Overfeeding and Substrate Availability, But Not Age or BMI, Alter Human Satellite Cell Function
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. High Substrate Media Challenge
2.3. Proliferation Assay
2.4. Myogenic Index
2.5. Substrate Oxidation
2.6. RNA Extraction and qRT-PCR
2.7. Statistical Analysis
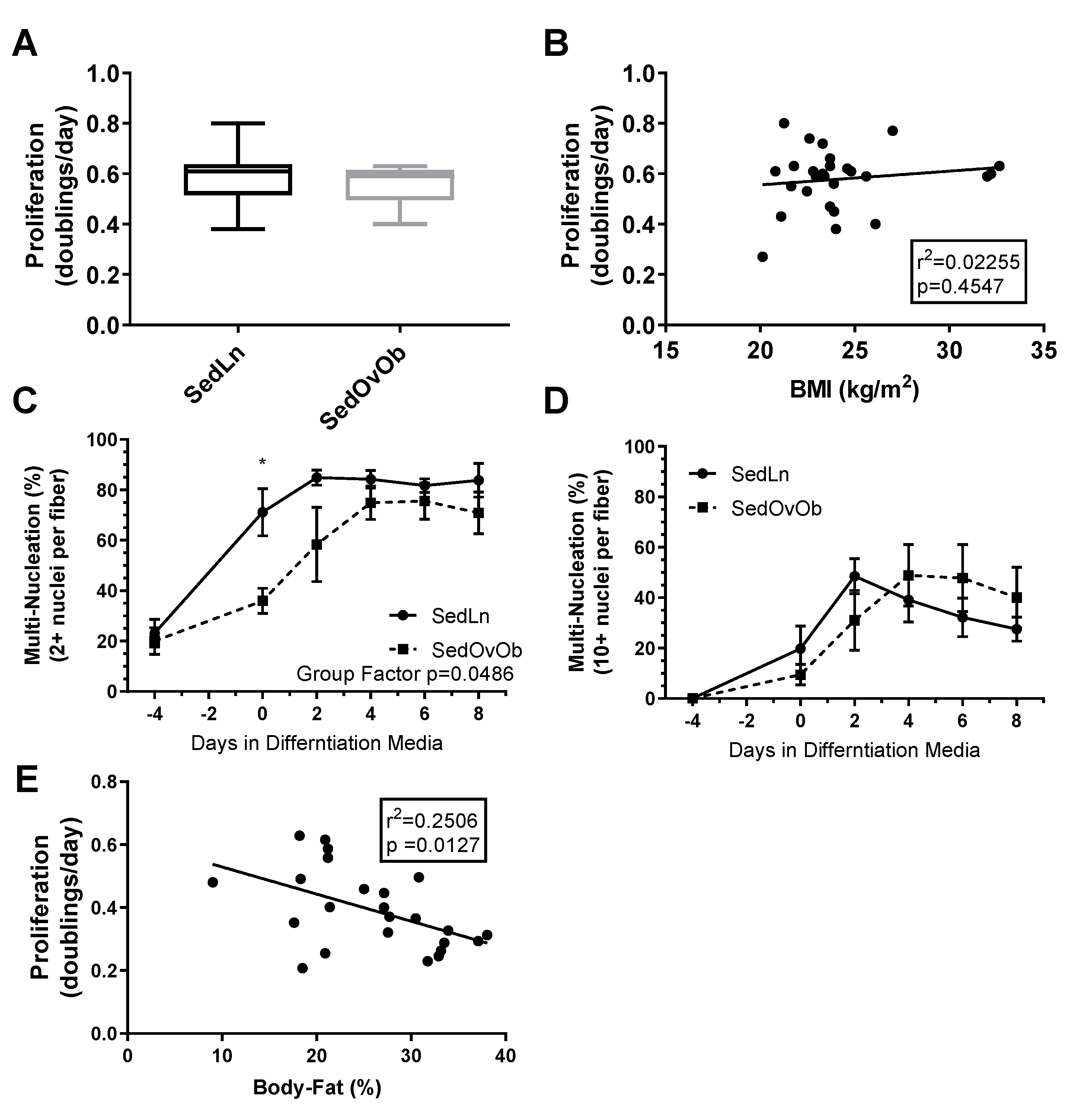
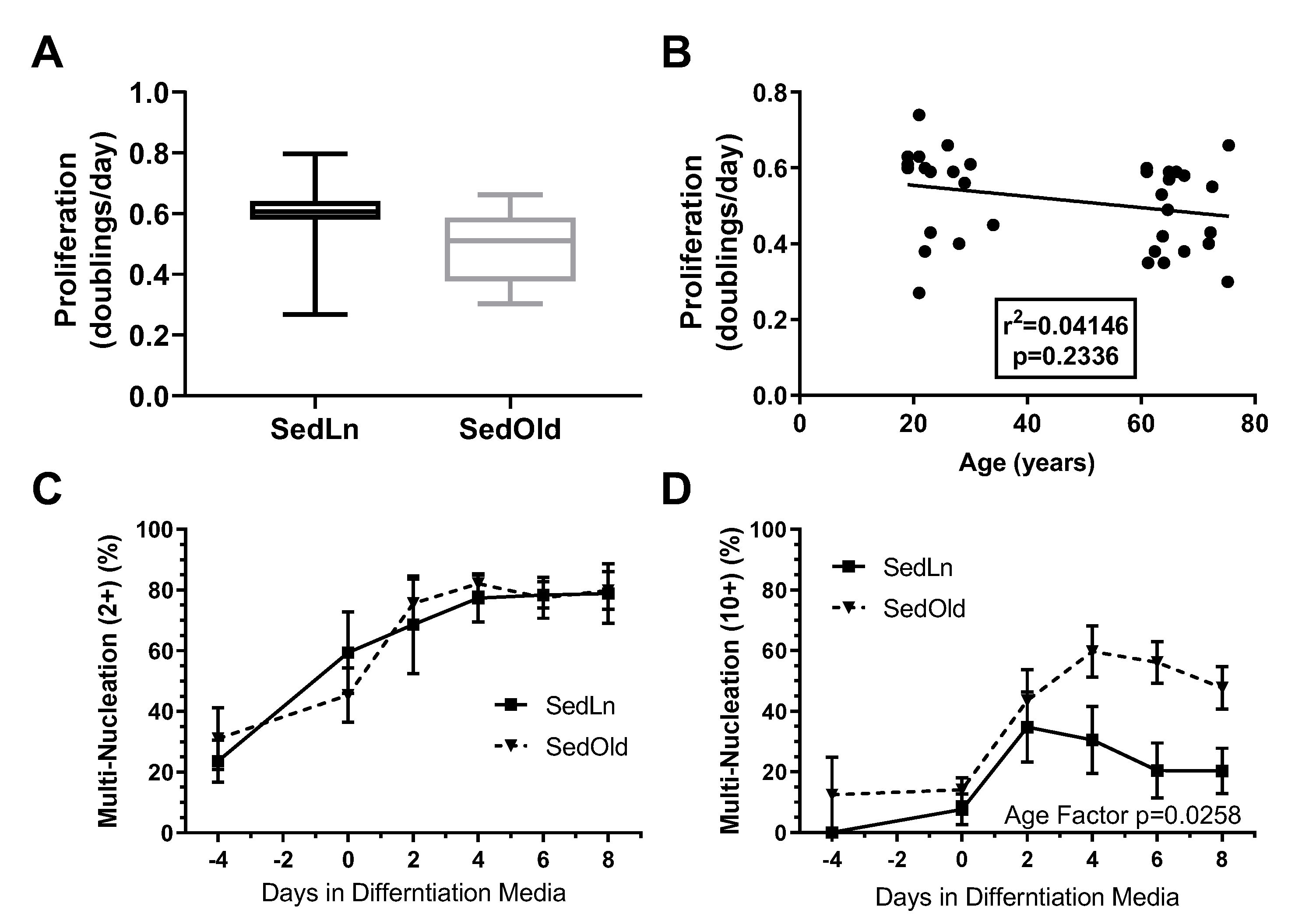
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Geliebter, A.; Maher, M.M.; Gerace, L.; Gutin, B.; Heymsfield, S.B.; Hashim, S.A. Effects of strength or aerobic training on body composition, resting metabolic rate, and peak oxygen consumption in obese dieting subjects. Am. J. Clin. Nutr. 1997, 66, 557–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stiegler, P.; Cunliffe, A. The role of diet and exercise for the maintenance of fat-free mass and resting metabolic rate during weight loss. Sports Med. 2006, 36, 239–262. [Google Scholar] [CrossRef] [PubMed]
- Izumiya, Y.; Hopkins, T.; Morris, C.; Sato, K.; Zeng, L.; Viereck, J.; Hamilton, J.A.; Ouchi, N.; LeBrasseur, N.K.; Walsh, K. Fast/Glycolytic muscle fiber growth reduces fat mass and improves metabolic parameters in obese mice. Cell Metab. 2008, 7, 159–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGregor, R.A.; Cameron-Smith, D.; Poppitt, S.D. It is not just muscle mass: A review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev. Healthspan 2014, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Landi, F.; Cruz-Jentoft, A.J.; Liperoti, R.; Russo, A.; Giovannini, S.; Tosato, M.; Capoluongo, E.; Bernabei, R.; Onder, G. Sarcopenia and mortality risk in frail older persons aged 80 years and older: Results from ilSIRENTE study. Age Ageing 2013, 42, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef]
- Keller, K.; Engelhardt, M. Strength and muscle mass loss with aging process. Age and strength loss. Muscles Ligaments Tendons J. 2013, 3, 346–350. [Google Scholar] [CrossRef]
- Alway, S.E.; Myers, M.J.; Mohamed, J.S. Regulation of Satellite Cell Function in Sarcopenia. Front. Aging Neurosci. 2014, 6, 246. [Google Scholar] [CrossRef] [Green Version]
