Serum Scoring and Quantitative Magnetic Resonance Imaging in Intestinal Failure-Associated Liver Disease: A Feasibility Study
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Laboratory Tests
2.3. MRI Scanning
2.4. Statistical Analysis
3. Results
3.1. Serum Cohort
3.1.1. Patient Demographics
3.1.2. Biochemical Parameters
3.1.3. Composite Serum Scores
3.2. MRI Cohort
3.2.1. Demographics
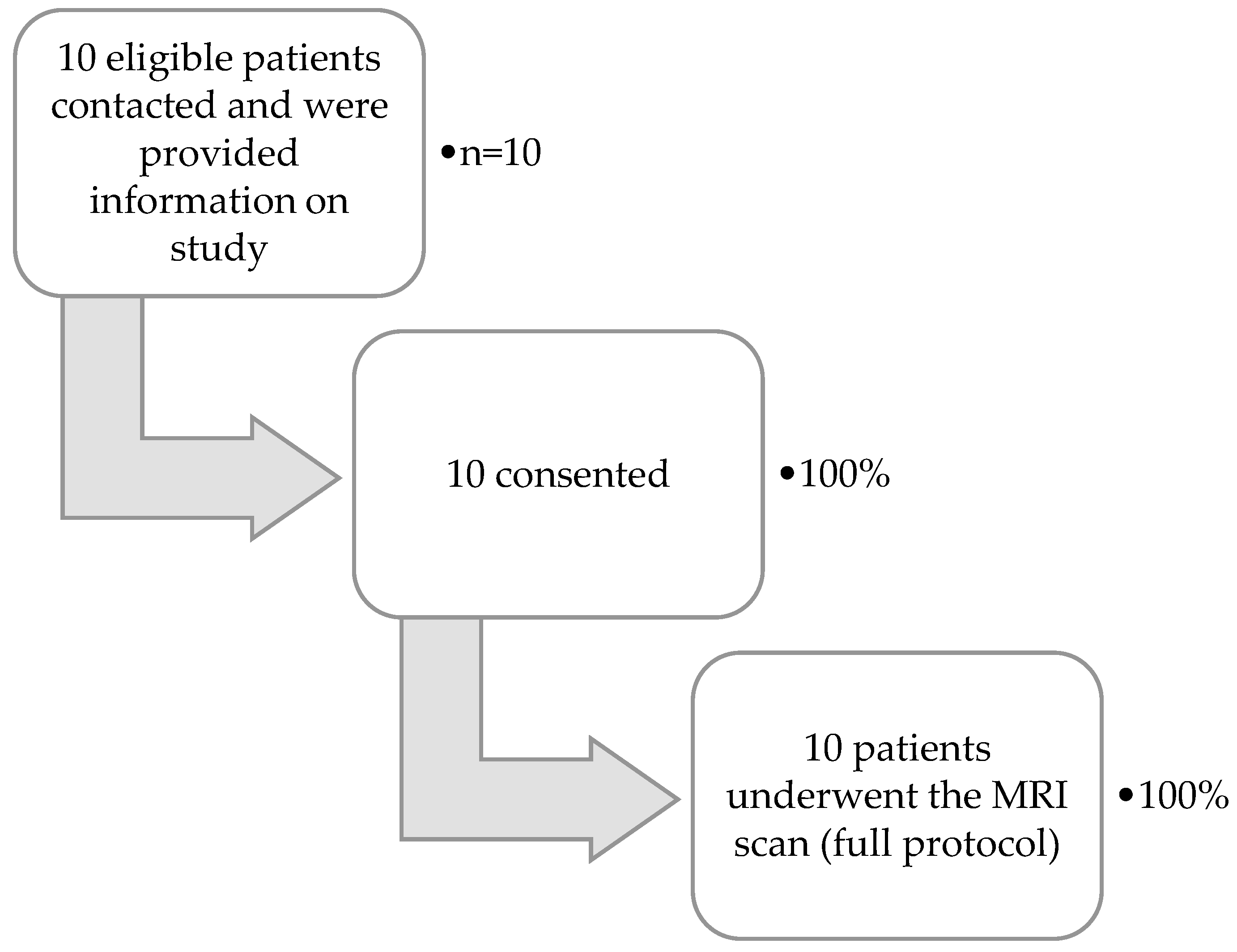
3.2.2. Feasibility Results
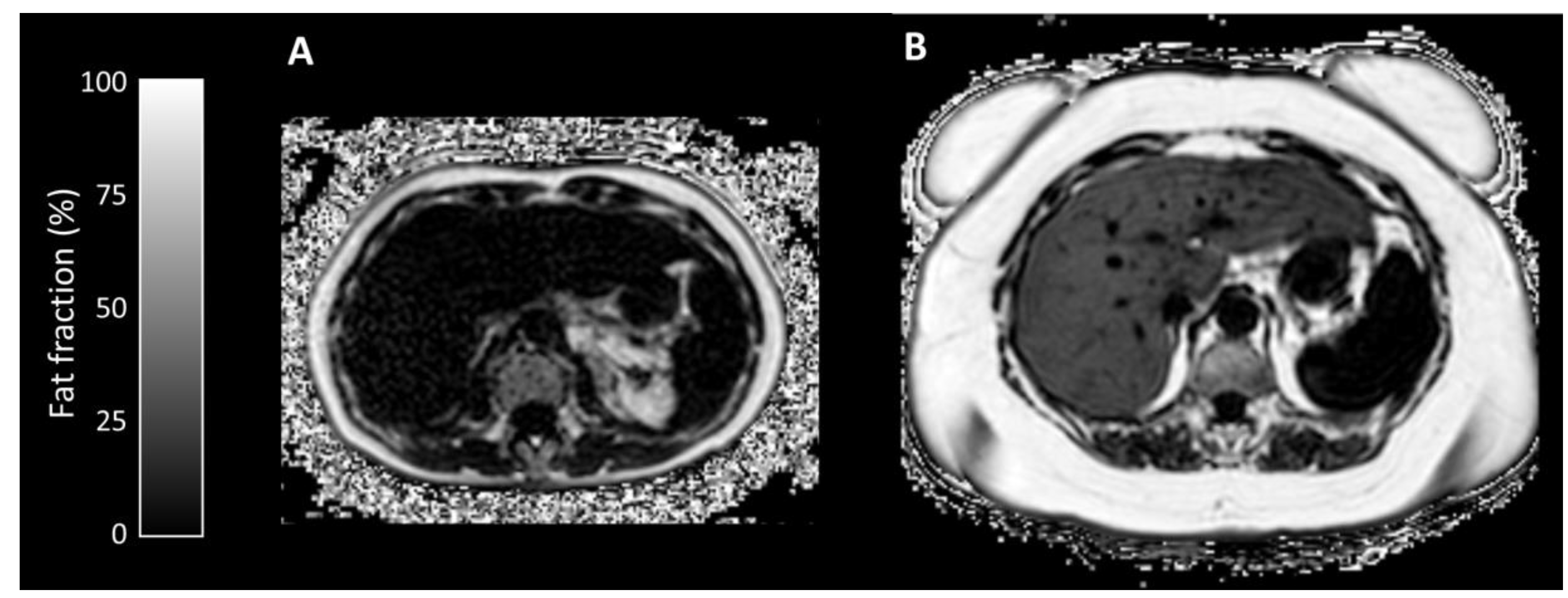
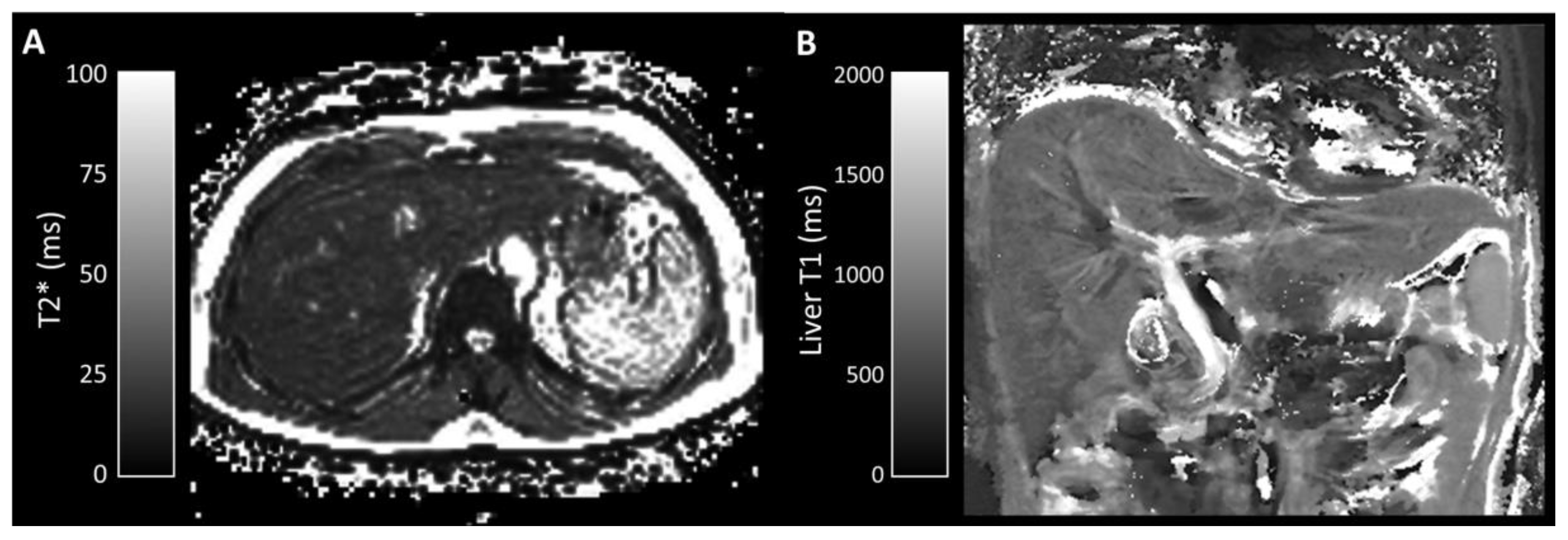
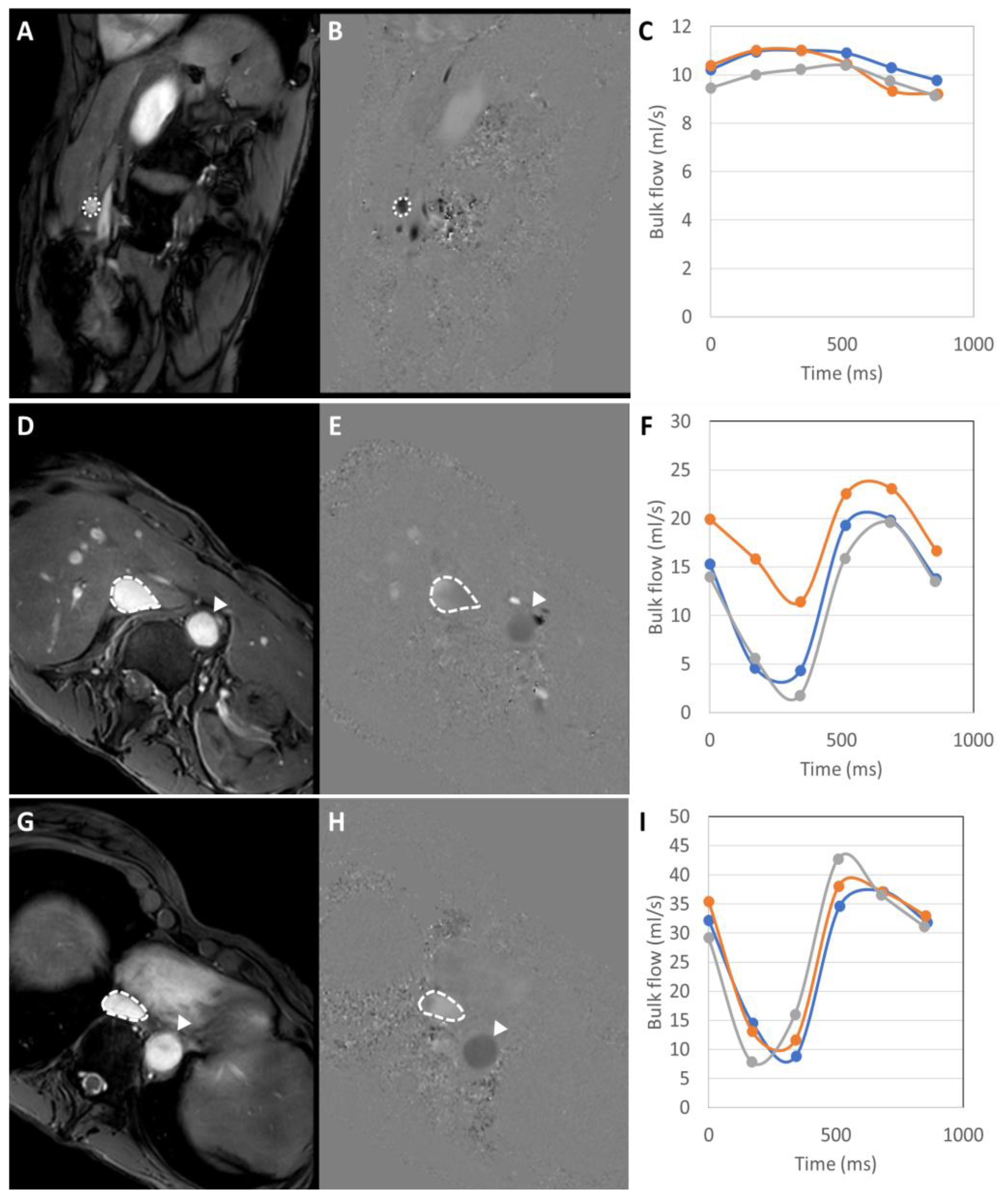
3.2.3. Quantitative Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
Appendix A.1. MRI Image Acquisition
Appendix A.1.1. Anatomical Imaging
Appendix A.1.2. Proton Density Fat Fraction/T2* Mapping
Appendix A.1.3. T1 Mapping
Appendix A.1.4. Phase-Contrast MRI
Appendix A.1.5. Small Bowel Motility
Appendix A.2. MRI Image Post-Processing
Appendix A.2.1. Proton Density Fat Fraction/T2* Mapping
Appendix A.2.2. T1 Mapping
Appendix A.2.3. Phase-Contrast MRI
Appendix A.2.4. Small Bowel Motility
References
- Pironi, L.; Arends, J.; Bozzetti, F.; Cuerda, C.; Gillanders, L.; Jeppesen, P.B.; Joly, F.; Kelly, D.; Lal, S.; Staun, M.; et al. ESPEN guidelines on chronic intestinal failure in adults. Clin. Nutr. 2016, 35, 247–307. [Google Scholar] [CrossRef] [PubMed]
- Sasdelli, A.S.; Agostini, F.; Pazzeschi, C.; Guidetti, M.; Lal, S.; Pironi, L. Assessment of Intestinal Failure Associated Liver Disease according to different diagnostic criteria. Clin. Nutr. 2019, 38, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.; Pironi, L.; Wanten, G.; Arends, J.; Bozzetti, F.; Cuerda, C.; Joly, F.; Kelly, D.; Staun, M.; Szczepanek, K.; et al. Clinical approach to the management of Intestinal Failure Associated Liver Disease (IFALD) in adults: A position paper from the Home Artificial Nutrition and Chronic Intestinal Failure Special Interest Group of ESPEN. Clin. Nutr. 2018, 37, 1794–1797. [Google Scholar] [CrossRef]
- Pironi, L.; Goulet, O.; Buchman, A.; Messing, B.; Gabe, S.; Candusso, M.; Bond, G.; Gupte, G.; Pertkiewicz, M.; Steiger, E.; et al. Outcome on home parenteral nutrition for benign intestinal failure: A review of the literature and benchmarking with the European prospective survey of ESPEN. Clin. Nutr. 2012, 31, 831–845. [Google Scholar] [CrossRef]
- Hagi, A.; Nakayama, M.; Shinzaki, W.; Haji, S.; Ohyanagi, H. Effects of the ω-6:ω-3 Fatty acid ratio of fat emulsions on the fatty acid composition in cell membranes and the anti-inflammatory action. JPEN J. Parenter. Enteral Nutr. 2010, 34, 263–270. [Google Scholar] [CrossRef]
- Dupont, I.E. Peroxidation of lipid emulsions: Effects of changes in fatty acid pattern and α-tocopherol content on the sensitivity to peroxidative damage. Clin. Nutr. 1999, 18, 113–116. [Google Scholar] [CrossRef]
- Mutanen, A.; Nissinen, M.J.; Lohi, J.; Heikkilä, P.; Gylling, H.; Pakarinen, M.P. Serum plant sterols, cholestanol, and cholesterol precursors associate with histological liver injury in pediatric onset intestinal failure. Am. J. Clin. Nutr. 2014, 100, 1085–1094. [Google Scholar] [CrossRef]
- Raman, M.; Almutairdi, A.; Mulesa, L.; Alberda, C.; Beattie, C.; Gramlich, L. Parenteral nutrition and lipids. Nutrients 2017, 9, 388. [Google Scholar] [CrossRef]
- Zaman, N.; Tam, Y.K.; Jewell, L.D.; Coutts, R.T. Effects of intravenous lipid as a source of energy in parenteral nutrition associated hepatic dysfunction and lidocaine elimination: A study using isolated rat liver perfusion. Biopharm. Drug Dispos. 1997, 18, 803–819. [Google Scholar] [CrossRef]
- Christensen, R.D.; Henry, E.; Wiedmeier, S.E.; Burnett, J.; Lambert, D.K. Identifying patients, on the first day of life, at high-risk of developing parenteral nutrition-associated liver disease. J. Perinatol. 2007, 27, 284–290. [Google Scholar] [CrossRef]
- Hwang, T.L.; Lue, M.C.; Chen, L.L. Early use of cyclic TPN prevents further deterioration of liver functions for the TPN patients with impaired liver function. Hepatogastroenterology 2000, 47, 1347–1350. [Google Scholar] [PubMed]
