Breakfast Consumption in Low-Income Hispanic Elementary School-Aged Children: Associations with Anthropometric, Metabolic, and Dietary Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
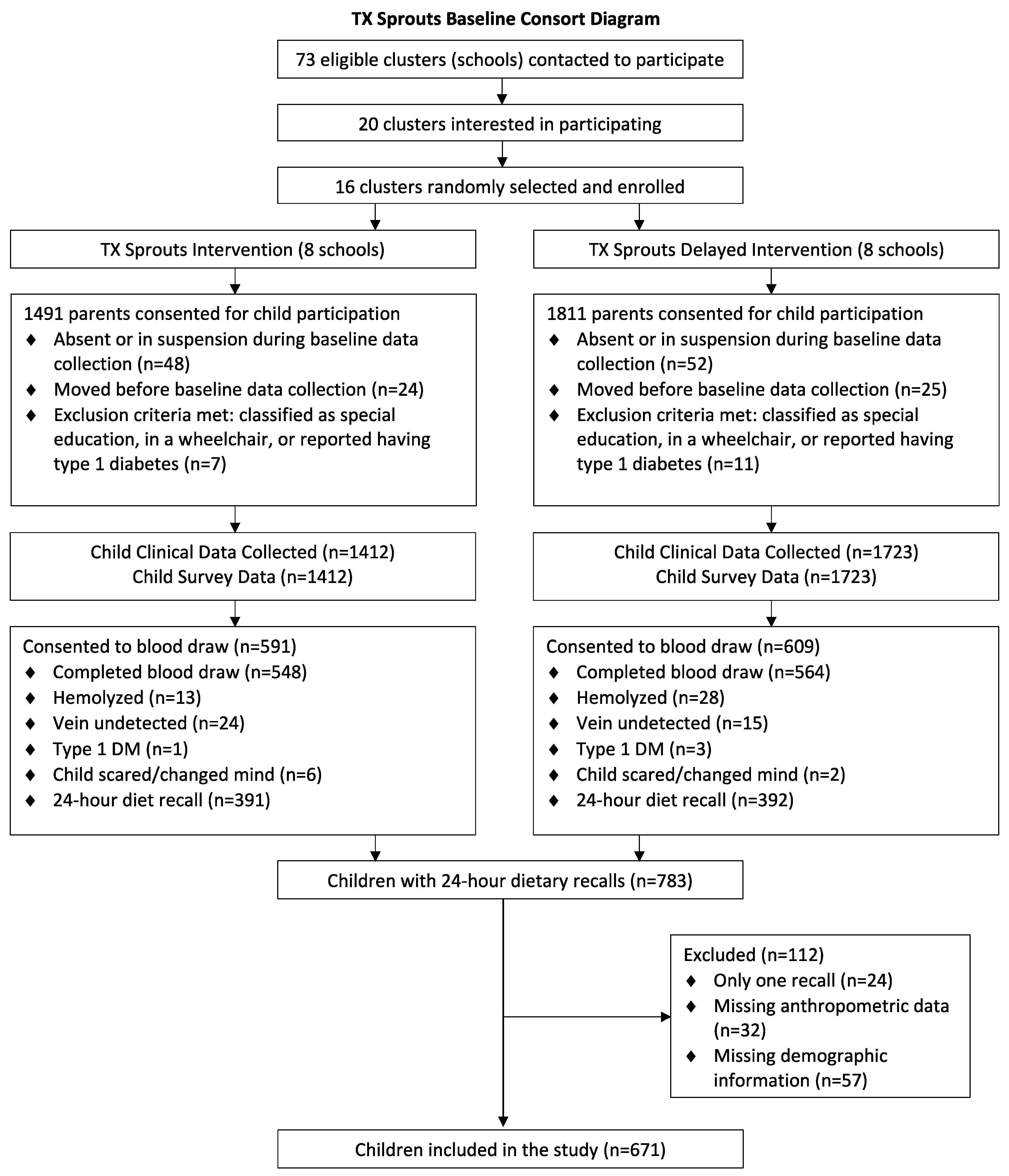
2.3. Recruitment
2.4. Anthropometric Parameters
2.5. Metabolic Parameters
2.6. Dietary Parameters
2.7. Breakfast Parameters
2.8. Statistical Analysis
3. Results
3.1. Study Sample
3.2. Relationships between Breakfast Consumption and Anthropometric and Metabolic Parameters
3.3. Relationships between Breakfast Consumption and Daily Dietary Parameters
3.4. Relationships between Breakfast Consumption and Breakfast Dietary Parameters
4. Discussion
Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief. 2017, 288, 1–8. [Google Scholar]
- Monzani, A.; Ricotti, R.; Caputo, M.; Solito, A.; Archero, F.; Bellone, S.; Prodam, F. A systematic review of the association of skipping breakfast with weight and cardiometabolic risk factors in children and adolescents. What should we better investigate in the future? Nutrients 2019, 11, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, K.J.; Gall, S.L.; McNaughton, S.A.; Blizzard, L.; Dwyer, T.; Venn, A.J. Skipping breakfast: Longitudinal associations with cardiometabolic risk factors in the Childhood determinants of adult health study. Am. J. Clin. Nutr. 2010, 92, 1316–1325. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, G.; Kelishadi, R.; Qorbani, M.; Motlagh, M.E.; Taheri, M.; Ardalan, G.; Taslimi, M.; Poursafa, P.; Heshmat, R.; Larijani, B. Association of breakfast intake with cardiometabolic risk factors. J. Pediatr. 2013, 89, 575–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, C.Y.; Huang, Y.C.; Lo, Y.T.; Wahlqvist, M.L.; Lee, M.S. Breakfast is associated with the metabolic syndrome and school performance among Taiwanese children. Res. Dev. Disabil. 2015, 43–44, 179–188. [Google Scholar] [CrossRef]
- Bauer, L.B.; Reynolds, L.J.; Douglas, S.M.; Kearney, M.L.; Hoertel, H.A.; Shafer, R.S.; Thyfault, J.P.; Leidy, H.J. A pilot study examining the effects of consuming a high-protein vs normal-protein breakfast on free-living glycemic control in overweight/obese ‘breakfast skipping’ adolescents. Int. J. Obes. 2015, 39, 1421–1424. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh-Taskar, P.; Nicklas, T.A.; Radcliffe, J.D.; O’Neil, C.E.; Liu, Y. The relationship of breakfast skipping and type of breakfast consumed with overweight/obesity, abdominal obesity, other cardiometabolic risk factors and the metabolic syndrome in young adults. The National Health and Nutrition Examination Survey (NHANES): 1999–2006. Public Health Nutr. 2013, 16, 2073–2082. [Google Scholar] [CrossRef] [Green Version]
- Monzani, A.; Rapa, A.; Fuiano, N.; Diddi, G.; Prodam, F.; Bellone, S.; Bona, G. Metabolic syndrome is strictly associated with parental obesity beginning from childhood. Clin. Endocrinol. 2014, 81, 45–51. [Google Scholar] [CrossRef]
- Osawa, H.; Sugihara, N.; Ukiya, T.; Ishizuka, Y.; Birkhed, D.; Hasegawa, M.; Matsukubo, T. Metabolic syndrome, lifestyle, and dental caries in Japanese school children. Bull. Tokyo Dent. Coll. 2015, 56, 233–241. [Google Scholar] [CrossRef] [Green Version]
- O’Neil, C.E.; Nicklas, T.A.; Fulgoni, V.L., 3rd. Nutrient intake, diet quality, and weight measures in breakfast patterns consumed by children compared with breakfast skippers: NHANES 2001–2008. AIMS Public Health 2015, 2, 441–468. [Google Scholar] [CrossRef]