- Kalyani, R.R.; Corriere, M.; Ferrucci, L. Age-related and disease-related muscle loss: The effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014, 2, 819–829. [Google Scholar] [CrossRef] [Green Version]
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- Abrigo, J.; Rivera, J.C.; Aravena, J.; Cabrera, D.; Simon, F.; Ezquer, F.; Ezquer, M.; Cabello-Verrugio, C. High Fat Diet-Induced Skeletal Muscle Wasting Is Decreased by Mesenchymal Stem Cells Administration: Implications on Oxidative Stress, Ubiquitin Proteasome Pathway Activation, and Myonuclear Apoptosis. Oxidative Med. Cell. Longev. 2016, 2016, 9047821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conboy, I.M.; Conboy, M.J.; Smythe, G.M.; Rando, T.A. Notch-Mediated Restoration of Regenerative Potential to Aged Muscle. Science 2003, 302, 1575–1577. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.D.; Dalbo, V.J.; Sunderland, K.; Poole, C.; Hassell, S.E.; Kerksick, C.M. Myogenic mRNA markers in young and old human skeletal muscle prior to and following sequential exercise bouts. Appl. Physiol. Nutr. Metab. 2011, 36, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Banerji, C.R.S.; Panamarova, M.; Hebaishi, H.; White, R.B.; Relaix, F.; Severini, S.; Zammit, P.S. PAX7 target genes are globally repressed in facioscapulohumeral muscular dystrophy skeletal muscle. Nat. Commun. 2017, 8, 2152. [Google Scholar] [CrossRef] [Green Version]
- Lindstrom, M.; Pedrosa-Domellof, F.; Thornell, L.E. Satellite cell heterogeneity with respect to expression of MyoD, myogenin, Dlk1 and c-Met in human skeletal muscle: Application to a cohort of power lifters and sedentary men. Histochem. Cell Biol. 2010, 134, 371–385. [Google Scholar] [CrossRef] [Green Version]
- Rayagiri, S.S.; Ranaldi, D.; Raven, A.; Mohamad Azhar, N.I.F.; Lefebvre, O.; Zammit, P.S.; Borycki, A.-G. Basal lamina remodeling at the skeletal muscle stem cell niche mediates stem cell self-renewal. Nat. Commun. 2018, 9, 1075. [Google Scholar] [CrossRef] [Green Version]
