A High-Protein Meal during a Night Shift Does Not Improve Postprandial Metabolic Response the Following Breakfast: A Randomized Crossover Study with Night Workers
Abstract
:1. Introduction
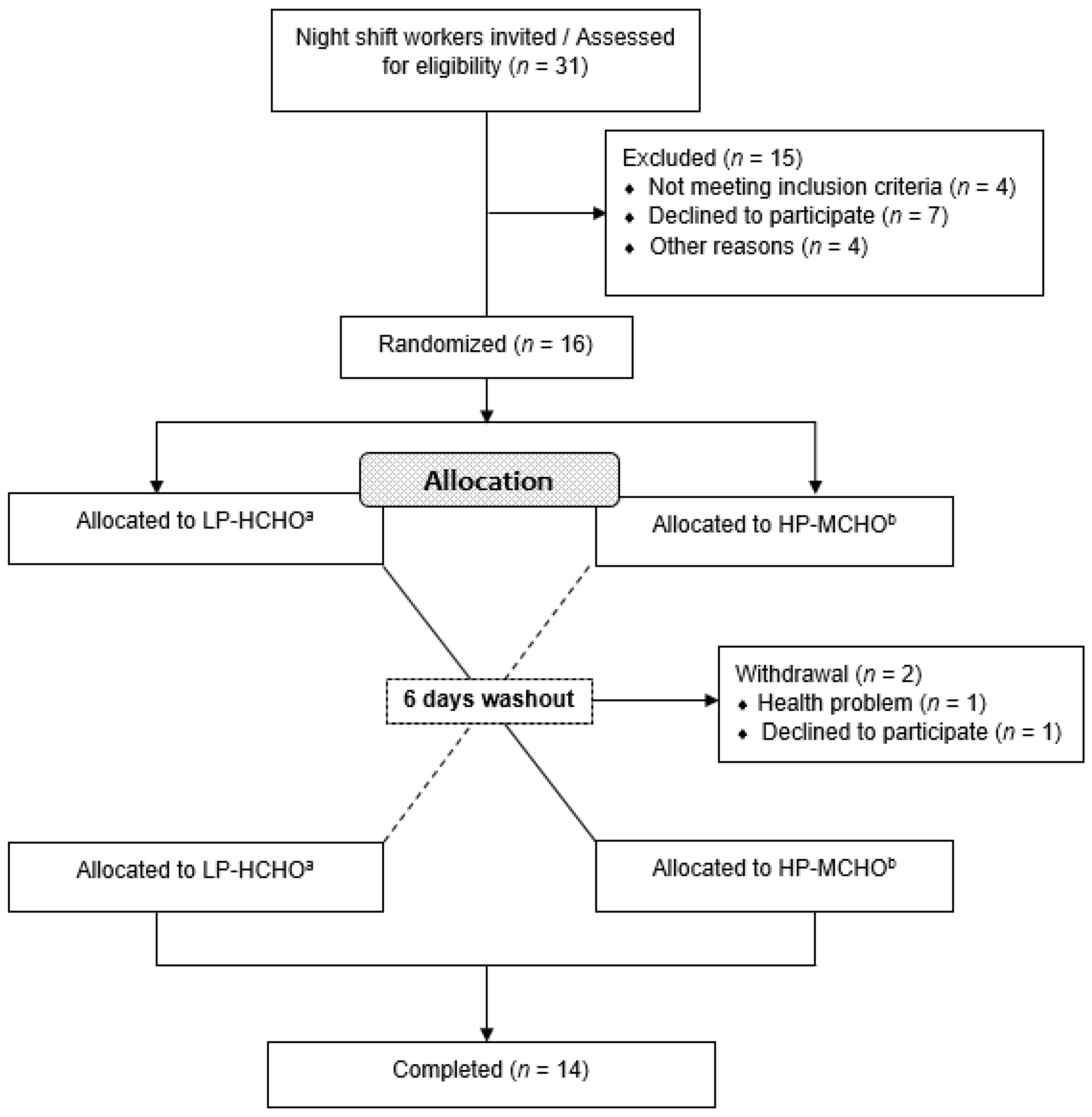
2. Methods
2.1. Participants and Ethics
2.2. Pre-Intervention Procedures
2.2.1. Initial Questionnaire
2.2.2. Anthropometric Evaluation
2.2.3. Basal Metabolic Assessment
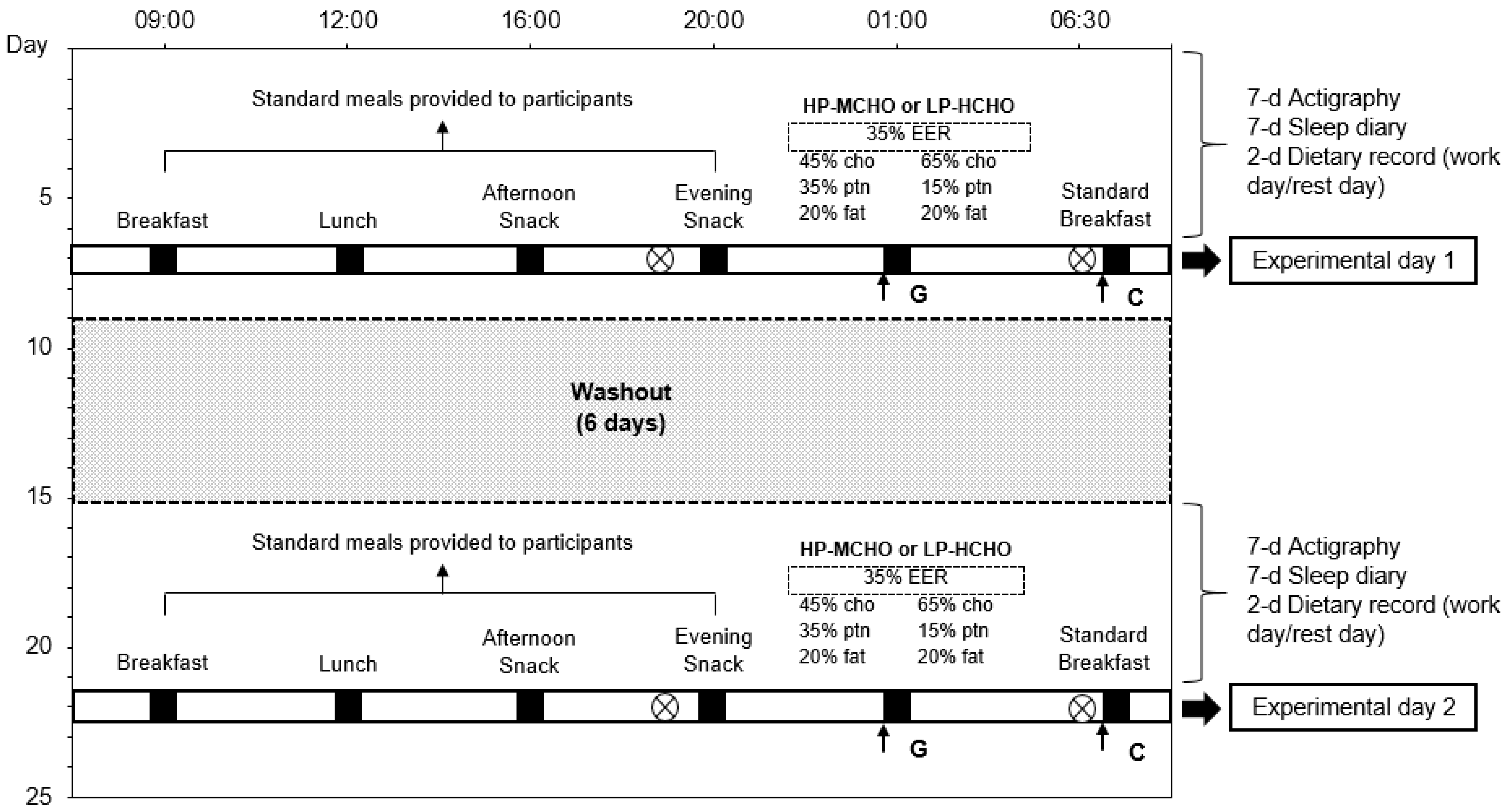
2.3. Experimental Protocol
2.3.1. Dietary Conditions
2.3.2. Metabolic Assessment
2.4. Statistical Analysis
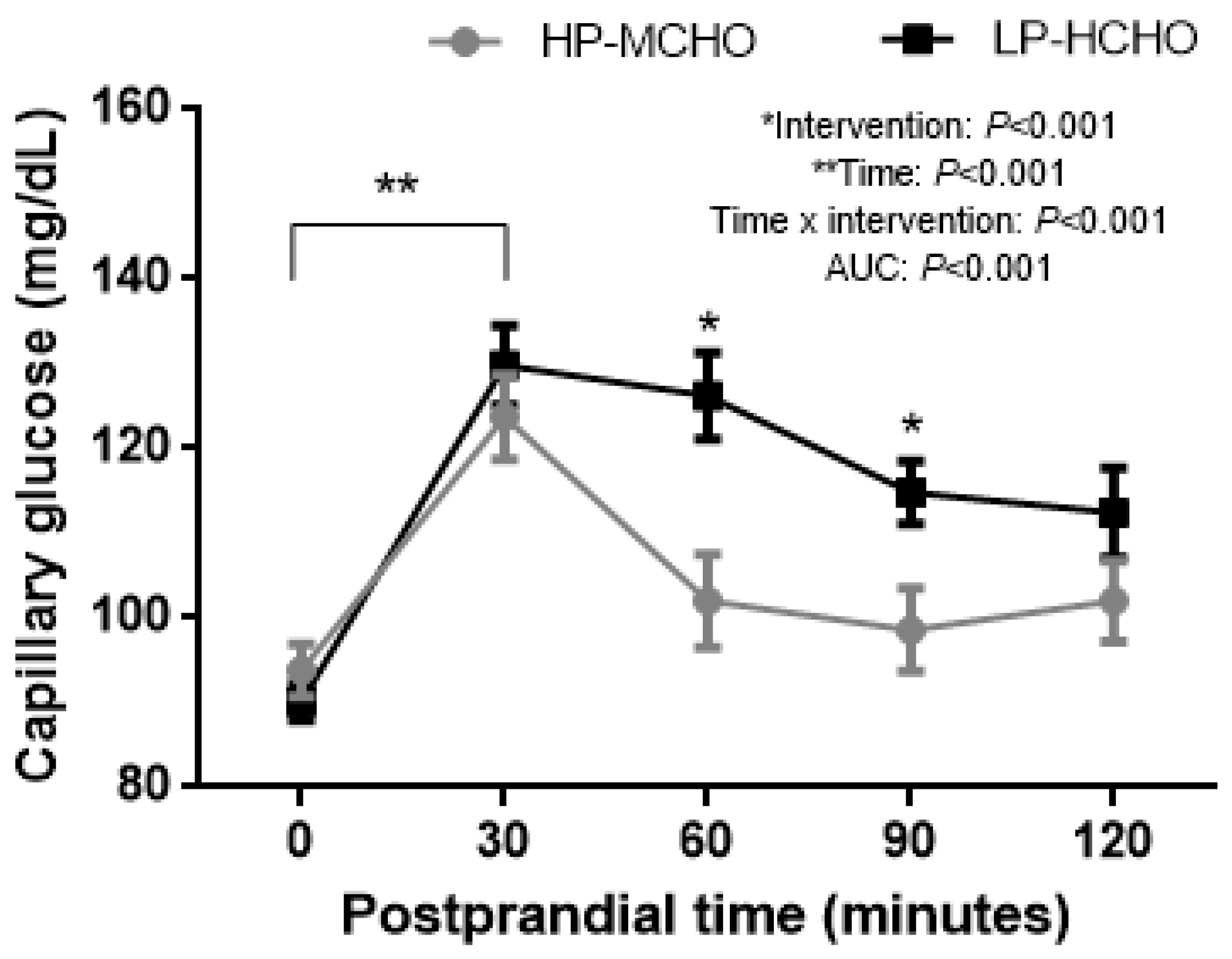
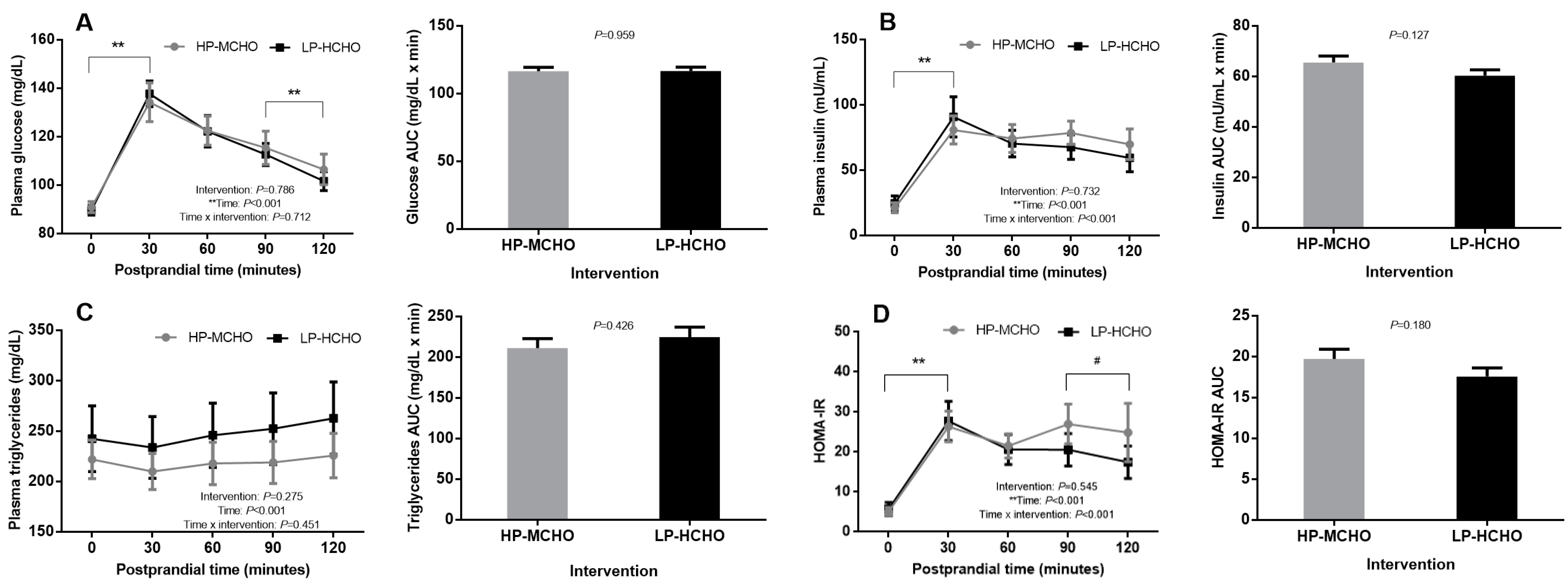
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Canuto, R.; Garcez, A.S.; Olinto, M.T.A. Metabolic syndrome and shift work: A systematic review. Sleep Med. Rev. 2013, 17, 425–431. [Google Scholar] [CrossRef]
- Nea, F.M.; Kearney, J.; Livingstone, M.B.E.; Pourshahidi, L.K.; Corish, A. Dietary and lifestyle habits and the associated health risks in shift workers. Nutr. Res. Rev. 2015, 28, 143–166. [Google Scholar] [CrossRef] [Green Version]
- Bonham, M.P.; Bonnell, E.K.; Huggins, C.E. Energy intake of shift workers compared to fixed day workers: A systematic review and meta-analysis. Chronobiol. Int. 2016, 33, 1086–1100. [Google Scholar] [CrossRef] [PubMed]
- Barbadoro, P.; Santarelli, L.; Croce, N.; Bracci, M.; Vincitorio, D.; Prospero, E.; Minelli, A. Rotating shift-work as an independent risk factor for overweight Italian workers: A cross-sectional study. PLoS ONE 2013, 8, 1–6. [Google Scholar] [CrossRef]
