Selenium Deficiency Is Associated with Mortality Risk from COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Trace Element Analysis
2.3. SELENOP Quantification by ELISA
2.4. Assessment of Glutathione Peroxidase-3 (GPx3) Activity
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
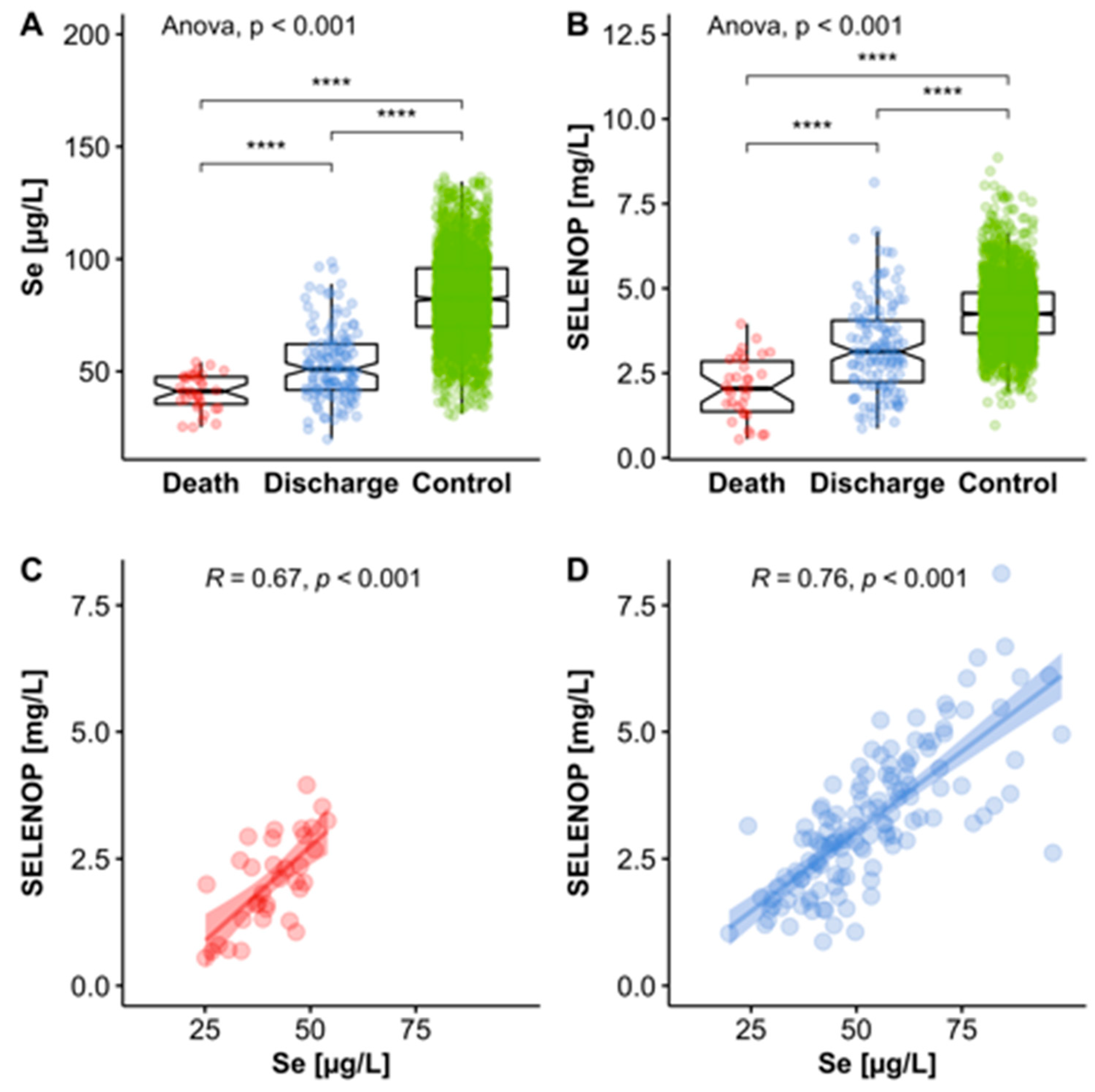
3.2. Selenium (Se) Status Analysis
3.3. Se Status of COVID-19 Patients in Relation to Reference Range of Healthy Control Subjects
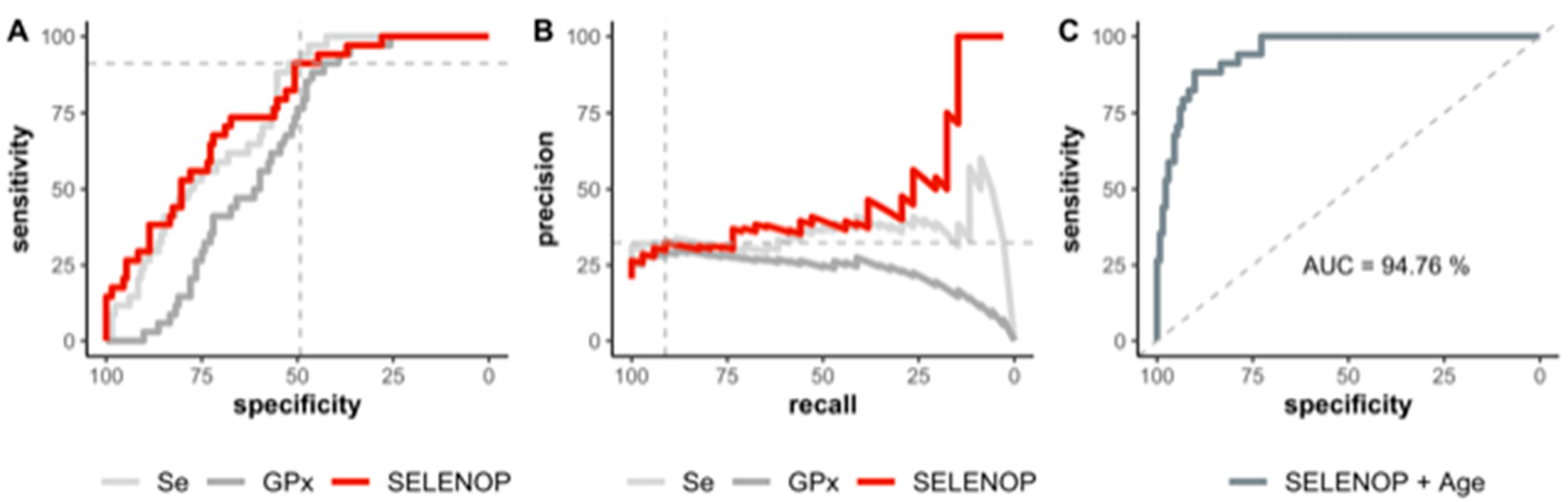
3.4. Se Status of COVID-19 Patients in Relation to Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guan, W.J.; Liang, W.H.; Zhao, Y.; Liang, H.R.; Chen, Z.S.; Li, Y.M.; Liu, X.Q.; Chen, R.C.; Tang, C.L.; Wang, T.; et al. Comorbidity and its impact on 1590 patients with covid-19 in china: A nationwide analysis. Eur. Respir. J. 2020, 55, 2000547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.L.; Li, R.B.; Lu, Z.; Huang, Y. Does comorbidity increase the risk of patients with COVID-19: Evidence from meta-analysis. Aging 2020, 12, 6049–6057. [Google Scholar] [CrossRef]
- Song, P.; Li, W.; Xie, J.; Hou, Y.; You, C. Cytokine storm induced by sars-cov-2. Clin. Chim. Acta 2020, 509, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. COVID-19: Demand for dexamethasone surges as recovery trial publishes preprint. BMJ 2020, 369, m2512. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Ferrando, C.; Martinez, D.; Ambros, A.; Munoz, T.; Soler, J.A.; Aguilar, G.; Alba, F.; Gonzalez-Higueras, E.; Conesa, L.A.; et al. Dexamethasone treatment for the acute respiratory distress syndrome: A multicentre, randomised controlled trial. Lancet Respir. Med. 2020, 8, 267–276. [Google Scholar] [CrossRef]
- Hoffman, S.L.; Punjabi, N.H.; Kumala, S.; Moechtar, M.A.; Pulungsih, S.P.; Rivai, A.R.; Rockhill, R.C.; Woodward, T.E.; Loedin, A.A. Reduction of mortality in chloramphenicol-treated severe typhoid fever by high-dose dexamethasone. N. Engl. J. Med. 1984, 310, 82–88. [Google Scholar] [CrossRef]