- Jensen, A.R.; Goldin, A.B.; Koopmeiners, J.S.; Stevens, J.; Waldhausen, J.H.T.; Kim, S.S. The association of cyclic parenteral nutrition and decreased incidence of cholestatic liver disease in patients with gastroschisis. J. Pediatr. Surg. 2009, 44, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Stout, S.M.; Cober, M.P. Metabolic effects of cyclic parenteral nutrition infusion in adults and children. Nutr. Clin. Pract. 2010, 25, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Lacaille, F.; Gupte, G.; Colomb, V.; D’antiga, L.; Hartman, C.; Hojsak, I.; Kolacek, S.; Puntis, J.; Shamir, R. Intestinal failure-associated liver disease: A position paper of the ESPGHAN working group of intestinal failure and intestinal transplantation. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 272–283. [Google Scholar] [CrossRef]
- Slagle, T.A.; Gross, S.J. Effect of early low-volume enteral substrate on subsequent feeding tolerance in very low birth weight infants. J. Pediatr. 1988, 113, 526–531. [Google Scholar] [CrossRef]
- McClure, R.J.; Newell, S.J. Randomised controlled study of clinical outcome following trophic feeding. Arch. Dis. Child. Fetal Neonatal Ed. 2000, 82, F29–F33. [Google Scholar] [CrossRef]
- Hermans, D.; Talbotec, C.; Lacaille, F.; Goulet, O.; Ricour, C.; Colomb, V. Early central catheter infections may contribute to hepatic fibrosis in children receiving long-term parenteral nutrition. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 459–463. [Google Scholar] [CrossRef]
- Cahova, M.; Bratova, M.; Wohl, P. Parenteral nutrition-associated liver disease: The role of the gut microbiota. Nutrients 2017, 9, 987. [Google Scholar] [CrossRef]
- Lee, W.S.; Sokol, R.J. Intestinal Microbiota, Lipids, and the Pathogenesis of Intestinal Failure-Associated Liver Disease. J. Pediatr. 2015, 167, 519–526. [Google Scholar] [CrossRef]
- Luman, W.; Shaffer, J.L. Prevalence, outcome and associated factors of deranged liver function tests in patients on home parenteral nutrition. Clin. Nutr. 2002, 21, 337–343. [Google Scholar] [CrossRef]
- Lloyd, D.A.J.; Zabron, A.A.; Gabe, S.M. Chronic biochemical cholestasis in patients receiving home parenteral nutrition: Prevalence and predisposing factors. Aliment. Pharmacol. Ther. 2008, 27, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Cavicchi, M.; Beau, P.; Crenn, P.; Degott, C.; Messing, B. Prevalence of liver disease and contributing factors in patients receiving home parenteral nutrition for permanent intestinal failure. Ann. Intern. Med. 2000, 132, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Cazals-Hatem, D.; Billiauws, L.; Rautou, P.E.; Bondjemah, V.; Poté, N.; Corcos, O.; Paradis, V.; Joly, F. Ultra-short bowel is an independent risk factor for liver fibrosis in adults with home parenteral nutrition. Liver Int. 2018, 38, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, S.S.; Loseke, C.A.; Lupo, J.V.; Young, R.J.; Murray, N.D.; Pinch, L.W.; Vanderhoof, J.A. Influence of bacterial overgrowth and intestinal inflammation on duration of parenteral nutrition in children with short bowel syndrome. J. Pediatr. 1997, 131, 356–361. [Google Scholar] [CrossRef]
- Kelly, D.A. Intestinal failure-associated liver disease: What do we know today? Gastroenterology 2006, 130, S70–S77. [Google Scholar] [CrossRef]
- Rangel, S.J.; Calkins, C.M.; Cowles, R.A.; Barnhart, D.C.; Huang, E.Y.; Abdullah, F.; Arca, M.J.; Teitelbaum, D.H. Parenteral nutrition-associated cholestasis: An American pediatric surgical association outcomes and clinical trials committee systematic review. J. Pediatr. Surg. 2012, 47, 225–240. [Google Scholar] [CrossRef]
- Fiel, M.I.; Wu, H.S.; Iyer, K.; Rodriguez-Laiz, G.; Schiano, T.D. Rapid reversal of parenteral-nutrition-associated cirrhosis following isolated intestinal transplantation. J. Gastrointest. Surg. 2009, 13, 1717–1723. [Google Scholar] [CrossRef]
- Leggett, G.; Butler, A.; Massey, D.; Middleton, S.; Russell, N.; Woodward, J.; Green, J.; Bond, D.; Duncan, S.; Woolner, L.; et al. A summary of 10 years of transplant activity and outcomes from a UK centre for intestinal and multivisceral transplantation. Clin. Nutr. 2018, 37, S304. [Google Scholar] [CrossRef]