- Wen, Y.; Bi, P.; Liu, W.; Asakura, A.; Keller, C.; Kuang, S. Constitutive Notch activation upregulates Pax7 and promotes the self-renewal of skeletal muscle satellite cells. Mol. Cell. Biol. 2012, 32, 2300–2311. [Google Scholar] [CrossRef] [Green Version]
- Von Maltzahn, J.; Jones, A.E.; Parks, R.J.; Rudnicki, M.A. Pax7 is critical for the normal function of satellite cells in adult skeletal muscle. Proc. Natl. Acad. Sci. USA 2013, 110, 16474–16479. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.; Yin, A.; Nichenko, A.S.; Beedle, A.M.; Call, J.A.; Yin, H. Transient HIF2A inhibition promotes satellite cell proliferation and muscle regeneration. J. Clin. Investig. 2018, 128, 2339–2355. [Google Scholar] [CrossRef] [Green Version]
- Day, K.; Shefer, G.; Shearer, A.; Yablonka-Reuveni, Z. The depletion of skeletal muscle satellite cells with age is concomitant with reduced capacity of single progenitors to produce reserve progeny. Dev. Biol. 2010, 340, 330–343. [Google Scholar] [CrossRef] [Green Version]
- Pelosi, M.; De Rossi, M.; Barberi, L.; Musaro, A. IL-6 impairs myogenic differentiation by downmodulation of p90RSK/eEF2 and mTOR/p70S6K axes, without affecting AKT activity. Biomed. Res. Int. 2014, 2014, 206026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Q.; Yang, S.T.; Wang, J.J.; Zhou, J.; Xing, S.S.; Shen, C.C.; Wang, X.X.; Yue, Y.X.; Song, J.; Chen, M.; et al. TNF alpha inhibits myogenic differentiation of C2C12 cells through NF-kappaB activation and impairment of IGF-1 signaling pathway. Biochem. Biophys. Res. Commun. 2015, 458, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Palacios, D.; Mozzetta, C.; Consalvi, S.; Caretti, G.; Saccone, V.; Proserpio, V.; Marquez, V.E.; Valente, S.; Mai, A.; Forcales, S.V.; et al. TNF/p38 alpha/Polycomb signalling to Pax7 locus in satellite cells links inflammation to the epigenetic control of muscle regeneration. Cell Stem Cell 2010, 7, 455–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKay, B.R.; Ogborn, D.I.; Bellamy, L.M.; Tarnopolsky, M.A.; Parise, G. Myostatin is associated with age-related human muscle stem cell dysfunction. FASEB J. 2012, 26, 2509–2521. [Google Scholar] [CrossRef] [PubMed]