- Joshi, S.; Joshi, M.; Degani, M.S. Tackling sars-cov-2: Proposed targets and repurposed drugs. Future Med. Chem. 2020. [Google Scholar] [CrossRef]
- Calder, P.C.; Carr, A.C.; Gombart, A.F.; Eggersdorfer, M. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, P.R.; Berry, M.J. The influence of selenium on immune responses. Mol. Nutr. Food Res. 2008, 52, 1273–1280. [Google Scholar] [CrossRef]
- Schomburg, L. The other view: The trace element selenium as a micronutrient in thyroid disease, diabetes, and beyond. Hormones 2020, 19, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Guillin, O.M.; Vindry, C.; Ohlmann, T.; Chavatte, L. Selenium, selenoproteins and viral infection. Nutrients 2019, 11, 2101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, M.A.; Handy, J.; Levander, O.A. Host nutritional status: The neglected virulence factor. Trends Microbiol. 2004, 12, 417–423. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, T.; Li, Q.; Li, D. Prevention of keshan disease by selenium supplementation: A systematic review and meta-analysis. Biol. Trace Elem. Res. 2018, 186, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Forceville, X.; Vitoux, D.; Gauzit, R.; Combes, A.; Lahilaire, P.; Chappuis, P. Selenium, systemic immune response syndrome, sepsis, and outcome in critically ill patients. Crit. Care Med. 1998, 26, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, M.; Kusmenkov, T.; Zuck, C.; Angstwurm, M.; Becker, N.P.; Bocker, W.; Schomburg, L.; Bogner-Flatz, V. Selenium and selenoprotein p deficiency correlates with complications and adverse outcome after major trauma. Shock 2020, 53, 63–70. [Google Scholar] [CrossRef]
- Zhang, J.; Taylor, E.W.; Bennett, K.; Saad, R.; Rayman, M.P. Association between regional selenium status and reported outcome of covid-19 cases in china. Am. J. Clin. Nutr. 2020, 111, 1297–1299. [Google Scholar] [CrossRef]
- Fakhrolmobasheri, M.; Nasr-Esfahany, Z.; Khanahmad, H.; Zeinalian, M. Selenium supplementation can relieve the clinical complications of covid-19 and other similar viral infections. Int. J. Vitam. Nutr. Res. 2020, 9, 1–3. [Google Scholar] [CrossRef]