- Woodward, J.M.; Massey, D.; Sharkey, L. The Long and Short of IT: Intestinal failure-associated liver disease (IFALD) in adults—Recommendations for early diagnosis and intestinal transplantation. Frontline Gastroenterol. 2020, 11, 34–39. [Google Scholar] [CrossRef]
- Naini, B.V.; Lassman, C.R. Total parenteral nutrition therapy and liver injury: A histopathologic study with clinical correlation. Hum. Pathol. 2012, 43, 826–833. [Google Scholar] [CrossRef]
- Buchman, A.L.; Ament, M.E.; Sohel, M.; Dubin, M.; Jenden, D.J.; Roch, M.; Pownall, H.; Farley, W.; Awal, M.; Ahn, C. Choline deficiency causes reversible hepatic abnormalities in patients receiving parenteral nutrition: Proof of a human choline requirement: A placebo-controlled trial. JPEN J. Parenter. Enteral Nutr. 2001, 25, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Picasso, M.C.; Fragkos, K.; Di Caro, S.; Rahman, F.; McNaughton, J.; Mehta, S.J. Composite serum liver Fibrosis/Cirrhosis scores may be associated with parenchymal liver disease in patients with chronic intestinal failure. Clin. Nutr. 2018, 37, S300. [Google Scholar] [CrossRef]
- Lin, Z.H.; Xin, Y.N.; Dong, Q.J.; Wang, Q.; Jiang, X.J.; Zhan, S.H.; Sun, Y.; Xuan, S.Y. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: An updated meta-analysis. Hepatology 2011, 53, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Wai, C.T.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S.F. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef]
- Shaheen, A.A.M.; Myers, R.P. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis C-related fibrosis: A systematic review. Hepatology 2007, 46, 912–921. [Google Scholar] [CrossRef]
- Mummadi, R.R.; Petersen, J.R.; Xiao, S.Y.; Snyder, N. Role of simple biomarkers in predicting fibrosis progression in HCV infection. World J. Gastroenterol. 2010, 16, 5710–5715. [Google Scholar] [CrossRef]
- Snyder, N.; Gajula, L.; Xiao, S.Y.; Grady, J.; Luxon, B.; Lau, D.T.Y.; Soloway, R.; Petersen, J. APRI: An easy and validated predictor of hepatic fibrosis in chronic hepatitis C. J. Clin. Gastroenterol. 2006, 40, 535–542. [Google Scholar] [CrossRef]
- Kruger, F.C.; Daniels, C.R.; Kidd, M.; Swart, G.; Brundyn, K.; van Rensburg, C.; Kotze, M. APRI: A simple bedside marker for advanced fibrosis that can avoid liver biopsy in patients with NAFLD/NASH. S. Afr. Med. J. 2011, 101, 477–480. [Google Scholar]
- Yilmaz, Y.; Yonal, O.; Kurt, R.; Bayrak, M.; Aktas, B.; Ozdogan, O. Noninvasive assessment of liver fibrosis with the aspartate transaminase to platelet ratio index (APRI): Usefulness in patients with chronic liver disease: APRI in chronic liver disease. Hepat. Mon. 2011, 11, 103. [Google Scholar]
- Rumbo, C.; Martinez, M.I.; Cabanne, A.; Trentadue, J.; Fernández, A.; Gondolesi, G. Utility of Aminotransferase/Platelet Ratio Index to Predict Liver Fibrosis in Intestinal Failure-Associated Liver Disease in Pediatric Patients. JPEN J. Parenter. Enteral Nutr. 2017, 41, 884–889. [Google Scholar] [CrossRef]
- Díaz, J.J.; Gura, K.M.; Roda, J.; Perez-Atayde, A.R.; Duggan, C.; Jaksic, T.; Lo, C.W. Aspartate aminotransferase to platelet ratio index correlates with hepatic cirrhosis but not with fibrosis in pediatric patients with intestinal failure. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Hukkinen, M.; Mutanen, A.; Nissinen, M.; Merras-Salmio, L.; Gylling, H.; Pakarinen, M.P. Parenteral Plant Sterols Accumulate in the Liver Reflecting Their Increased Serum Levels and Portal Inflammation in Children with Intestinal Failure. JPEN J. Parenter. Enteral Nutr. 2017, 41, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Mangus, R.S.; O’Connor, M.G.; Tector, A.J.; Lim, J.D.; Vianna, R.M. Use of the aspartate aminotransferase to platelet ratio index to follow liver fibrosis progression in infants with short gut. J. Pediatr. Surg. 2010, 45, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Van Gossum, A.; Pironi, L.; Messing, B.; Moreno, C.; Colecchia, A.; Derrico, A.; Demetter, P.; De Gos, F.; Cazals-Halem, D.; Joly, F. Transient Elastography (FibroScan) Is Not Correlated with Liver Fibrosis but with Cholestasis in Patients with Long-Term Home Parenteral Nutrition. JPEN J. Parenter. Enteral Nutr. 2015, 39, 719–724. [Google Scholar] [CrossRef]
- Xiao, G.; Yang, J.; Yan, L. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: A systemic review and meta-analysis. Hepatology 2015, 61, 292–302. [Google Scholar] [CrossRef]
- Kim, B.K.; Kim, D.Y.; Park, J.Y.; Ahn, S.H.; Chon, C.Y.; Kim, J.K.; Paik, Y.H.; Lee, K.S.; Park, Y.N.; Han, K.H. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int. 2010, 30, 546–553. [Google Scholar] [CrossRef]
- Shah, A.G.; Lydecker, A.; Murray, K.; Tetri, B.N.; Contos, M.J.; Sanyal, A.J.; Abrams, S.; Arceo, D.; Espinosa, D.; Fairly, L.; et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2009, 7, 1104–1112. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Kayadibi, H.; Yasar, B.; Ozkara, S.; Serdar, M.A.; Kurdas, O.O.; Gonen, C. The diagnostic accuracy of the Forns index, platelet count and AST to Platelet Ratio Index derived fibrosis index for the prediction of Hepatitis C virus-related significant liver fibrosis and cirrhosis. Scand. J. Clin. Lab. Investig. 2014, 74, 240–247. [Google Scholar] [CrossRef]
- Wong, G.L.H.; Wong, V.W.S.; Choi, P.C.L.; Chan, A.W.H.; Chan, H.L.Y. Development of a non-invasive algorithm with transient elastography (Fibroscan) and serum test formula for advanced liver fibrosis in chronic hepatitis B. Aliment. Pharmacol. Ther. 2010, 31, 1095–1103. [Google Scholar] [CrossRef]
- Forns, X.; Ampurdanès, S.; Llovet, J.M.; Aponte, J.; Quintó, L.; Martínez-Bauer, E.; Bruguera, M.; Sánchez-Tapias, J.M.; Rodés, J. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology 2002, 36, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Mayo, M.J.; Parkes, J.; Adams-Huet, B.; Combes, B.; Mills, A.S.; Markin, R.S.; Rubin, R.; Wheeler, D.; Contos, M.; West, A.B.; et al. Prediction of clinical outcomes in primary biliary cirrhosis by serum enhanced liver fibrosis assay. Hepatology 2008, 48, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Lichtinghagen, R.; Pietsch, D.; Bantel, H.; Manns, M.P.; Brand, K.; Bahr, M.J. The Enhanced Liver Fibrosis (ELF) score: Normal values, influence factors and proposed cut-off values. J. Hepatol. 2013, 59, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Zhou, X.; Huang, P.; Wei, J.; Wang, W.; Zheng, S. The performance of enhanced liver fibrosis (ELF) test for the staging of liver fibrosis: A meta-analysis. PLoS ONE 2014, 9, e92772. [Google Scholar] [CrossRef] [PubMed]
- Fagan, K.J.; Pretorius, C.J.; Horsfall, L.U.; Irvine, K.M.; Wilgen, U.; Choi, K.; Fletcher, L.M.; Tate, J.; Melino, M.; Nusrat, S.; et al. ELF score ≥9.8 indicates advanced hepatic fibrosis and is influenced by age, steatosis and histological activity. Liver Int. 2015, 35, 1673–1681. [Google Scholar] [CrossRef]
- Guha, I.N.; Parkes, J.; Roderick, P.; Chattopadhyay, D.; Cross, R.; Harris, S.; Kaye, P.; Burt, A.D.; Ryder, S.D.; Aithal, G.P.; et al. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: Validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology 2007, 47, 455–460. [Google Scholar] [CrossRef]
- Lemoine, M.; Shimakawa, Y.; Nayagam, S.; Khalil, M.; Suso, P.; Lloyd, J.; Goldin, R.; Njai, H.F.; Ndow, G.; Taal, M.; et al. The gamma-glutamyl transpeptidase to platelet ratio (GPR) predicts significant liver fibrosis and cirrhosis in patients with chronic HBV infection in West Africa. Gut 2016, 65, 1369–1376. [Google Scholar] [CrossRef]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef]
- Aykut, U.E.; Akyuz, U.; Yesil, A.; Eren, F.; Gerin, F.; Ergelen, R.; Celikel, C.A.; Yilmaz, Y. A comparison of fibrometerTM NAFLD score, NAFLD fibrosis score, and transient elastography as noninvasive diagnostic tools for hepatic fibrosis in patients with biopsy-proven non-alcoholic fatty liver disease. Scand. J. Gastroenterol. 2014, 49, 1343–1348. [Google Scholar] [CrossRef]
- Treeprasertsuk, S.; Björnsson, E.; Enders, F.; Suwanwalaikorn, S.; Lindor, K.D. NAFLD fibrosis score: A prognostic predictor for mortality and liver complications among NAFLD patients. World J. Gastroenterol. 2013, 19, 1219–1229. [Google Scholar] [CrossRef]