- Proto, J.D.; Tang, Y.; Lu, A.; Chen, W.C.; Stahl, E.; Poddar, M.; Beckman, S.A.; Robbins, P.D.; Nidernhofer, L.J.; Imbrogno, K.; et al. NF-kappaB inhibition reveals a novel role for HGF during skeletal muscle repair. Cell Death Dis. 2015, 6, e1730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharples, A.P.; Polydorou, I.; Hughes, D.C.; Owens, D.J.; Hughes, T.M.; Stewart, C.E. Skeletal muscle cells possess a ’memory’ of acute early life TNF-alpha exposure: Role of epigenetic adaptation. Biogerontology 2016, 17, 603–617. [Google Scholar] [CrossRef]
- Shefer, G.; Rauner, G.; Stuelsatz, P.; Benayahu, D.; Yablonka-Reuveni, Z. Moderate-intensity treadmill running promotes expansion of the satellite cell pool in young and old mice. FEBS J. 2013, 280, 4063–4073. [Google Scholar] [CrossRef] [Green Version]
- D’Souza, D.M.; Trajcevski, K.E.; Al-Sajee, D.; Wang, D.C.; Thomas, M.; Anderson, J.E.; Hawke, T.J. Diet-induced obesity impairs muscle satellite cell activation and muscle repair through alterations in hepatocyte growth factor signaling. Physiological reports 2015, 3, e12506. [Google Scholar] [CrossRef]
- Antico Arciuch, V.G.; Elguero, M.E.; Poderoso, J.J.; Carreras, M.C. Mitochondrial regulation of cell cycle and proliferation. Antioxid. Redox Signal. 2012, 16, 1150–1180. [Google Scholar] [CrossRef] [Green Version]
- Kondoh, H.; Lleonart, M.E.; Nakashima, Y.; Yokode, M.; Tanaka, M.; Bernard, D.; Gil, J.; Beach, D. A high glycolytic flux supports the proliferative potential of murine embryonic stem cells. Antioxid. Redox Signal. 2007, 9, 293–299. [Google Scholar] [CrossRef]
- Urbani, L.; Piccoli, M.; Franzin, C.; Pozzobon, M.; De Coppi, P. Hypoxia Increases Mouse Satellite Cell Clone Proliferation Maintaining both In Vitro and In Vivo Heterogeneity and Myogenic Potential. PLoS ONE 2012, 7, e49860. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.M.; Bryner, R.W.; Alway, S.E. Satellite cell proliferation is reduced in muscles of obese Zucker rats but restored with loading. Am. J. Physiol. Cell Physiol. 