- Smetanina, N.; Albaviciute, E.; Babinska, V.; Karinauskiene, L.; Albertsson-Wikland, K.; Petrauskiene, A.; Verkauskiene, R. Prevalence of overweight/obesity in relation to dietary habits and lifestyle among 7–17 years old children and adolescents in Lithuania. BMC Public Health 2015, 15, 1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakrzewski, J.K.; Gillison, F.B.; Cumming, S.; Church, T.S.; Katzmarzyk, P.T.; Broyles, S.T.; Champagne, C.M.; Chaput, J.P.; Denstel, K.D.; Fogelholm, M.; et al. Associations between breakfast frequency and adiposity indicators in children from 12 countries. Int. J. Obes. Suppl. 2015, 5, S80–S88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fayet-Moore, F.; Kim, J.; Sritharan, N.; Petocz, P. Impact of breakfast skipping and breakfast choice on the nutrient intake and body mass index of australian children. Nutrients 2016, 8, 487. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.J.; Breslin, M.C.; McNaughton, S.A.; Gall, S.L.; Blizzard, L.; Venn, A.J. Skipping breakfast among Australian children and adolescents; findings from the 2011–12 national nutrition and physical activity survey. Aust. N. Z. J. Public Health 2017, 41, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Gotthelf, S.J.; Tempestti, C.P. Breakfast, nutritional status, and socioeconomic outcome measures among primary school students from the City of Salta: A cross-sectional study. Arch. Argent. Pediatr. 2017, 115, 424–431. [Google Scholar] [CrossRef]
- Nilsen, B.B.; Yngve, A.; Monteagudo, C.; Tellstrom, R.; Scander, H.; Werner, B. Reported habitual intake of breakfast and selected foods in relation to overweight status among seven- to nine-year-old Swedish children. Scand. J. Public Health 2017, 45, 886–894. [Google Scholar] [CrossRef]
- Tee, E.S.; Nurliyana, A.R.; Norimah, A.K.; Mohamed, H.; Tan, S.Y.; Appukutty, M.; Hopkins, S.; Thielecke, F.; Ong, M.K.; Ning, C.; et al. Breakfast consumption among Malaysian primary and secondary school children and relationship with body weight status—Findings from the MyBreakfast Study. Asia Pac. J. Clin. Nutr. 2018, 27, 421–432. [Google Scholar] [CrossRef]
- Archero, F.; Ricotti, R.; Solito, A.; Carrera, D.; Civello, F.; Di Bella, R.; Bellone, S.; Prodam, F. Adherence to the mediterranean diet among school children and adolescents living in northern italy and unhealthy food behaviors associated to overweight. Nutrients 2018, 10, 1322. [Google Scholar] [CrossRef] [Green Version]
- Gibney, M.J.; Barr, S.I.; Bellisle, F.; Drewnowski, A.; Fagt, S.; Livingstone, B.; Masset, G.; Varela Moreiras, G.; Moreno, L.A.; Smith, J.; et al. Breakfast in human nutrition: The international breakfast research initiative. Nutrients 2018, 10, 559. [Google Scholar] [CrossRef] [Green Version]
- Currie, C.Z.C.; Morgan, A.; Currie, D.; de Looze, M.; Roberts, C.; Samdal, O.; Smith, O.R.F.; Barnekow, V. (Eds.) Social determinants of health and well-being among young people. In Health Behaviour in School-Aged Children (HBSC) Study: International Report from the 2009/2010 Survey; World Health Organization: Copenhagen, Danmark, 2012; p. 252. [Google Scholar]
- Murata, M. Secular trends in growth and changes in eating patterns of Japanese children. Am. J. Clin. Nutr. 2000, 72, 1379S–1383S. [Google Scholar] [CrossRef] [Green Version]
- Dwyer, J.T.; Evans, M.; Stone, E.J.; Feldman, H.A.; Lytle, L.; Hoelscher, D.; Johnson, C.; Zive, M.; Yang, M. Child and adolescents′ eating patterns influence their nutrient intakes. J. Am. Diet. Assoc. 2001, 101, 798–802. [Google Scholar] [CrossRef]