- Kieliszek, M.; Lipinski, B. Selenium supplementation in the prevention of coronavirus infections (covid-19). Med. Hypotheses 2020, 143, 109878. [Google Scholar] [CrossRef]
- Seale, L.A.; Torres, D.J.; Berry, M.J.; Pitts, M.W. A role for selenium-dependent gpx1 in sars-cov-2 virulence. Am. J. Clin. Nutr. 2020. [Google Scholar] [CrossRef]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brunink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-ncov) by real-time rt-pcr. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef] [Green Version]
- Hughes, D.J.; Fedirko, V.; Jenab, M.; Schomburg, L.; Meplan, C.; Freisling, H.; Bueno-de-Mesquita, H.B.; Hybsier, S.; Becker, N.P.; Czuban, M.; et al. Selenium status is associated with colorectal cancer risk in the european prospective investigation of cancer and nutrition cohort. Int. J. Cancer 2015, 136, 1149–1161. [Google Scholar] [CrossRef]
- Hybsier, S.; Schulz, T.; Wu, Z.; Demuth, I.; Minich, W.B.; Renko, K.; Rijntjes, E.; Kohrle, J.; Strasburger, C.J.; Steinhagen-Thiessen, E.; et al. Sex-specific and inter-individual differences in biomarkers of selenium status identified by a calibrated elisa for selenoprotein p. Redox Biol. 2017, 11, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, L.; Schweizer, U.; Holtmann, B.; Flohe, L.; Sendtner, M.; Kohrle, J. Gene disruption discloses role of selenoprotein p in selenium delivery to target tissues. Biochem. J. 2003, 370, 397–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehl, S.; Sun, Q.; Gorlich, C.L.; Hackler, J.; Kopp, J.F.; Renko, K.; Mittag, J.; Schwerdtle, T.; Schomburg, L. Cross-sectional analysis of trace element status in thyroid disease. J. Trace Elem. Med. Biol. 2020, 58, 126430. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; The R Foundation for Statistical Computing: Vienna, Austria, 2020; Volume 1. [Google Scholar]
- Wickham, H.; Henry, L. Tidyr: Tidy Messy Data; R Package Version 1.1.0; The R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://CRAN.R-project.org/package=tidyr (accessed on 14 July 2020).