- Friedrich-Rust, M.; Rosenberg, W.; Parkes, J.; Herrmann, E.; Zeuzem, S.; Sarrazin, C. Comparison of ELF, FibroTest and FibroScan for the non-invasive assessment of liver fibrosis. BMC Gastroenterol. 2010, 10. [Google Scholar] [CrossRef] [PubMed]
- Poynard, T.; Morra, R.; Halfon, P.; Castera, L.; Ratziu, V.; Imbert-Bismut, F.; Naveau, S.; Thabut, D.; Lebrec, D.; Zoulim, F.; et al. Meta-analyses of FibroTest diagnostic value in chronic liver disease. BMC Gastroenterol. 2007, 7. [Google Scholar] [CrossRef] [PubMed]
- Calès, P.; Lainé, F.; Boursier, J.; Deugnier, Y.; Moal, V.; Oberti, F.; Hunault, G.; Rousselet, M.C.; Hubert, I.; Laafi, J.; et al. Comparison of blood tests for liver fibrosis specific or not to NAFLD. J. Hepatol. 2009, 50, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.A.; Bulsara, M.; Rossi, E.; DeBoer, B.; Speers, D.; George, J.; Kench, J.; Farrell, G.; McCaughan, G.W.; Jeffrey, G.P. Hepascore: An accurate validated predictor of liver fibrosis in chronic hepatitis C infection. Clin. Chem. 2005, 51, 1867–1873. [Google Scholar] [CrossRef]
- Strauss, S.; Gavish, E.; Gottlieb, P.; Katsnelson, L. Interobserver and intraobserver variability in the sonographic assessment of fatty liver. Am. J. Roentgenol. 2007, 189, 1449. [Google Scholar] [CrossRef]
- Cengiz, M.; Sentürk, S.; Cetin, B.; Bayrak, A.H.; Bilek, S.U. Sonographic assessment of fatty liver: Intraobserver and interobserver variability. Int. J. Clin. Exp. Med. 2014, 7, 5453–5460. [Google Scholar]
- Huijbers, A.; Wanten, G.; Dekker, H.M.; van der Graaf, M. Noninvasive Quantitative Assessment of Hepatic Steatosis by Proton Magnetic Resonance Spectroscopy Among Adult Patients Receiving Home Parenteral Nutrition. JPEN J. Parenter. Enteral Nutr. 2018, 42, 778–785. [Google Scholar] [CrossRef]
- Weijers, G.; Wanten, G.; Thijssen, J.M.; van der Graaf, M.; de Korte, C.L. Quantitative Ultrasound for Staging of Hepatic Steatosis in Patients on Home Parenteral Nutrition Validated with Magnetic Resonance Spectroscopy: A Feasibility Study. Ultrasound Med. Biol. 2016, 42, 637–644. [Google Scholar] [CrossRef][Green Version]
- Bond, A.; Hayes, S.; Abraham, A.; Teubner, A.; Farrer, K.; Pironi, L.; Lal, S. Reversal of intestinal failure associated liver disease fibrosis in a patient receiving long term home parenteral nutrition. Clin. Nutr. ESPEN 2018, 28, 228–231. [Google Scholar] [CrossRef]
- Lawrence, A.E.; Dienhart, M.; Cooper, J.N.; Lodwick, D.; Lopez, J.J.; Fung, B.; Smith, S.; Warren, P.; Mezoff, E.; Balint, J.; et al. Ultrasound Elastography as a Non-Invasive Method to Monitor Liver Disease in Children with Short Bowel Syndrome: Updated Results. J. Pediatr. Surg. 2019, 54, 1179–1183. [Google Scholar] [CrossRef]
- Marchesini, G.; Day, C.P.; Dufour, J.F.; Canbay, A.; Nobili, V.; Ratziu, V.; Tilg, H.; Roden, M.; Gastaldelli, A.; Yki-Järvinen, H.; et al. EASL-EASD-EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. Obes. Facts 2016, 9, 65–90. [Google Scholar] [CrossRef] [PubMed]
- Jordan, T.; Popovič, P.; Rotovnik Kozjek, N. Liver steatosis in adult patients on home parenteral nutrition. Eur. J. Clin. Nutr. 2020, 74, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Bray, T.J.P.; Chouhan, M.D.; Punwani, S.; Bridge, A.; Hall-Craggs, M.A. Fat fraction mapping using magnetic resonance imaging: Insight into pathophysiology. Br. J. Radiol. 2018, 91, 20170344. [Google Scholar] [CrossRef] [PubMed]
- Zummo, J.; Jeppesen, P.B.; Lin, T.; Buchman, A. P2.54 Re-analysis of a randomized placebo (PBO) controlled trial of intravenous (IV) choline chloride for IFALD using state-of-the-art analytic and imaging methods and contemporary definition of IFALD. Transplantation 2019, 103, S111. [Google Scholar] [CrossRef]
- Paisant, A.; D’Assignies, G.; Bannier, E.; Bardou-Jacquet, E.; Gandon, Y. MRI for the measurement of liver iron content, and for the diagnosis and follow-up of iron overload disorders. Press. Medicale 2017, 46, e279–e287. [Google Scholar] [CrossRef]
- Massironi, S.; Cavalcoli, F.; Rausa, E.; Invernizzi, P.; Braga, M.; Vecchi, M. Understanding short bowel syndrome: Current status and future perspectives. Dig. Liver Dis. 2020, 52, 253–261. [Google Scholar] [CrossRef]
- Bradley, C.R.; Cox, E.F.; Scott, R.A.; James, M.W.; Kaye, P.; Aithal, G.P.; Francis, S.T.; Guha, I.N. Multi-organ assessment of compensated cirrhosis patients using quantitative magnetic resonance imaging. J. Hepatol. 2018, 69, 1015–1024. [Google Scholar] [CrossRef]
- Chouhan, M.D.; Mookerjee, R.P.; Bainbridge, A.; Punwani, S.; Jones, H.; Davies, N.; Walker-Samuel, S.; Patch, D.; Jalan, R.; Halligan, S.; et al. Caval Subtraction 2D Phase-Contrast MRI to Measure Total Liver and Hepatic Arterial Blood Flow. Investig. Radiol. 2017, 52, 170–176. [Google Scholar] [CrossRef]
- Menys, A.; Puylaert, C.; Nolthenius, C.E.T.; Plumb, A.A.; Makanyanga, J.; Tielbeek, J.; Pendse, D.; Brosens, L.A.; Rodriguez-Justo, M.; Atkinson, D.; et al. Quantified terminal ileal motility during MR enterography as a biomarker of Crohn disease activity: Prospective multi-institution study. Radiology 2018, 289, 428–435. [Google Scholar] [CrossRef]
- Tappenden, K.A. Pathophysiology of short bowel syndrome: Considerations of resected and residual anatomy. JPEN J. Parenter. Enteral Nutr. 2014, 38, 14S–22S. [Google Scholar] [CrossRef]
- Rosenberg, W.M.C.; Voelker, M.; Thiel, R.; Becka, M.; Burt, A.; Schuppan, D.; Hubscher, S.; Roskams, T.; Pinzani, M.; Arthur, M.J.P. Serum markers detect the presence of liver fibrosis: A cohort study. Gastroenterology 2004, 127, 1704–1713. [Google Scholar] [CrossRef] [PubMed]
- Hoad, C.L.; Palaniyappan, N.; Kaye, P.; Chernova, Y.; James, M.W.; Costigan, C.; Austin, A.; Marciani, L.; Gowland, P.A.; Guha, I.N.; et al. A study of T1 relaxation time as a measure of liver fibrosis and the influence of confounding histological factors. NMR Biomed. 2015, 28, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, M.D.; Mookerjee, R.P.; Bainbridge, A.; Walker-Samuel, S.; Davies, N.; Halligan, S.; Lythgoe, M.F.; Taylor, S.A. Use of caval subtraction 2d phase-contrast mr imaging to measure total liver and hepatic arterial blood flow: Preclinical validation and initial clinical translation. Radiology 2016, 280, 916–923. [Google Scholar] [CrossRef]
- de Jonge, C.S.; Menys, A.; van Rijn, K.L.; Bredenoord, A.J.; Nederveen, A.J.; Stoker, J. Detecting the effects of a standardized meal challenge on small bowel motility with MRI in prepared and unprepared bowel. Neurogastroenterol. Motil. 2019, 31, e13506. [Google Scholar] [CrossRef] [PubMed]
- Hariz, M.B.; Goulet, O.; De Potter, S.; Girot, M.; Rambaud, C.; Colomb, V.; Corriol, O.; Ricour, C. Iron overload in children receiving prolonged parenteral nutrition. J. Pediatr. 1993, 123, 238–241. [Google Scholar] [CrossRef]
- Yang, C.J.; Duro, D.; Zurakowski, D.; Lee, M.; Jaksic, T.; Duggan, C. High prevalence of multiple micronutrient deficiencies in children with intestinal failure: A longitudinal study. J. Pediatr. 2011, 159, 39–44. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Fracanzani, A.L.; Fargion, S.; Valenti, L. Iron in fatty liver and in the metabolic syndrome: A promising therapeutic target. J. Hepatol. 2011, 55, 920–932. [Google Scholar] [CrossRef]
- Gilligan, L.A.; Dillman, J.R.; Tkach, J.A.; Xanthakos, S.A.; Gill, J.K.; Trout, A.T. Magnetic resonance imaging T1 relaxation times for the liver, pancreas and spleen in healthy children at 1.5 and 3 tesla. Pediatr. Radiol. 2019, 49, 1018–1024. [Google Scholar] [CrossRef]
- Chouhan, M.D.; Lythgoe, M.F.; Mookerjee, R.P.; Taylor, S.A. Vascular assessment of liver disease-towards a new frontier in MRI. Br. J. Radiol. 2016, 89, 20150675. [Google Scholar] [CrossRef]
- Chouhan, M.; Bainbridge, A.; Lythgoe, M.; Mookerjee, R.; Halligan, S.; Taylor, S.A. SS 4.9 The negative hepatic arterial buffer response: Pre and post-prandial changes in total liver and hepatic arterial blood flow measured using caval subtraction phase contrast MRI in normal volunteers. Insights Imaging 2017, 8, S600. [Google Scholar]
- Iwata, T.; Kanematsu, T. Prolonged enteral fast with parenteral nutrition decreases PCO2 and flow volume in portal vein: Its association with bile secretion. Eur. Surg. Res. 1996, 28, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Plauth, M.; Roske, A.E.; Romaniuk, P.; Roth, E.; Ziebig, R.; Lochs, H. Post-feeding hyperammonaemia in patients with transjugular intrahepatic portosystemic shunt and liver cirrhosis: Role of small intestinal ammonia release and route of nutrient administration. Gut 2000, 46, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Cowles, R.A.; Ventura, K.A.; Martinez, M.; Lobritto, S.J.; Harren, P.A.; Brodlie, S.; Carroll, J.; Jan, D.M. Reversal of intestinal failure-associated liver disease in infants and children on parenteral nutrition: Experience with 93 patients at a referral center for intestinal rehabilitation. J. Pediatr. Surg. 2010, 45, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, A.H.; Pimentel, M. Gastrointestinal bacterial overgrowth: Pathogenesis and clinical significance. Ther. Adv. Chronic Dis. 2013, 4, 223–231. [Google Scholar] [CrossRef]
- Pande, C.; Kumar, A.; Sarin, S.K. Small-intestinal bacterial overgrowth in cirrhosis is related to the severity of liver disease. Aliment. Pharmacol. Ther. 2009, 29, 1273–1281. [Google Scholar] [CrossRef]
- Kuo, P.C.; Li, K.; Alfrey, E.J.; Jeffrey, R.B.; Garcia, G.; Dafoe, D.C. Magnetic resonance imaging and hepatic hemodynamics: Correlation with metabolic function in liver transplantation candidates. Surgery 1995, 117, 373–379. [Google Scholar] [CrossRef]


| Name | Components | Liver Diseases in Which the Biomarkers Have Been Studied | Score Calculation |
|---|---|---|---|
| APRI | AST and Platelet Count | Chronic Hepatitis C, Chronic Hepatitis B, Non-alcoholic steatohepatitis (NASH), NAFLD, biliary atresia and IFALD | ((AST level/AST upper level of normal)/platelet count)) × 100 − [AST upper level of normal = 40] [34] |
| AST/ALT Ratio | AST and ALT | Alcoholic liver disease, primary biliary cirrhosis, NAFLD and IFALD | AST/ALT |
| ELF | Hyaluronic Acid (HA), PIIINP and Tissue inhibitor of metalloproteinase 1 (TIMP-1) | Mixed chronic liver diseases, Chronic Hepatitis C, and primary biliary cirrhosis | (2.494 + 0.84 ln (CHA) + 0.735 ln(CPIIINP) + 0.391 ln(CTIMP-1) [81] |
| FIB-4 | ALT, AST and Platelet Count | HIV/HCV coinfection, Chronic Hepatitis B, NAFLD and IFALD | (age × AST level/platelet count × √ALT) [48] |
| Forns Index | Age, GGT, Cholesterol and Platelet Count | Chronic Hepatitis C, Chronic Hepatitis B, and alcoholic liver disease. | (7.811 − 3.131 × ln(platelet count) + 0.781 × ln(GGT) + 3.467 × ln(age) − 0.014 × cholesterol) [51] |
| Fibrosis Index | Age, GGT, Cholesterol and Platelet Count | NAFLD | (−2.948 + 0.562 × Forns index + 0.288 × APRI + 0.006 × Platelet count (109/L)) [49] |
| GPR | GGT and Platelet count | HIV/Chronic Hepatitis B, coinfection | ((GGT level/GGT upper level of normal)/platelet count) × 100 GGT upper limit of normal: 40 (women) and 60 (men) [57] |
| NAFLD Fibrosis Score | Age, Hyperglycaemia, BMI, Platelet Count, Albumin, AST and ALT | NAFLD | (−1.675 + 0.037 × age (years) + 0.094 × BMI (kg/m2) + 1.13 × impaired fasting glucose/diabetes (yes = 1, no = 0) + 0.99 × AST/ALT ratio − 0.013 × platelet count − 0.66 × albumin) [58] |
| Parameters | Total (n = 20) | IFALD (n = 8) | Non-IFALD (n = 12) | p-Value |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age (years) | 51.15 ± 17.30 | 58.13 ± 15.90 | 46.50 ± 17.23 | 0.145 |
| Gender (Males:Females) | 8:12 | 4:4 | 4:8 | 0.648 |
| BMI (kg/m2) | 21.27 ± 3.63 | 23.73 ± 3.36 | 19.91 ± 2.95 | 0.036 |
| Oral Diet (Yes:No) | 15:5 | 7:1 | 8:4 | 0.292 |
| Pathophysiological classification of IF | ||||
| SBS-I—n (%) | 2 (10%) | 1 (8.3%) | 1 (8.3%) | 0.833 |
| SBS-JC—n (%) | 1 (5%) | 1 (8.3%) | 0 | |
| SBS-JIC—n (%) | 1 (5%) | 0 | 1 (8.3%) | |
| Dysmotility—n (%) | 8 (40%) | 3 (37.5%) | 5 (41.7%) | |
| Mechanical obstruction—n (%) | 2 (10%) | 0 | 2 (16.7%) | |
| Mucosal disease—n (%) | 6 (30%) | 3 (37.5%) | 3 (50%) | |
| Small bowel length (cm) | 100.56 ± 52.05 | 75.00 ± 49.12 | 132.50 ± 39.48 | 0.100 |
| Nutritional characteristics | ||||
| PN duration (months) | 88.90 (77.33) | 85.50 (78.75) | 57.50 (64.75) | 0.980 |
| Age PN started (years) | 43.55 ± 17.61 | 47.50 ± 17.80 | 40.92 ± 17.76 | 0.428 |
| PN mean energy (kcal/day) | 1730.91 ± 372.02 | 1687.90 ± 419.00 | 1759.58 ± 353.67 | 0.685 |
| PN mean lipids (g/kg/day) | 0.41 ± 0.28 | 0.39 ± 0.30 | 0.41 ± 0.28 | 0.887 |
| Days of PN/week | 7 (2.50) | 7 (0.25) | 6.5 (3) | 0.257 |
| Days of PN lipids/week | 2.80 ± 1.58 | 2.25 ± 1.16 | 3.17 ± 1.75 | 0.211 |
| Biochemical Parameters | Normal Range | Total (n = 20) | IFALD (n = 8) | Non-IFALD (n = 12) | p-Value |
|---|---|---|---|---|---|
| Platelet Count (× 109/L) | 150–400 | 219.80 ± 65.53 | 172.00 ± 42.04 | 251.67 ± 59.35 | 0.040 |
| C-Reactive Protein (mg/L) | 0–5.0 | 4.30 (8.00) | 0.90 (1.23) | 8.20 (9.70) | 0.005 |
| PIIINP (μg/L) | 1.7–4.2 | 4.72 ± 2.26 | 6.20 ± 1.93 | 3.68 ± 1.92 | 0.018 |
| Haptoglobin (g/L) | 0.3–2.0 | 1.59 ± 0.96 | 0.88 ± 0.70 | 2.07 ± 0.81 | 0.003 |
| Bilirubin(μmol/L) | 0–20 | 7.35 (5.83) | 11.84 (6.54) | 6.07 (3.34) | 0.005 |
| AST (IU/L) | 0–31 | 28.30 ± 11.41 | 38.88 ± 9.08 | 21.25 ± 6.06 | <0.001 |
| ALT (IU/L) | 10–35 | 24.20 (19.48) | 36.67 (18.72) | 18.94 (10.82) | 0.040 |
| GGT (IU/L) | 6–42 | 35.00 (84.46) | 130.00 (93.25) | 23.25 (25.75) | 0.040 |
| Hepatic Fibrosis Scores | Total (n = 20) | IFALD (n = 8) | Non-IFALD (n = 12) | p-Value |
|---|---|---|---|---|
| ELF | 8.91 ± 1.34 | 9.71 ± 1.24 | 8.34 ± 1.14 | 0.032 |
| APRI | 0.30 (0.40) | 0.63 (0.32) | 0.19 (0.13) | <0.001 |
| FIB-4 | 1.23 (0.60) | 2.21 (1.63) | 0.96 (0.71) | 0.010 |
| Forns Index | 5.36 ± 2.55 | 7.48 ± 1.94 | 3.94 ± 1.83 | 0.001 |
| GPR | 0.35 (0.82) | 1.20 (1.37) | 0.20 (0.22) | 0.002 |
| Fibrosis Index | 1.49 ± 1.21 | 2.46 ± 0.98 | 0.84 ± 0.87 | 0.001 |
| Parameters | |
|---|---|
| Clinical characteristics | |
| Age (years) | 52.3 ± 18.0 |
| Gender (Males:Females) | 2:8 |
| BMI (kg/m2) | 20.95 ± 4.38 |
| Pathophysiological classification of IF | |
| SBS—n (%) | 8 (80.0%) |
| Dysmotility—n (%) | 2 (20.0%) |
| Nutritional characteristics | |
| PN duration (months) | 120 ± 87 |
| PN mean energy (kcal/day) | 1151 ± 398 |
| PN mean lipids (g/kg/day) | 3.85 ±3.54 |
| Non-IFALD (n = 5) | IFALD Steatosis (n = 5) | p-Value | |
|---|---|---|---|
| Median (range) | Median (range) | ||
| Liver Fat Fraction (%) | 2.14 (1.4–4.2) | 10.90 (2.2–27.4) | 0.032 |
| Liver Iron Concentration (μmol/g) | 11.3 (10.4–38.9) | 16.0 (11.3–46.7) | 0.222 |
| Liver T1 (ms) | 715 (544–848) | 740 (594–919) | 0.873 |
| Portal venous flow (qPV) (mL/min/100 g) | 64.2 (45.6–137.6) | 56.2 (37.5–93.0) | 0.667 |
| Estimated Total Liver Blood Flow (eTLBF) (mL/min/100 g) | 91.8 (63.5–145.7) | 62.1 (27.9–119.3) | 0.151 |
| Small Bowel Mean motility (a.u.) | 0.19 (0.07–0.21) | 0.16 (0.11–0.21) | 0.999 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fragkos, K.C.; Picasso Bouroncle, M.C.; Kumar, S.; Caselton, L.; Menys, A.; Bainbridge, A.; Taylor, S.A.; Torrealdea, F.; Kumagai, T.; Di Caro, S.; et al. Serum Scoring and Quantitative Magnetic Resonance Imaging in Intestinal Failure-Associated Liver Disease: A Feasibility Study. Nutrients 2020, 12, 2151. https://doi.org/10.3390/nu12072151
Fragkos KC, Picasso Bouroncle MC, Kumar S, Caselton L, Menys A, Bainbridge A, Taylor SA, Torrealdea F, Kumagai T, Di Caro S, et al. Serum Scoring and Quantitative Magnetic Resonance Imaging in Intestinal Failure-Associated Liver Disease: A Feasibility Study. Nutrients. 2020; 12(7):2151. https://doi.org/10.3390/nu12072151
Chicago/Turabian StyleFragkos, Konstantinos C., María Claudia Picasso Bouroncle, Shankar Kumar, Lucy Caselton, Alex Menys, Alan Bainbridge, Stuart A. Taylor, Francisco Torrealdea, Tomoko Kumagai, Simona Di Caro, and et al. 2020. "Serum Scoring and Quantitative Magnetic Resonance Imaging in Intestinal Failure-Associated Liver Disease: A Feasibility Study" Nutrients 12, no. 7: 2151. https://doi.org/10.3390/nu12072151
APA StyleFragkos, K. C., Picasso Bouroncle, M. C., Kumar, S., Caselton, L., Menys, A., Bainbridge, A., Taylor, S. A., Torrealdea, F., Kumagai, T., Di Caro, S., Rahman, F., Macnaughtan, J., Chouhan, M. D., & Mehta, S. (2020). Serum Scoring and Quantitative Magnetic Resonance Imaging in Intestinal Failure-Associated Liver Disease: A Feasibility Study. Nutrients, 12(7), 2151. https://doi.org/10.3390/nu12072151