- Van Kleef, E.; Vingerhoeds, M.H.; Vrijhof, M.; van Trijp, H.C.M. Breakfast barriers and opportunities for children living in a Dutch disadvantaged neighbourhood. Appetite 2016, 107, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Papas, M.A.; Trabulsi, J.C.; Dahl, A.; Dominick, G. Food insecurity increases the odds of obesity among young hispanic children. J. Immigr. Minor. Health 2016, 18, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Potochnick, S.; Perreira, K.M.; Bravin, J.I.; Castaneda, S.F.; Daviglus, M.L.; Gallo, L.C.; Isasi, C.R. Food insecurity among hispanic/latino youth: Who is at risk and what are the health correlates? J. Adolesc. Health 2019, 64, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Kuriyan, R.; Thomas, T.; Sumithra, S.; Lokesh, D.P.; Sheth, N.R.; Joy, R.; Bhat, S.; Kurpad, A.V. Potential factors related to waist circumference in urban South Indian children. Indian Pediatr. 2012, 49, 124–128. [Google Scholar] [CrossRef]
- Kupers, L.K.; de Pijper, J.J.; Sauer, P.J.; Stolk, R.P.; Corpeleijn, E. Skipping breakfast and overweight in 2- and 5-year-old Dutch children-the GECKO Drenthe cohort. Int. J. Obes. 2014, 38, 569–571. [Google Scholar] [CrossRef]
- Fayet-Moore, F.; McConnell, A.; Tuck, K.; Petocz, P. Breakfast and breakfast cereal choice and its impact on nutrient and sugar intakes and anthropometric measures among a nationally representative sample of australian children and adolescents. Nutrients 2017, 9, 1045. [Google Scholar] [CrossRef]
- Polonsky, H.M.; Bauer, K.W.; Fisher, J.O.; Davey, A.; Sherman, S.; Abel, M.L.; Hanlon, A.; Ruth, K.J.; Dale, L.C.; Foster, G.D. Effect of a breakfast in the classroom initiative on obesity in urban school-aged children: A cluster randomized clinical trial. JAMA Pediatr. 2019, 173, 326–333. [Google Scholar] [CrossRef]
- Coulthard, J.D.; Palla, L.; Pot, G.K. Breakfast consumption and nutrient intakes in 4–18-year-olds: UK national diet and nutrition survey rolling programme (2008–2012). Br. J. Nutr. 2017, 118, 280–290. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh-Taskar, P.R.; Nicklas, T.A.; O’Neil, C.E.; Keast, D.R.; Radcliffe, J.D.; Cho, S. The relationship of breakfast skipping and type of breakfast consumption with nutrient intake and weight status in children and adolescents: The National health and nutrition examination survey 1999–2006. J. Am. Diet. Assoc. 2010, 110, 869–878. [Google Scholar] [CrossRef]
- Drewnowski, A.; Rehm, C.D.; Vieux, F. Breakfast in the United States: Food and nutrient intakes in relation to diet quality in national health and examination survey 2011–2014. a study from the international breakfast research initiative. Nutrients 2018, 10, 1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lepicard, E.M.; Maillot, M.; Vieux, F.; Viltard, M.; Bonnet, F. Quantitative and qualitative analysis of breakfast nutritional composition in French schoolchildren aged 9–11 years. J. Hum. Nutr. Diet. 2017, 30, 151–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, J.; Nikah, K.; Asigbee, F.M.; Landry, M.J.; Vandyousefi, S.; Ghaddar, R.; Hoover, A.; Jeans, M.; Pont, S.J.; Richards, D.; et al. Design and participant characteristics of TX sprouts: A school-based cluster randomized gardening, nutrition, and cooking intervention. Contemp Clin. Trials 2019, 85, 105834. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Anthropometry Procedures Manual. 2007. Available online: https://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/manual_an.pdf (accessed on 7 July 2020).
- Centers for Disease Control and Prevention. Clinical Growth Charts. 2000. Available online: https://www.cdc.gov/growthcharts/clinical_charts.htm (accessed on 7 July 2020).
- American Diabetes, A. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37 (Suppl. 1), S81–S90. [Google Scholar] [CrossRef] [Green Version]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Burrows, T.L.; Martin, R.J.; Collins, C.E. A systematic review of the validity of dietary assessment methods in children when compared with the method of doubly labeled water. J. Am. Diet. Assoc. 2010, 110, 1501–1510. [Google Scholar] [CrossRef]
- Feskanich, D.; Sielaff, B.H.; Chong, K.; Buzzard, I.M. Computerized collection and analysis of dietary intake information. Comput. Methods Programs Biomed. 1989, 30, 47–57. [Google Scholar] [CrossRef]
- National Cancer Institute. Developing the Healthy Eating Index. 2015. Available online: https://epi.grants.cancer.gov/hei/developing.html (accessed on 7 July 2020).
- Kirkpatrick, S.I.; Reedy, J.; Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Wilson, M.M.; Lerman, J.L.; Tooze, J.A. Applications of the healthy eating index for surveillance, epidemiology, and intervention research: Considerations and caveats. J. Acad. Nutr. Diet. 2018, 118, 1603–1621. [Google Scholar] [CrossRef]
- Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Kirkpatrick, S.I.; Lerman, J.L.; Tooze, J.A.; Wilson, M.M.; Reedy, J. Update of the healthy eating index: HEI-2015. J. Acad. Nutr. Diet. 2018, 118, 1591–1602. [Google Scholar] [CrossRef] [Green Version]
- Reedy, J.; Lerman, J.L.; Krebs-Smith, S.M.; Kirkpatrick, S.I.; Pannucci, T.E.; Wilson, M.M.; Subar, A.F.; Kahle, L.L.; Tooze, J.A. Evaluation of the healthy eating index-2015. J. Acad. Nutr. Diet. 2018, 118, 1622–1633. [Google Scholar] [CrossRef]
- Johnson, R.K.; Driscoll, P.; Goran, M.I. Comparison of multiple-pass 24-hour recall estimates of energy intake with total energy expenditure determined by the doubly labeled water method in young children. J. Am. Diet. Assoc. 1996, 96, 1140–1144. [Google Scholar] [CrossRef]
- Leech, R.M.; Worsley, A.; Timperio, A.; McNaughton, S.A. Characterizing eating patterns: A comparison of eating occasion definitions. Am. J. Clin. Nutr. 2015, 102, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- O′Neil, C.E.; Byrd-Bredbenner, C.; Hayes, D.; Jana, L.; Klinger, S.E.; Stephenson-Martin, S. The role of breakfast in health: Definition and criteria for a quality breakfast. J. Acad. Nutr. Diet. 2014, 114, S8–S26. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.A.; Erickson, E.; McKee, P.; Schrankler, K.; Raatz, S.K.; Lytle, L.A.; Pellegrini, A.D. Breakfast frequency and quality may affect glycemia and appetite in adults and children. J. Nutr. 2011, 141, 163–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siega-Riz, A.M.; Popkin, B.M.; Carson, T. Trends in breakfast consumption for children in the United States from 1965–1991. Am. J. Clin. Nutr. 1998, 67, 748S–756S. [Google Scholar] [CrossRef]
- Cho, S.; Dietrich, M.; Brown, C.J.; Clark, C.A.; Block, G. The effect of breakfast type on total daily energy intake and body mass index: Results from the third national health and nutrition examination survey (NHANES III). J. Am. Coll. Nutr. 2003, 22, 296–302. [Google Scholar] [CrossRef]
- Deshmukh-Taskar, P.R.; Radcliffe, J.D.; Liu, Y.; Nicklas, T.A. Do breakfast skipping and breakfast type affect energy intake, nutrient intake, nutrient adequacy, and diet quality in young adults? NHANES 1999–2002. J. Am. Coll. Nutr. 2010, 29, 407–418. [Google Scholar] [CrossRef]
- Lyerly, J.E.; Huber, L.R.; Warren-Findlow, J.; Racine, E.F.; Dmochowski, J. Is breakfast skipping associated with physical activity among U.S. adolescents? A cross-sectional study of adolescents aged 12–19 years, National Health and Nutrition Examination Survey (NHANES). Public Health Nutr. 2014, 17, 896–905. [Google Scholar] [CrossRef] [Green Version]
- United States Department of Agriculture. HEI Scores for Americans. Average Healthy Eating Index-2015 Scores for Americans by Age Group, WWEIA/NHANES 2015–2016. 2016. Available online: https://fns-azureedge.net/sites/default/files/media/file/HEI-2015_1516_web.pdf (accessed on 7 July 2020).
- Gaal, S.; Kerr, M.A.; Ward, M.; McNulty, H.; Livingstone, M.B.E. Breakfast consumption in the UK: Patterns, nutrient intake and diet quality. A study from the international breakfast research initiative group. Nutrients 2018, 10, 999. [Google Scholar] [CrossRef] [Green Version]
- Barr, S.I.; Vatanparast, H.; Smith, J. Breakfast in Canada: Prevalence of consumption, contribution to nutrient and food group intakes, and variability across tertiles of daily diet quality. A study from the international breakfast research initiative. Nutrients 2018, 10, 985. [Google Scholar] [CrossRef] [Green Version]
- Chaumontet, C.; Even, P.C.; Schwarz, J.; Simonin-Foucault, A.; Piedcoq, J.; Fromentin, G.; Azzout-Marniche, D.; Tome, D. High dietary protein decreases fat deposition induced by high-fat and high-sucrose diet in rats. Br. J. Nutr. 2015, 114, 1132–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- French, W.W.; Dridi, S.; Shouse, S.A.; Wu, H.; Hawley, A.; Lee, S.O.; Gu, X.; Baum, J.I. A High-protein diet reduces weight gain, decreases food intake, decreases liver fat deposition, and improves markers of muscle metabolism in obese zucker Rats. Nutrients 2017, 9, 587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Du, S.; Wang, H.; Popkin, B. Influence of dietary fat intake on bodyweight and risk of obesity among Chinese adults, 1991–2015: A prospective cohort study. Lancet 2018, 392. [Google Scholar] [CrossRef]
- Harland, J.I.; Garton, L.E. Whole-grain intake as a marker of healthy body weight and adiposity. Public Health Nutr. 2008, 11, 554–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Shang, L.; Light, K.; O′Loughlin, J.; Paradis, G.; Gray-Donald, K. Associations between added sugar (solid vs. liquid) intakes, diet quality, and adiposity indicators in Canadian children. Appl. Physiol. Nutr. Metab. 2015, 40, 835–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigornia, S.J.; LaValley, M.P.; Noel, S.E.; Moore, L.L.; Ness, A.R.; Newby, P.K. Sugar-sweetened beverage consumption and central and total adiposity in older children: A prospective study accounting for dietary reporting errors. Public Health Nutr. 2015, 18, 1155–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laverty, A.A.; Magee, L.; Monteiro, C.A.; Saxena, S.; Millett, C. Sugar and artificially sweetened beverage consumption and adiposity changes: National longitudinal study. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 137. [Google Scholar] [CrossRef] [Green Version]
- Seferidi, P.; Millett, C.; Laverty, A.A. Sweetened beverage intake in association to energy and sugar consumption and cardiometabolic markers in children. Pediatr. Obes. 2018, 13, 195–203. [Google Scholar] [CrossRef]
- Mattes, R. Soup and satiety. Physiol. Behav. 2005, 83, 739–747. [Google Scholar] [CrossRef]
- Bolton, R.P.; Heaton, K.W.; Burroughs, L.F. The role of dietary fiber in satiety, glucose, and insulin: Studies with fruit and fruit juice. Am. J. Clin. Nutr. 1981, 34, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Flood-Obbagy, J.E.; Rolls, B.J. The effect of fruit in different forms on energy intake and satiety at a meal. Appetite 2009, 52, 416–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- US Department of Agriculture. Percentages of Selected Nutrients Contributed by Food and Beverages Consumed at Breakfast, by Race/Ethnicity and Age. What We Eat. In America NHANES 2015–2016. Available online: https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/1516/Table_14_BRK_RAC_15.pdf (accessed on 7 July 2020).