- Sun, M.; Feng, W.; Wang, F.; Li, P.; Li, Z.; Li, M.; Tse, G.; Vlaanderen, J.; Vermeulen, R.; Tse, L.A. Meta-analysis on shift work and risks of specific obesity types. Obes. Rev. 2018, 19, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.S.; Andrade, R.Z.; Silva, G.C.; Mota, M.C.; Resende, S.G.; Teixeira, K.R.; Gonçalves, B.F.; Crispim, C.A. Social jetlag among night workers is negatively associated with the frequency of moderate or vigorous physical activity and with energy expenditure related to physical activity. J. Biol. Rhythm. 2017, 32, 83–93. [Google Scholar] [CrossRef]
- Balieiro, L.C.T.; Rossato, L.T.; Waterhouse, J.; Paim, S.L.; Mota, M.C.; Crispim, C.A. Nutritional status and eating habits of bus drivers during the day and night. Chronobiol. Int. 2014, 31, 1123–1129. [Google Scholar] [CrossRef]
- Lowden, A.; Moreno, C.; Holmbäck, U.; Lennernäs, M.; Tucker, P. Eating and shift work – effects on habits, metabolism and performance. Scand. J. Work Environ. Health 2010, 36, 150–162. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, J.; Duan, P.; Liu, B.; Li, T.; Wang, C.; Li, H.; Yang, T.; Gan, Y.; Wang, X.; et al. Is shift work associated with a higher risk of overweight or obesity? A systematic review of observational studies with meta-analysis. Int. J. Epidemiol. 2018, 47, 1956–1971. [Google Scholar] [CrossRef] [Green Version]
- Vyas, M.V.; Garg, A.X.; Iansavichus, A.V.; Costella, J.P.; Donner, A.; Laugsand, L.E.; Janszky, I.; Mrkobrada, M.; Parraga, G.; Hackam, D.G. Shift work and vascular events: Systematic review and meta-analysis. BMJ 2012, 345, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Monk, T.H.; Buysse, D.J. Exposure to shift work as a risk factor for diabetes. J. Biol. Rhythm. 2013, 28, 356–359. [Google Scholar] [CrossRef] [Green Version]