- Wickham, H.; Francois, R.; Henry, L.; Müller, K. Dplyr: A Grammar of Data Manipulation; R Package Version 1.0.0; The R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://CRAN.R-project.org/package=dplyr (accessed on 14 July 2020).
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.C.; Muller, M. Proc: An open-source package for r and s+ to analyze and compare roc curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; R Package Version 2.1.0; Springer: New York, NY, USA, 2009; pp. 1–195. [Google Scholar]
- Boschloo, R.D. Raised conditional level of significance for 2x2 table when testing equality of 2 probabilities. Ann. Math. Stat. 1968, 39, 1094. [Google Scholar]
- Saito, T.; Rehmsmeier, M. The precision-recall plot is more informative than the roc plot when evaluating binary classifiers on imbalanced datasets. PLoS ONE 2015, 10, e0118432. [Google Scholar] [CrossRef] [Green Version]
- Saito, Y.; Misu, H.; Takayama, H.; Takashima, S.I.; Usui, S.; Takamura, M.; Kaneko, S.; Takamura, T.; Noguchi, N. Comparison of human selenoprotein p determinants in serum between our original methods and commercially available kits. Biol. Pharm. Bull. 2018, 41, 828–832. [Google Scholar] [CrossRef] [Green Version]
- Schomburg, L.; Melander, O. Letter by schomburg and melander regarding article, “selenoprotein p promotes the development of pulmonary arterial hypertension: A possible novel therapeutic target”. Circulation 2019, 139, 722–723. [Google Scholar] [CrossRef]
- Harthill, M. Review: Micronutrient selenium deficiency influences evolution of some viral infectious diseases. Biol. Trace Elem. Res. 2011, 143, 1325–1336. [Google Scholar] [CrossRef]
- Wu, J.T.; Leung, K.; Bushman, M.; Kishore, N.; Niehus, R.; de Salazar, P.M.; Cowling, B.J.; Lipsitch, M.; Leung, G.M. Estimating clinical severity of covid-19 from the transmission dynamics in Wuhan, China. Nat. Med. 2020, 26, 506–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renko, K.; Hofmann, P.J.; Stoedter, M.; Hollenbach, B.; Behrends, T.; Kohrle, J.; Schweizer, U.; Schomburg, L. Down-regulation of the hepatic selenoprotein biosynthesis machinery impairs selenium metabolism during the acute phase response in mice. FASEB J. 2009, 23, 1758–1765. [Google Scholar] [CrossRef]
- Becker, N.P.; Martitz, J.; Renko, K.; Stoedter, M.; Hybsier, S.; Cramer, T.; Schomburg, L. Hypoxia reduces and redirects selenoprotein biosynthesis. Metallomics 2014, 6, 1079–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martitz, J.; Becker, N.P.; Renko, K.; Stoedter, M.; Hybsier, S.; Schomburg, L. Gene-specific regulation of hepatic selenoprotein expression by interleukin-6. Metallomics 2015, 7, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, W.; Langlois, P.L.; Heyland, D.K. Pharmaconutrition with selenium in critically ill patients: What do we know? Nutr. Clin. Pract. 2015, 30, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, J.; Arner, E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Al-Quraishy, S.; Dkhil, M.A.; Wunderlich, F.; Sies, H. Dietary selenium in adjuvant therapy of viral and bacterial infections. Adv. Nutr. 2015, 6, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Sies, H.; Parnham, M.J. Potential therapeutic use of ebselen for covid-19 and other respiratory viral infections. Free Radic. Biol. Med. 2020, 156, 107–112. [Google Scholar] [CrossRef]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of m(pro) from sars-cov-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alehagen, U.; Johansson, P.; Bjornstedt, M.; Rosen, A.; Post, C.; Aaseth, J. Relatively high mortality risk in elderly swedish subjects with low selenium status. Eur. J. Clin. Nutr. 2016, 70, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Schomburg, L.; Orho-Melander, M.; Struck, J.; Bergmann, A.; Melander, O. Selenoprotein-p deficiency predicts cardiovascular disease and death. Nutrients 2019, 11, 1852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bomer, N.; Grote Beverborg, N.; Hoes, M.F.; Streng, K.W.; Vermeer, M.; Dokter, M.M.; Ijmker, J.; Anker, S.D.; Cleland, J.G.F.; Hillege, H.L.; et al. Selenium and outcome in heart failure. Eur. J. Heart Fail. 2019. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.F.; Stranges, S.; Chan, L.H.M. Circulating selenium concentration is inversely associated with the prevalence of stroke: Results from the canadian health measures survey and the national health and nutrition examination survey. J. Am. Heart Assoc. 2019, 8, e012290. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Schomburg, L.; Hughes, D.J. The missing link? The potential role of selenium in the development of liver cancer and significance for the general population. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 707–709. [Google Scholar] [CrossRef] [PubMed]



| Death | Discharge | Total | |
|---|---|---|---|
| Sex | |||
| female | 4 (67%) | 15 (56%) | 19 (58%) |
| male | 2 (33%) | 12 (44%) | 14 (42%) |
| Age | |||
| median (IQR) | 89 (81, 94) | 69 (38, 91) | 77 (38, 94) |
| Comorbidities | |||
| hypertension | 4 (67%) | 18 (67%) | 22 (67%) |
| diabetes | 2 (33%) | 4 (15%) | 6 (18%) |
| COPD | 0 (0%) | 1 (4%) | 1 (3%) |
| CVD | 3 (50%) | 14 (52%) | 17 (52%) |
| cerebrovascular disease | 1 (17%) | 5 (19%) | 6 (18%) |
| adipositas | 1 (17%) | 6 (22%) | 7 (21%) |
| Time to discharge or death * [d] | |||
| median (IQR) | 10 (2, 32) | 19 (3, 46) | 15 (2, 46) |
| All Samples | Discharge | Death | p-Value * | |
|---|---|---|---|---|
| serum Se [µg/L] | n = 166 | n= 132 | n= 34 | p< 0.001 |
| 50.8 ± 15.7 | 53.3 ± 16.2 | 40.8 ± 8.1 | ||
| serum SELENOP [mg/L] | 3.0 ± 1.4 | 3.3 ± 1.3 | 2.1 ± 0.9 | p< 0.001 |
| serum GPx3 [U/L] | 246.1 ± 64.4 | 251.6 ± 69.6 | 224.8 ± 30.3 | p< 0.001 |
| Serum Se | Serum SELENOP | GPx3 Activity | All | |
|---|---|---|---|---|
| (intercept) | −1.70 *** [−2.20, −1.20] | −1.75 *** [−2.27, −1.24] | −1.42 *** [−1.81, −1.02] | −1.80 *** [−2.34, −1.26] |
| Se | −1.19 *** [−1.79, −0.60] | −0.55 [−1.39, 0.30] | ||
| SELENOP | −1.28 *** [−1.86, −0.70] | −0.94 * [−1.72, −0.16] | ||
| GPx3 | −0.46 * [−0.89, −0.04] | 0.09 [−0.37, 0.54] | ||
| N | 166 | 166 | 166 | 166 |
| AIC | 150.5 | 146.3 | 167.3 | 148.5 |
| Pseudo R2 | 0.19 | 0.23 | 0.05 | 0.24 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moghaddam, A.; Heller, R.A.; Sun, Q.; Seelig, J.; Cherkezov, A.; Seibert, L.; Hackler, J.; Seemann, P.; Diegmann, J.; Pilz, M.; et al. Selenium Deficiency Is Associated with Mortality Risk from COVID-19. Nutrients 2020, 12, 2098. https://doi.org/10.3390/nu12072098
Moghaddam A, Heller RA, Sun Q, Seelig J, Cherkezov A, Seibert L, Hackler J, Seemann P, Diegmann J, Pilz M, et al. Selenium Deficiency Is Associated with Mortality Risk from COVID-19. Nutrients. 2020; 12(7):2098. https://doi.org/10.3390/nu12072098
Chicago/Turabian StyleMoghaddam, Arash, Raban Arved Heller, Qian Sun, Julian Seelig, Asan Cherkezov, Linda Seibert, Julian Hackler, Petra Seemann, Joachim Diegmann, Maximilian Pilz, and et al. 2020. "Selenium Deficiency Is Associated with Mortality Risk from COVID-19" Nutrients 12, no. 7: 2098. https://doi.org/10.3390/nu12072098
APA StyleMoghaddam, A., Heller, R. A., Sun, Q., Seelig, J., Cherkezov, A., Seibert, L., Hackler, J., Seemann, P., Diegmann, J., Pilz, M., Bachmann, M., Minich, W. B., & Schomburg, L. (2020). Selenium Deficiency Is Associated with Mortality Risk from COVID-19. Nutrients, 12(7), 2098. https://doi.org/10.3390/nu12072098