- US. Department of Health and Human Services; U.S. Department of Agriculture. Dietary Guidelines for Americans, 2015–2020, 8th ed.; U.S. Government Printing Office: Washington, DC, USA, 2015.
- Lennerz, B.; Lennerz, J.K. Food Addiction, High-Glycemic-Index Carbohydrates, and Obesity. Clin. Chem. 2018, 64, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Filgueiras, A.R.; Pires de Almeida, V.B.; Koch Nogueira, P.C.; Alvares Domene, S.M.; Eduardo da Silva, C.; Sesso, R.; Sawaya, A.L. Exploring the consumption of ultra-processed foods and its association with food addiction in overweight children. Appetite 2019, 135, 137–145. [Google Scholar] [CrossRef]
- Afeiche, M.C.; Taillie, L.S.; Hopkins, S.; Eldridge, A.L.; Popkin, B.M. Breakfast dietary patterns among mexican children are related to total-day diet quality. J. Nutr. 2017, 147, 404–412. [Google Scholar] [CrossRef] [PubMed]
- US Department of Agriculture, A.R.S. Distribution of Meal Patterns and Snack Occasions, by Race/Ethnicity and Age. What We Eat. In America NHANES 2015–2016. Available online: https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/1516/Table_34_DMP_RAC_15.pdf (accessed on 8 July 2020).
| Variable | Value a |
|---|---|
| Sex (F) | 364 (54.3%) |
| Age (years) | 9.3 (9.2, 9.3) |
| Ethnicity | |
| Hispanic | 392 (58.4%) |
| Non-Hispanic | 279 (41.6%) |
| Free/Reduced Lunch | 446 (66.5%) |
| Breakfast Consumption Groups | |
| Skippers | 114 (17.0%) |
| Intermittent Consumers | 249 (37.1%) |
| Regular Consumers | 308 (45.9%) |
| Height (cm) | 138.4 (137.8, 139.1) |
| Weight (kg) | 39.4 (38.5, 40.3) |
| BMI z-score | 0.8 (0.8, 0.9) |
| BMI categories | |
| Overweight | 129 (19.2%) |
| Obese | 198 (29.5%) |
| Variable | Mean a | 95% CIs |
|---|---|---|
| Eating occasions | 3.3 | (3.3, 3.4) |
| Healthy Eating Index-2015 | 53.8 | (52.8, 54.7) |
| Total energy (kcal/day) | 1446.2 | (1405.1, 1487.3) |
| Carbohydrate (% kcal) | 49.7 | (49.0, 50.3) |
| Protein (% kcal) | 16.2 | (15.9, 16.5) |
| Fat (% kcal) | 33.3 | (32.8, 33.8) |
| Total fiber (g) | 12.1 | (11.7, 12.6) |
| Soluble fiber (g) | 3.8 | (3.7, 4.0) |
| Insoluble fiber (g) | 8.2 | (7.9, 8.5) |
| Total sugar (% kcal) | 20.7 | (20.2, 21.3) |
| Added sugar (% kcal) | 10.1 | (9.6,10.5) |
| Total vegetables (serving/day) b | 1.7 | (1.6, 1.8) |
| Excluding 100% juice | 1.7 | (1.6, 1.8) |
| Excluding 100% juice and potatoes | 1.5 | (1.4, 1.6) |
| Total Fruits (serving/day) | 1.5 | (1.4, 1.7) |
| Excluding 100% juice | 0.9 | (0.8, 1.0) |
| Dairy (serving/day) | 1.7 | (1.6, 1.8) |
| Excluding flavored milk | 1.5 | (1.4, 1.6) |
| Sugar-sweetened beverages (serving/day) | 0.7 | (0.7, 0.8) |
| Excluding flavored milk | 0.5 | (0.4, 0.6) |
| Whole grains (serving/day) | 1.1 | (1.0, 1.2) |
| Refined grains (serving/day) | 4.6 | (4.4, 4.9) |
| Meat (serving/day) | 3.7 | (3.5, 3.8) |
| Legumes (serving/day) | 0.2 | (0.16, 0.23) |
| Overall † | <-------------------- Skipper --------------------> | <-------------------- Intermittent --------------------> | |||||
|---|---|---|---|---|---|---|---|
| Variable | p | β | (95% CI) | p | β | (95% CI) | p |
| Anthropometric Parameters (n = 671) | |||||||
| Waist circumference (cm) a | 0.09 | 0.59 | (−1.98, 3.16) | 0.62 | 2.09 | (0.08, 4.10) | 0.03 |
| Total body fat (%) a | 0.39 | −0.05 | (−1.95, 1.86) | 0.76 | 0.82 | (−0.67, 2.31) | 0.17 |
| BMI z-score a | 0.12 | 0.12 | (−0.12, 0.36) | 0.33 | 0.19 | (0.01, 0.38) | 0.04 |
| Systolic blood pressure (mmHg) a | 0.58 | −0.10 | (−2.51, 2.31) | 0.99 | 0.84 | (−1.05, 2.73) | 0.33 |
| Diastolic blood pressure (mmHg) a | 0.72 | 0.63 | (−1.46, 2.72) | 0.64 | 0.43 | (−1.21, 2.06) | 0.43 |
| Metabolic Parameters (n = 344) | |||||||
| Fasting glucose (mg/dL) b,c,d | 0.89 | 0.47 | (−2.23, 3.17) | 0.73 | −0.21 | (−2.42, 2.01) | 0.85 |
| Insulin (µIU/mL) b,c,d | 0.67 | 0.89 | (−2.29, 4.08) | 0.39 | 1.26 | (−1.35, 3.87) | 0.90 |
| Triglycerides (mg/dL) b,c,d | 0.37 | 4.14 | (−7.46, 15.74) | 0.61 | −5.98 | (−15.49, 3.52) | 0.32 |
| Total cholesterol (mg/dL) b,c,d | 0.95 | 0.42 | (−6.82, 7.66) | 0.91 | 0.93 | (−5.00, 6.87) | 0.76 |
| HDL (mg/dL) c | 0.96 | −0.30 | (−2.95, 2.36) | 0.89 | −0.31 | (−2.49, 1.87) | 0.77 |
| Non-HDL (mg/dL) c | 0.91 | 0.69 | (−5.86, 7.24) | 0.84 | 1.18 | (−4.19, 6.54) | 0.67 |
| LDL (mg/dL) c | 0.54 | −0.15 | (−6.05, 5.74) | 0.96 | 2.45 | (−2.38, 7.28) | 0.32 |
| HbA1c (%) d | 0.49 | −0.09 | (−0.15, 0.09) | 0.50 | 0.04 | (−0.06, 0.15) | 0.53 |
| Overall † | <----------------- Skipper -----------------> | <--------------- Intermittent ---------------> | |||||
|---|---|---|---|---|---|---|---|
| Variable | p | β | (95% CI) | p | β | (95% CI) | p |
| Dietary Intake Parameters | |||||||
| Eating occasions | <0.001 * | −0.87 | (−1.01, −0.73) | <0.001 * | −0.42 | (−0.53, −0.30) | <0.001 * |
| HEI-2015 | <0.005 * | −3.88 | (−6.52, −1.24) | 0.004 * | −2.69 | (−4.76, −0.62) | 0.01 * |
| Total energy (kcal) | <0.004 * | 111.35 | (−5.11, 227.81) | 0.14 | 174.73 | (84.25, 265.20) | 0.001 * |
| Protein (%kcal) a,b | <0.001 * | −1.46 | (−2.26, −0.66) | <0.001 * | −0.96 | (−1.59, −0.34) | 0.001 * |
| Fat (%kcal) a,b | <0.005 * | 2.48 | (1.00, 3.95) | 0.001 * | 0.90 | (−0.25, 2.06) | 0.13 |
| Carbohydrate (%kcal) a,b | <0.001 * | −7.28 | (−9.10, −5.46) | <0.001 * | −2.82 | (−4.24, −1.39) | <0.001 * |
| Total fiber (g) a,b | <0.007 * | −1.03 | (−1.97, −0.09) | <0.02 * | −0.98 | (−1.72, −0.25) | 0.004 * |
| Total sugar (%kcal) a,b | <0.001 * | −5.91 | (−7.55, −4.26) | <0.001 * | −2.03 | (−3.32, −0.75) | 0.002 * |
| Soluble fiber (g) b | <0.001 * | −0.62 | (−0.93, −0.31) | <0.001 * | −0.39 | (−0.63, −0.14) | 0.001 * |
| Insoluble fiber (g) b | 0.051 | −0.46 | (−1.23, 0.30) | 0.12 | −0.61 | (−1.21, −0.01) | 0.02 |
| Added sugar (%kcal) b | 0.003 * | −1.64 | (−2.91, −0.37) | 0.002 * | 0.31 | (−0.68, 1.30) | 0.81 |
| Food and Beverage Servings | |||||||
| Whole grains (serving/day) c,d,e | 0.002 * | −0.14 | (−0.40, 0.13) | 0.001 * | −0.05 | (−0.26, 0.16) | 0.37 |
| Refined grains (serving/day) c,d,e | 0.16 | −0.21 | (−0.69, 0.28) | 0.06 | −0.27 | (−0.65, 0.11) | 0.32 |
| Meats (serving/day) c,d,e | 0.98 | 0.17 | (−0.30, 0.65) | 0.94 | 0.10 | (−0.27, 0.48) | 0.88 |
| Legumes (serving/day) c,d,e | 0.07 | 0.10 | (0.01, 0.19) | 0.02 | 0.01 | (−0.06, 0.08) | 0.64 |
| Vegetables, including 100% juice & potatoes (serving/day) c | 0.87 | −0.001 | (−0.26, 0.26) | 0.86 | −0.09 | (−0.29, 0.12) | 0.69 |
| Fruit, including 100% juice (serving/day) c | 0.001 * | −0.43 | (−0.77, −0.08) | 0.002 * | −0.24 | (−0.51, 0.03) | 0.003 * |
| Dairy, including flavored milk (serving/day) c | 0.09 | −0.18 | (−0.40, 0.03) | 0.03 | −0.09 | (−0.26, 0.08) | 0.30 |
| SSBs, including flavored milk (serving/day) c | 0.44 | −0.09 | (−0.28, 0.09) | 0.42 | 0.10 | (−0.04, 0.25) | 0.52 |
| Vegetables, excluding 100% juice (serving/day) d,e | 0.85 | 0.0004 | (−0.26, 0.26) | 0.85 | −0.09 | (−0.26, 0.26) | 0.66 |
| Fruit, excluding 100% juice (serving/day) d,e | 0.29 | −0.10 | (−0.40, 0.19) | 0.27 | −0.05 | (−0.28, 0.18) | 0.15 |
| Dairy, excluding flavored milk (serving/day) d,e | 0.40 | −0.07 | (−0.28, 0.14) | 0.27 | −0.09 | (−0.28, 1.4) | 0.26 |
| SSBs, excluding flavored milk (serving/day) d,e | 0.65 | 0.02 | (−0.14, 0.18) | 0.61 | 0.10 | (−0.03, 0.22) | 0.37 |
| Vegetables, excluding 100% juice & potatoes (serving/day) e | 0.57 | 0.03 | (−0.22, 0.28) | 0.66 | −0.12 | (−0.31, 0.07) | 0.46 |
| Intermittent | |||
|---|---|---|---|
| Variable | β | (95% CI) | p |
| Breakfast Dietary Intake Parameters | |||
| Breakfast total energy (kcal) | −207.11 | (−208.88, −159.76) | <0.001 * |
| Breakfast protein (%kcal) a,b | −0.34 | (−1.13, 0.44) | 0.13 |
| Breakfast fat (%kcal) a,b | 0.51 | (−1.80, 2.82) | 0.59 |
| Breakfast carbohydrate (%kcal) a,b | −0.37 | (−3.24, 2.51) | 0.43 |
| Breakfast total fiber (g) a | −1.75 | (−2.07, −1.43) | <0.001 * |
| Breakfast total sugar (%kcal) a | −0.61 | (−3.12, 1.89) | 0.63 |
| Breakfast soluble fiber (g) b | −0.62 | (−0.74, −0.51) | <0.001 * |
| Breakfast insoluble fiber (g) b | −1.11 | (−1.34, −0.87) | <0.001 * |
| Breakfast added sugar (%kcal) b | 1.38 | (−0.52, 3.29) | 0.81 |
| Breakfast Food Servings | |||
| Breakfast whole grains (serving/day) c | −0.22 | (−0.31, −0.13) | <0.001 * |
| Breakfast refined grains (serving/day) c | −0.03 | (−0.12, 0.07) | 0.14 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeans, M.R.; Asigbee, F.M.; Landry, M.J.; Vandyousefi, S.; Ghaddar, R.; Leidy, H.J.; Davis, J.N. Breakfast Consumption in Low-Income Hispanic Elementary School-Aged Children: Associations with Anthropometric, Metabolic, and Dietary Parameters. Nutrients 2020, 12, 2038. https://doi.org/10.3390/nu12072038
Jeans MR, Asigbee FM, Landry MJ, Vandyousefi S, Ghaddar R, Leidy HJ, Davis JN. Breakfast Consumption in Low-Income Hispanic Elementary School-Aged Children: Associations with Anthropometric, Metabolic, and Dietary Parameters. Nutrients. 2020; 12(7):2038. https://doi.org/10.3390/nu12072038
Chicago/Turabian StyleJeans, Matthew R., Fiona M. Asigbee, Matthew J. Landry, Sarvenaz Vandyousefi, Reem Ghaddar, Heather J. Leidy, and Jaimie N. Davis. 2020. "Breakfast Consumption in Low-Income Hispanic Elementary School-Aged Children: Associations with Anthropometric, Metabolic, and Dietary Parameters" Nutrients 12, no. 7: 2038. https://doi.org/10.3390/nu12072038