- Vimalananda, V.G.; Palmer, J.R.; Gerlovin, H.; Wise, L.A.; Rosenzweig, J.L.; Rosenberg, L. Night-shift and incident diabetes among African-American women. Diabetologia 2015, 58, 699–706. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Laurenti, M.C.; Man, C.D.; Varghese, R.T.; Cobelli, C.; Rizza, R.A.; Matveyenko, A.V.; Vella, A. Glucose metabolism during rotational shift-work in healthcare workers. Diabetologia 2017, 60, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.P., Jr.; Bogan, R.K.; Wyatt, J.K. Shift work and the assessment and management of shift work disorder (SWD). Sleep Med. Rev. 2013, 17, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.C.; Waterhouse, J.; De-Souza, D.A.; Rossato, L.T.; Silva, C.M.; Araújo, M.B.J.; Tufik, S.; de Mello, M.T.; Crispim, C.A. Sleep pattern is associated with adipokine levels and nutritional markers in resident physicians. Chronobiol. Int. 2014, 31, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Crispim, C.A.; Zimberg, I.Z.; Reis, B.G.; Diniz, R.M.; Tufik, S.; De-Mello, M.T. Relationship between food intake and sleep pattern in health induviduals. J. Clin. Sleep Med. 2011, 7, 659–664. [Google Scholar] [CrossRef] [Green Version]
- Mota, M.C.; De-Souza, D.A.; Rossato, L.T.; Silva, C.M.; Araújo, M.B.J.; Tufik, S.; de Mello, M.T.; Crispim, C.A. Dietary patterns, metabolic markers and subjective sleep measures in resident physicians. Chronobiol. Int. 2013, 10, 1032–1041. [Google Scholar] [CrossRef]
- Leung, G.K.W.; Huggins, C.E.; Bonham, M.P. Effect of meal timing on postprandial glucose responses to a low glycemic index meal: A crossover trial in healthy volunteers. Clin. Nutr. 2017, 38, 465–471. [Google Scholar] [CrossRef]
- Crispim, C.A.; Mota, M.C. New perspectives on chrononutrition. Biol. Rhythm Res. 2018, 1, 1–15. [Google Scholar]
- Lowden, A.; Holmbäck, U.; Akerstedt, T.; Forslund, A.; Forslund, J.; Lennernäs, M. Time of day type of food – relation to mood and hunger during 24 hours of constant conditions. J. Human Ergol. 2001, 30, 381–386. [Google Scholar]
- Waterhouse, J.; Buckley, P.; Edwards, B.; Reilly, T. Measurement of, and some reasons for, differences in eating habits between night and day workers. Chronobiol. Int. 2003, 20, 1075–1092. [Google Scholar] [CrossRef] [PubMed]
- Buxton, O.M.; Cain, S.W.; O’Connor, S.P.; Porter, J.H.; Duffy, J.F.; Wang, W.; Czeisler, C.A.; Shea, S.A. Metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci. Transl. Med. 2012, 4, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Grant, C.L.; Coates, A.M.; Dorrian, J.; Kennaway, D.R.; Wittert, G.A.; Heilbronn, L.K.; Pajcin, M.; della Vedova, C.; Gupta, C.C.; Banks, S. Timing of food intake during simulated night shift impacts glucose metabolism: A controlled study. Chronobiol. Int. 2017, 34, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Reutrakul, S.; Van Cauter, E. Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metab. Clin. Exp. 2018, 84, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Jebb, S.A.; Prentice, A.M.; Goldberg, G.R.; Murgatroyd, P.R.; Black, A.E.; Coward, W.A. Changes in macronutrient balance during over- and underfeeding assessed by 12-d continuous whole-body calorimetry. Am. J. Clin. Nutr. 1996, 64, 259–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Layman, D.K.; Shiue, H.; Sather, C.; Erickson, D.J.; Baum, J. Increased dietary protein modifies glucose and insulin homeostasis in adult women during weight loss. Human Nutr. Metab. 2003, 133, 405–410. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioural Sciences; Academic Press: New York, NY, USA, 1969. [Google Scholar]
- Al-Naimi, S.; Hampton, S.M.; Richard, P.; Tzung, C.; Morgan, L.M. Postprandial metabolic profiles following meals and snacks eaten during simulated night and day shift work. Chronobiol. Int. 2004, 21, 937–947. [Google Scholar] [CrossRef]
- Sato, M.; Nakamura, K.; Ogata, H.; Miyashita, A.; Nagasaka, S.; Omi, N.; Yamaguchi, S.; Hibi, M.; Umeda, T.; Nakaji, S.; et al. Acute effect of late evening meal on diurnal variation of blood glucose and energy metabolism. Obes. Res. Clin. Pract. 2011, 5, e220–e228. [Google Scholar] [CrossRef] [PubMed]
- Reutrakul, S.; Hood, M.M.; Crowley, S.J.; Morgan, M.K.; Teodori, M.; Knutson, K.L. The relationship between breakfast skipping, chronotype, and glycemic control in type 2 diabetes. Chronobiol. Int. 2014, 31, 71. [Google Scholar] [CrossRef]
- Lohman, T.G.; Roche, A.F.; Martorrel, R. Anthropometrics standardization reference manual. Medicine 1988. [Google Scholar] [CrossRef] [Green Version]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, J.A.; Benedict, F.G. A biometric study of human basal metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cedernaes, J.; Brandell, J.; Ros, O.; Broman, J.E.; Hogenkamp, O.S.; Schiöth, H.B.; Benedict, C. Increased impulsivity in response to food cues after sleep loss in healthy young men. Obesity 2014, 22, 1786–1791. [Google Scholar] [CrossRef]
- NEPA-UNICAMP—Núcleo de estudos e pesquisas em alimentação—NEPA/Universidade Estadual de Campinas—UNICAMP. In Tabela Brasileira de Composição de Alimentos—TACO, 4th ed.; NEPA-UNICAMP: Campinas, Portuguese, 2011.
- US Department of Agriculture, Agricultural Research Service, Nutrient Data Laboratory. USDA National Nutrient Database for Standard Reference, Release 28 (Slightly Revised). Version Current: May 2016. Available online: http://www.ars.usda.gov/ba/bhnrc/ndl (accessed on 10 July 2020).
- Ziegler, A.; Vens, M. Generalized estimating equations: Notes on the choice of the working correlation matrix. Methods Inf. Med. 2010, 49, 421–425. [Google Scholar] [PubMed]
- American Diabetes Association (ADA). Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37, 581–590. [Google Scholar]
- Lopez-Mingues, J.; Saxena, R.; Bandín, C.; Scheer, F.A.; Garaulet, M. Late dinner impairs glucose tolerance in MTNR1B risk allele carriers: A randomized, cross-over study. Clin. Nutr. 2018, 37, 1133–1140. [Google Scholar] [CrossRef]
- Knutson, K.L.; Spiegel, K.; Penev, P.; Van Cauter, E. The metabolic consequences of sleep deprivation. Sleep Med. Rev. 2007, 11, 163–178. [Google Scholar] [CrossRef] [Green Version]
- Scheer, F.A.J.L.; Hilton, M.F.; Mantzoros, C.S.; Shes, S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 2009, 106, 4453–4458. [Google Scholar] [CrossRef] [Green Version]
- Spiegel, K.; Leproult, R.; Van Cauter, E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999, 354, 1435–1439. [Google Scholar] [CrossRef]
- Linn, T.; Santosa, B.A.S.; Groenemeyer, D.; Aygen, S.; Scholz, N.; Busch, M.; Bretzel, R.G. Effect of long-term dietary protein intake on glucose metabolism in humans. Diabetologia 2000, 43, 1257–1265. [Google Scholar] [CrossRef] [Green Version]
- Holmbäck, U.; Forslund, A.; Lowden, A.; Forslund, J.M.; Åkerstedt, T.; Lennernäs, M.; Hambraeus, L.; Stridsberg, M. Endocrine responses to nocturnal eating—Possible implications for night work. Eur. J. Nutr. 2003, 42, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Geloneze, B.; Vasques, A.C.J.; Stabe, C.F.C.; Pareja, J.C.; Rosado, L.E.F.P.d.; de Queiroz, E.C.; Tambascia, M.A. HOMA1-IR and HOMA2-IR indexes in identifying insulin resistance and metabolic syndrome: Brazilian Metabolic Syndrome Study (BRAMS). Med. Arq. Bras. Endocrinol. Metabol. 2009, 53, 281–287. [Google Scholar] [CrossRef] [Green Version]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Lund, J.; Arendt, J.; Hampton, S.M.; English, J.; Morgan, L.M. Postprandial hormone and metabolic responses amongst shift workers in Antarctica. J. Endocrinol. 2001, 171, 557–564. [Google Scholar] [CrossRef] [Green Version]
- Ginsberg, H.N. Hypertriglyceridemia: New insights and new approaches to pharmacologic therapy. Am. J. Cardiol. 2001, 87, 1174–1180. [Google Scholar] [CrossRef]
- Crispim, C.A.; Zalcman, I.; Dáttilo, M.; Padilha, H.G.; Edwards, B.R.; Waterhouse, J.; Tufik, S.; de Mello, M.T. The influence of sleep and sleep loss upon food intake and metabolism. Nutr. Res. Rev. 2007, 20, 195–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmbäck, U.; Forslund, A.; Forslund, J.M.; Hambraeus, L.; Lennernäs, M.; Lowden, A.; Stridsberg, M.; Åkerstedt, T. Metabolic responses to nocturnal eating in men are affected by sources of dietary energy. Human Nutr. Metab. 2002, 132, 1892–1899. [Google Scholar] [CrossRef]
- Barclay, J.L.; Husse, J.; Bode, B.; Naujokat, N.; Meyer-Kovac, J.; Schmid, S.M.; Lehnert, H.; Oster, H. Circadian desynchrony promotes metabolic disruption in a mouse model of shiftwork. PLoS ONE 2012, 7, e37150. [Google Scholar] [CrossRef] [Green Version]
- Sherman, H.; Genzer, Y.; Cohen, R.; Chapnik, N.; Madar, Z.; Froy, O. Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J. 2012, 26, 3493–3502. [Google Scholar] [CrossRef]
- Gupta, C.C.; Dorrian, J.; Grant, C.L.; Pajcin, M.; Coates, A.M.; Kennaway, D.J.; Wittert, G.A.; Heilbronn, L.K.; della Vedova, C.; Banks, S. It’s not just what you eat but when: The impact of eating a meal during simulated shift work on driving performance. Chronobiol. Int. 2017, 34, 66–77. [Google Scholar] [CrossRef]
| Energy (kcal) | Energy (kcal/kg) | Carbohydrate (g) | Carbohydrate (g/kg) | Carbohydrate (en%) | Protein (g) | Protein (g/kg) | Protein (en%) | Fat (g) | Fat (g/kg) | Fat (en%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Breakfast (09:00) | |||||||||||
| White bread, mozzarella cheese, whole milk and coffee with sugar | 371.3 ± 61.9 | 4.3 ± 0.2 | 42.1 ± 7.0 | 0.5 ± 0.0 | 45.3 ± 0.0 | 16.5 ± 2.7 | 0.2 ± 0.0 | 17.8 ± 0.0 | 14.9 ± 2.5 | 0.1 ± 0.0 | 36.2 ± 0.0 |
| Lunch (12:00) | |||||||||||
| Rice, beans, beef, string bean, lettuce, tomato, raw carrot and pineapple juice with sugar | 601.4 ± 100.3 | 6.9 ± 0.4 | 84.4 ± 14.1 | 1.0 ± 0.0 | 56.1 ± 0.0 | 40.7 ± 6.8 | 0.5 ± 0.0 | 27.1 ± 0.0 | 11.3 ± 1.9 | 0.1 ± 0.0 | 16.9 ± 0.0 |
| Afternoon Snack (16:00) | |||||||||||
| Strawberry yogurt, salt biscuit and banana | 206.1 ± 34.4 | 2.4 ± 0.1 | 40.0 ± 6.7 | 0.5 ± 0.0 | 77.7 ± 0.0 | 4.6 ± 0.8 | 0.1 ± 0.0 | 8.9 ± 0.0 | 4.4 ± 0.7 | 0.1 ± 0.0 | 19.2 ± 0.0 |
| Evening Snack (20:00) | |||||||||||
| White bread, mozzarella cheese, ham, whole grape juice | 432.0 ± 72.1 | 5.0 ± 0.3 | 65.8 ± 11.0 | 0.7 ± 0.0 | 60.9 ± 0.0 | 15.2 ± 2.5 | 0.2 ± 0.0 | 14.1 ± 0.0 | 11.8 ± 2.0 | 0.1 ± 0.0 | 24.6 ± 0.0 |
| Test meal HP-MCHO (01:00) - 35% EER | |||||||||||
| Chicken, broccoli, carrot, lettuce, tomato, sugar free orange juice and pineapple | 753.2 ± 126.7 | 8.7 ± 0.5 | 83.0 ± 13.8 | 1.0 ± 0.0 | 44.1 ± 0.0 | 69.6 ± 11.6 | 0.8 ± 0.0 | 36.9 ± 0.0 | 16.1 ± 2.7 | 0.2 ± 0.0 | 19.2 ± 0.0 |
| Total intake of the day - HP-MCHO | 2364.0 ± 394.4 | 27.2 ± 1.5 | 315.3 ± 52.6 | 3.6 ± 0.2 | 53.3 ± 0.0 | 146.6 ± 24.5 | 1.7 ± 0.1 | 24.8 ± 0.0 | 58.5 ± 9.8 | 0.7 ± 0.0 | 22.3 ± 0.0 |
| Test meal LP-HCHO (01:00) - 35% EER | |||||||||||
| Pasta, tomato sauce, beef, raw carrot, lettuce, arugula, orange juice with sugar and guava jam | 769.0 ± 128.3 | 8.8 ± 0.5 | 124.1 ± 20.7 | 1.4 ± 0.1 | 64.6 ± 0.0 | 28.8 ± 4.8 | 0.3 ± 0.0 | 15.0 ± 0.0 | 18.0 ± 3.0 | 0.2 ± 0.0 | 21.1 ± 0.0 |
| Total intake of the day - LP-HCHO | 2379.8 ± 397.0 | 27.4 ± 1.5 | 356.4 ± 59.5 | 4.1 ± 0.2 | 60.0 ± 0.0 | 105.8 ± 17.7 | 1.2 ± 0.1 | 17.8 ± 0.0 | 60.4 ± 10.1 | 0.7 ± 0.0 | 22.8 ± 0.0 |
| Standard breakfast | |||||||||||
| Potato bread, mozzarella cheese, wheat flour cake, papaya, orange juice with sugar | 444.5 ± 0.0 | 5.3 ± 1.0 | 75.2 ± 0.0 | 0.9 ± 0.2 | 67.7 ± 0.0 | 11.6 ± 0.0 | 0.1 ± 0.0 | 10.4 ± 0.0 | 11.9 ± 0.0 | 0.1 ± 0.0 | 24.1 ± 0.0 |
| Variable | Night Shift Workers (n = 14) |
|---|---|
| Demographic | |
| Age (y) * | 40.9 ± 8.9 |
| Anthropometry | |
| Weight (kg) * | 87.7 ± 18.8 |
| Height (m) ** | 1.71 (1.69–1.77) |
| BMI (kg/m2) * | 29.1 ± 5.3 |
| Eutrophy % (n) | 21.43 (3) |
| Overweight % (n) | 42.85 (6) |
| Obesity % (n) | 35.72 (5) |
| WC (cm) * | 99.6 ± 10.8 |
| Sleep characteristics | |
| Average sleep (h) * | 5.5 ± 1.1 |
| Metabolic parameters | |
| Glucose (mg/dL) ** | 81.5 (76–92) |
| Insulin (mU/mL) * | 14.9 ± 7.9 |
| HOMA-IR * | 3.2 ± 1.8 |
| Total cholesterol (mg/dL) * | 199.2 ± 48.6 |
| HDL (mg/dL) * | 38.8 ± 7.5 |
| LDL (mg/dL) * | 127.1 ± 35.8 |
| Triglycerides (mg/dL) ** | 157.0 (116.2–244.0) |
| Variable | Night Shift Workers (n = 14) | ||
|---|---|---|---|
| Week before HP-MCHO | Week before LP-HCHO | p Value | |
| Sleep duration (hh:mm) | 06:52 ± 1:02 | 07:24 ± 1:20 | 0.248 |
| Energy (kcal) | 1816.68 (1472.66–2662.95) | 2145.00 (1485.05–2482.72) | 0.910 |
| Energy (kcal/kg) | 20.92 (15.76–31.82) | 22.97 (17.72–29.07) | 0.874 |
| Carbohydrate (g/kg) | 2.62 (1.93–3.99) | 2.52 (1.88–3.35) | 0.541 |
| Carbohydrate (%) | 49.31 (35.55–53.13) | 45.09 (31.69–57.91) | 0.769 |
| Protein (g/kg) | 1.19 (0.78–1.71) | 1.21 (0.90–2.11) | 0.769 |
| Protein (%) | 20.38 (17.42–26.29) | 24.00 (19.16–28.58) | 0.376 |
| Fat (g/kg) | 0.76 (0.44–1.17) | 0.71 (0.38–1.12) | 1.000 |
| Fat (%) | 30.75 (23.06–41.07) | 28.63 (18.91–39.98) | 0.769 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cunha, N.B.; Silva, C.M.; Mota, M.C.; Lima, C.A.; Teixeira, K.R.C.; Cunha, T.M.; Crispim, C.A. A High-Protein Meal during a Night Shift Does Not Improve Postprandial Metabolic Response the Following Breakfast: A Randomized Crossover Study with Night Workers. Nutrients 2020, 12, 2071. https://doi.org/10.3390/nu12072071
Cunha NB, Silva CM, Mota MC, Lima CA, Teixeira KRC, Cunha TM, Crispim CA. A High-Protein Meal during a Night Shift Does Not Improve Postprandial Metabolic Response the Following Breakfast: A Randomized Crossover Study with Night Workers. Nutrients. 2020; 12(7):2071. https://doi.org/10.3390/nu12072071
Chicago/Turabian StyleCunha, Nayara B., Catarina M. Silva, Maria C. Mota, Caio A. Lima, Kely R. C. Teixeira, Thulio M. Cunha, and Cibele A. Crispim. 2020. "A High-Protein Meal during a Night Shift Does Not Improve Postprandial Metabolic Response the Following Breakfast: A Randomized Crossover Study with Night Workers" Nutrients 12, no. 7: 2071. https://doi.org/10.3390/nu12072071
APA StyleCunha, N. B., Silva, C. M., Mota, M. C., Lima, C. A., Teixeira, K. R. C., Cunha, T. M., & Crispim, C. A. (2020). A High-Protein Meal during a Night Shift Does Not Improve Postprandial Metabolic Response the Following Breakfast: A Randomized Crossover Study with Night Workers. Nutrients, 12(7), 2071. https://doi.org/10.3390/nu12072071