2008, 295, C521–C528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajpeyi, S.; Myrland, C.K.; Covington, J.D.; Obanda, D.; Cefalu, W.T.; Smith, S.R.; Rustan, A.C.; Ravussin, E. Lipid in Skeletal Muscle Myotubes is associated to the Donors’ Insulin Sensitivity and Physical Activity Phenotypes. Obesity 2014, 22, 426–434. [Google Scholar] [CrossRef]
- Scarda, A.; Franzin, C.; Milan, G.; Sanna, M.; Dal Pra, C.; Pagano, C.; Boldrin, L.; Piccoli, M.; Trevellin, E.; Granzotto, M.; et al. Increased adipogenic conversion of muscle satellite cells in obese Zucker rats. Int. J. Obes. 2010, 34, 1319–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.S.; Park, J.Y.; Yu, R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res. Clin. Pract. 2005, 69, 29–35. [Google Scholar] [CrossRef]
- Schrauwen, P.; Wagenmakers, A.J.; van Marken Lichtenbelt, W.D.; Saris, W.H.; Westerterp, K.R. Increase in fat oxidation on a high-fat diet is accompanied by an increase in triglyceride-derived fatty acid oxidation. Diabetes 2000, 49, 640–646. [Google Scholar] [CrossRef] [Green Version]
- Flack, K.D.; Davy, B.M.; DeBerardinis, M.; Boutagy, N.E.; McMillan, R.P.; Hulver, M.W.; Frisard, M.I.; Anderson, A.S.; Savla, J.; Davy, K.P. Resistance exercise training and in vitro skeletal muscle oxidative capacity in older adults. Physiol. Rep. 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Osterberg, K.L.; Boutagy, N.E.; McMillan, R.P.; Stevens, J.R.; Frisard, M.I.; Kavanaugh, J.W.; Davy, B.M.; Davy, K.P.; Hulver, M.W. Probiotic supplementation attenuates increases in body mass and fat mass during high-fat diet in healthy young adults. Obesity 2015, 23, 2364–2370. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2018, 48, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Gould, H.; Brennan, S.L.; Kotowicz, M.A.; Nicholson, G.C.; Pasco, J.A. Total and Appendicular Lean Mass Reference Ranges for Australian Men and Women: The Geelong Osteoporosis Study. Calcif. Tissue Int. 2014, 94, 363–372. [Google Scholar] [CrossRef]
- Muoio, D.M.; Way, J.M.; Tanner, C.J.; Winegar, D.A.; Kliewer, S.A.; Houmard, J.A.; Kraus, W.E.; