008, 87, 1558S–1561S. [Google Scholar] [CrossRef] [Green Version]
- Fulgoni, V.L. Current protein intake in America: Analysis of the National Health and Nutrition Examination Survey, 2003-2004. Am. J. Clin. Nutr. 2008, 87, 1554S–1557S. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.Y.; Zhang, Z.L.; Wang, P.Y.; Qin, L.Q. Effects of high-protein diets on body weight, glycaemic control, blood lipids and blood pressure in type 2 diabetes: Meta-analysis of randomised controlled trials. Br. J. Nutr. 2013, 110, 781–789. [Google Scholar] [CrossRef]
- Westerterp-Plantenga, M.S.; Lemmens, S.G.; Westerterp, K.R. Dietary protein—Its role in satiety, energetics, weight loss and health. Br. J. Nutr. 2012, 108 (Suppl. 2), S105–S112. [Google Scholar] [CrossRef] [Green Version]
- Clifton, P. Effects of a high protein diet on body weight and comorbidities associated with obesity. Br. J. Nutr. 2012, 108 (Suppl. 2), S122–S129. [Google Scholar] [CrossRef] [Green Version]
- Layman, D.K.; Shiue, H.; Sather, C.; Erickson, D.J.; Baum, J. Increased dietary protein modifies glucose and insulin homeostasis in adult women during weight loss. J. Nutr. 2003, 133, 405–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Layman, D.K.; Evans, E.M.; Erickson, D.; Seyler, J.; Weber, J.; Bagshaw, D.; Griel, A.; Psota, T.; Kris-Etherton, P. A moderate-protein diet produces sustained weight loss and long-term changes in body composition and blood lipids in obese adults. J. Nutr. 2009, 139, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Clifton, P.M.; Bastiaans, K.; Keogh, J.B. High protein diets decrease total and abdominal fat and improve CVD risk profile in overweight and obese men and women with elevated triacylglycerol. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, F.Q.; Mooradian, A.D.; Gannon, M.C.; Billington, C.; Krezowski, P. Effect of protein ingestion on the glucose and insulin response to a standardized oral glucose load. Diabetes Care 1984, 7, 465–470. [Google Scholar] [CrossRef]
- Markova, M.; Pivovarova, O.; Hornemann, S.; Sucher, S.; Frahnow, T.; Wegner, K.; Machann, J.; Petzke, K.J.; Hierholzer, J.; Lichtinghagen, R.; et al. Isocaloric Diets High in Animal or Plant Protein Reduce Liver Fat and Inflammation in Individuals With Type 2 Diabetes. Gastroenterology 2017, 152, 571–585.e8. [Google Scholar] [CrossRef] [Green Version]
- Papakonstantinou, E.; Triantafillidou, D.; Panagiotakos, D.B.; Koutsovasilis, A.; Saliaris, M.; Manolis, A.; Melidonis, A.; Zampelas, A. A high-protein low-fat diet is more effective in improving blood pressure and triglycerides in calorie-restricted obese individuals with newly diagnosed type 2 diabetes. Eur. J. Clin. Nutr. 2010, 64, 595–602. [Google Scholar] [CrossRef]
- Malik, V.S.; Li, Y.; Tobias, D.K.; Pan, A.; Hu, F.B. Dietary Protein Intake and Risk of Type 2 Diabetes in US Men and Women. Am. J. Epidemiol. 2016, 183, 715–728. [Google Scholar] [CrossRef]
- Yoon, M.S. The Emerging Role of Branched-Chain Amino Acids in Insulin Resistance and Metabolism. Nutrients 2016, 8, 405. [Google Scholar] [CrossRef] [Green Version]
- Organization, W.H. Waist Circumference and Waist-Hip Ratio Report of a WHO Expert Consultation; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Park, K.B.; Park, H.A.; Kang, J.H.; Kim, K.; Cho, Y.G.; Jang, J. Animal and Plant Protein Intake and Body Mass Index and Waist Circumference in a Korean Elderly Population. Nutrients 2018, 10, 577. [Google Scholar] [CrossRef] [Green Version]
- Azadbakht, L.A.S.; Esmaillzadeh, A. Soy Protein Intake, Cardiorenal Indices, and C-Reactive Protein in Type 2 Diabetes With Nephropathy: A longitudinal randomized clinical trial. Diabetes Care 2008, 31, 648–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noakes, M.; Keogh, J.B.; Foster, P.R.; Clifton, P.M. Effect of an energy-restricted, high-protein, low-fat diet relative to a conventional high-carbohydrate, low-fat diet on weight loss, body composition, nutritional status, and markers of cardiovascular health in obese women. Am. J. Clin. Nutr. 2005, 81, 1298–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasiakos, S.M.; Lieberman, H.R.; Fulgoni, V.L. Higher-protein diets are associated with higher HDL cholesterol and lower BMI and waist circumference in US adults. J. Nutr. 2015, 145, 605–614. [Google Scholar] [CrossRef] [Green Version]
- McAuley, K.A.; Hopkins, C.M.; Smith, K.J.; McLay, R.T.; Williams, S.M.; Taylor, R.W.; Mann, J.I. Comparison of high-fat and high-protein diets with a high-carbohydrate diet in insulin-resistant obese women. Diabetologia 2005, 48, 8–16. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Wong, J.M.; Kendall, C.W.; Esfahani, A.; Ng, V.W.; Leong, T.C.; Faulkner, D.A.; Vidgen, E.; Greaves, K.A.; Paul, G.; et al. The effect of a plant-based low-carbohydrate (“Eco-Atkins”) diet on body weight and blood lipid concentrations in hyperlipidemic subjects. Arch. Intern. Med. 2009, 169, 1046–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Sun, C.; Liu, S.; Li, Y. Dietary Protein Intake and Type 2 Diabetes Among Women and Men in Northeast China. Sci. Rep. 2016, 6, 37604. [Google Scholar] [CrossRef] [Green Version]
- Odegaard, A.O.; Pereira, M.A. Trans fatty acids, insulin resistance, and type 2 diabetes. Nutr. Rev. 2006, 64, 364–372. [Google Scholar] [CrossRef]
- Von Frankenberg, A.D.; Marina, A.; Song, X.; Callahan, H.S.; Kratz, M.; Utzschneider, K.M. A high-fat, high-saturated fat diet decreases insulin sensitivity without changing intra-abdominal fat in weight-stable overweight and obese adults. Eur. J. Nutr. 2017, 6, 431–443. [Google Scholar] [CrossRef] [Green Version]
- Wiebe, S.L.; Bruce, V.M.; McDonald, B.E. A comparison of the effect of diets containing beef protein and plant proteins on blood lipids of healthy young men. Am. J. Clin. Nutr. 1984, 40, 982–989. [Google Scholar] [CrossRef] [Green Version]
- Park, M.S.; Kudchodkar, B.J.; Liepa, G.U. Effects of dietary animal and plant proteins on the cholesterol metabolism in immature and mature rats. J. Nutr. 1987, 117, 30–35. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Hosseini-Esfahabni, F.; Sadeghi, M.; Azizi, F. Dietary Protein, Protein to Carbohydrate Ratio and Subsequent Changes in Lipid Profile after a 3-Year Follow-Up: Tehran Lipid and Glucose Study. Iran. J. Public Health 2013, 42, 1232–1241. [Google Scholar]
- Hernández-Alonso, P.; Salas-Salvadó, J.; Ruiz-Canela, M.; Corella, D.; Estruch, R.; Fitó, M.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; Lapetra, J.; et al. High dietary protein intake is associated with an increased body weight and total death risk. Clin. Nutr. 2016, 35, 496–506. [Google Scholar] [CrossRef]
- Green, K.K.; Shea, J.L.; Vasdev, S.; Randell, E.; Gulliver, W.; Sun, G. Higher Dietary Protein Intake is Associated with Lower Body Fat in the Newfoundland Population. Clin. Med. Insights Endocrinol. Diabetes 2010, 3, 25–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.S.; Park, K.S.; Kim, M.J.; Kim, S.K.; Cho, Y.W.; Park, S.W. Type 2 diabetes is associated with low muscle mass in older adults. Geriatr. Gerontol. Int. 2014, 14 (Suppl. 1), 115–121. [Google Scholar] [CrossRef]
- Bassil, M.S.; Gougeon, R. Muscle protein anabolism in type 2 diabetes. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Nowson, C.; O’Connell, S. Protein Requirements and Recommendations for Older People: A Review. Nutrients 2015, 7, 6874–6899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezeh, U.; Pall, M.; Mathur, R.; Azziz, R. Association of fat to lean mass ratio with metabolic dysfunction in women with polycystic ovary syndrome. Hum. Reprod. 2014, 29, 1508–1517. [Google Scholar] [CrossRef] [Green Version]
- De Luis, D.A.; Izaola, O.; Aller, R.; de la Fuente, B.; Bachiller, R.; Romero, E. Effects of a high-protein/low carbohydrate versus a standard hypocaloric diet on adipocytokine levels and insulin resistance in obese patients along 9 months. J. Diabetes Complicat. 2015, 29, 950–954. [Google Scholar] [CrossRef]
- Johnston, C.S.; Sears, B.; Perry, M.; Knurick, J.R. Use of Novel High-Protein Functional Food Products as Part of a Calorie-Restricted Diet to Reduce Insulin Resistance and Increase Lean Body Mass in Adults: A Randomized Controlled Trial. Nutrients 2017, 9, 1182. [Google Scholar] [CrossRef] [Green Version]
- Segal, N.A.; Glass, N.A.; Baker, J.L.; Torner, J.C. Correcting for fat mass improves DXA quantification of quadriceps specific strength in obese adults aged 50–59 years. J. Clin. Densitom. 2009, 12, 299–305. [Google Scholar] [CrossRef] [Green Version]





| Variable: Medications | Below Recommended Protein (n = 17) | Meeting Recommended Protein (n = 22) | Above Recommended Protein (n = 23) | Total (n = 62) |
|---|---|---|---|---|
| Antihypertensive: n (%) | 11 (65) | 9 (41) | 10 (43) | 30 (48) |
| Hyperglycemic: n (%) | 9 (53) | 8 (36) | 6 (26) | 23 (37) |
| Lipid Lowering: n (%) | 6 (35) | 10 (45) | 7 (30) | 23 (37) |
| Hypothyroid: n (%) | 3 (18) | 2 (9) | 2 (9) | 7 (11) |
| NSAID: n (%) | 0 (0) b | 7 (32) | 3 (13) | 10 (16) |
| Variable: | Below Recommended Protein (n = 17) | Meeting Recommended Protein (n = 22) | Above Recommended Protein (n = 23) | Total (n = 62) |
|---|---|---|---|---|
| Male/Female (n/n) | 7/10 | 7/15 | 8/15 | 22/40 |
| Pre-DM/ T2D (n/n) | 8/9 | 16/6 | 19/4 | 43/19 |
| Age (years) | 60 ± 1.7 | 63 ± 1.4 | 62 ± 1.4 | 62 ± 0.8 |
| Height (cm) | 164 ± 1.7 | 165 ± 1.4 | 164 ± 1.5 | 165 ± 0.9 |
| Weight (kg) | 95 ± 3.3 c | 89 ± 2.9 c | 76 ± 2.4 | 86 ± 1.9 |
| BMI (kg/m2) | 35 ± 1.3 c | 33 ± 0.9 c | 28 ± 0.9 | 32 ± 0.7 |
| Waist Circumference (cm) | 109 ± 2.7 c | 104 ± 2.3 c | 97 ± 2.5 | 103 ± 1.5 |
| Hip Circumference (cm) | 119 ± 3.3 c | 115 ± 2.1 c | 106 ± 1.8 | 112 ± 1.5 |
| Waist-to-Hip Ratio (cm) | 0.92 ± 0.01 | 0.91 ± 0.02 | 0.92 ± 0.02 | 0.92 ± 0.01 |
| HbA1c (%) | 6.7 ± 0.20 | 6.4 ± 0.23 | 6.2 ± 0.20 | 6.4 ± 0.12 |
| SBP (mmHg) | 132 ± 4.3 | 133 ± 4.0 | 130 ± 2.9 | 131 ± 2.1 |
| DBP (mmHg) | 80 ± 3.0 | 79 ± 1.8 | 77 ± 1.8 | 78 ± 1.2 |
| Variable: | Below Recommended Protein (n = 17) | Meeting Recommended Protein (n = 22) | Above Recommended Protein (n = 23) | Total (n = 62) |
|---|---|---|---|---|
| Energy Intake (Kcal) | 1627 ± 69 | 2024 ± 105 a | 2163 ± 125 a | 1967 ± 67 |
| Fat (g) | 68 ± 3.6 d | 83 ± 5.6 | 86 ± 7.6 | 80 ± 3.6 |
| Saturated Fat (g) | 23 ± 1.9 | 25 ± 2.4 | 27 ± 2.9 | 25 ± 1.5 |
| Monounsaturated Fat (g) | 7.7 ± 1.3 c | 9.2 ± 1.7 | 12.7 ± 1.7 | 10.1 ± 1.0 |
| Polyunsaturated Fat (g) | 4.6 ± 0.86 c | 4.2 ± 0.75c | 8.1 ± 0.95 | 5.8 ± 0.55 |
| Trans Fat (g) | 1.1 ± 0.27 | 1.1 ± 0.27 | 1.3 ± 0.34 | 1.2 ± 0.17 |
| Cholesterol (mg) | 243 ± 22 | 367 ± 38 a | 411 ± 51 a | 349 ± 25 |
| Carbohydrates (g) | 190 ± 15 c | 234 ± 19 | 253 ± 19 | 228 ± 11 |
| Dietary Fiber (g) | 16 ± 1.5 c | 16 ± 1.5c | 23 ± 2.2 | 19 ± 1.1 |
| Total Sugar (g) | 84 ± 11 | 91 ± 14 | 91 ± 8 | 89 ± 6 |
| Protein (g) | 59 ± 3.5 b, c | 78 ± 2.2 a, c | 97 ± 4.1 a, b | 80 ± 2.7 |
| Animal Protein (g) | 35 ± 2.3 b, c | 51 ± 3.0 a, c | 68 ± 5.9 a, b | 53 ± 3.0 |
| Plant Protein (g) | 21 ± 2.9 c | 22 ± 1.9 c | 32.4 | 26 ± 1.8 |
| Animal/Plant Protein Ratio | 2.2 ± 0.33 | 2.6 ± 0.25 | 2.9 ± 0.43 | 2.6 ± 0.21 |
| Variables: | Total Energy Intake for BRP (kcals) | Total Energy Intake for MRP (kcals) | Total Energy Intake for ARP (kcals) |
|---|---|---|---|
| BMI (kg/m2) | 0.236 | 0.460 * | 0.190 |
| Total Body Fat (g) | 0.302 | 0.416 | 0.161 |
| Protein Intake (g) | 0.372 | 0.505 * | 0.433 |
| Variable: | Below Recommended Protein (n = 17) | Meeting Recommended Protein (n = 22) | Above Recommended Protein (n = 23) | Total (n = 62) |
|---|---|---|---|---|
| Blood Glucose (mg/dL) | 119 ± 5.7 | 121 ± 6.9 | 116 ± 5.5 | 119 ± 3.5 |
| Total Cholesterol (mg/dL) | 178 ± 8.1 | 212 ± 10 a | 197 ± 7.7 | 197 ± 5.3 |
| LDL-Cholesterol (mg/dL) | 91 ± 8.1 | 130 ± 12 a, c | 103 ± 7.4 | 109 ± 5.8 |
| HDL-Cholesterol (mg/dL) | 46 ± 1.9 | 55 ± 3.3 a | 51 ± 3.0 | 51 ± 1.7 |
| Triglycerides (mg/dL) | 142 ± 13 | 147 ± 12 | 128 ± 12 | 138 ± 7.1 |
| Insulin (uIU/dL) | 17 ± 2.7 | 15 ± 1.8 | 13 ± 1.5 | 15 ± 1.1 |
| Oxidized-LDL (U/L) | 9.3 ± 1.2 | 9.6 ± 0.75 | 8.0 ± 0.63 | 8.9 ± 0.47 |
| HOMA-IR | 5.2 ± 0.94 | 4.5 ± 0.62 | 3.5 ± 0.55 d | 4.3 ± 0.40 |
| HOMA-β | 123 ± 18 | 116 ± 20 | 87 ± 9.8 | 106 ± 9.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhavan, N.S.; Pourafshar, S.; Johnson, S.A.; Foley, E.M.; George, K.S.; Munoz, J.; Siebert, S.; Clark, E.A.; Basiri, R.; Hickner, R.C.; et al. The Relationship between Protein Intake and Source on Factors Associated with Glycemic Control in Individuals with Prediabetes and Type 2 Diabetes. Nutrients 2020, 12, 2031. https://doi.org/10.3390/nu12072031
Akhavan NS, Pourafshar S, Johnson SA, Foley EM, George KS, Munoz J, Siebert S, Clark EA, Basiri R, Hickner RC, et al. The Relationship between Protein Intake and Source on Factors Associated with Glycemic Control in Individuals with Prediabetes and Type 2 Diabetes. Nutrients. 2020; 12(7):2031. https://doi.org/10.3390/nu12072031
Chicago/Turabian StyleAkhavan, Neda S., Shirin Pourafshar, Sarah A. Johnson, Elizabeth M. Foley, Kelli S. George, Joseph Munoz, Shalom Siebert, Elizabeth A. Clark, Raedeh Basiri, Robert C. Hickner, and et al. 2020. "The Relationship between Protein Intake and Source on Factors Associated with Glycemic Control in Individuals with Prediabetes and Type 2 Diabetes" Nutrients 12, no. 7: 2031. https://doi.org/10.3390/nu12072031






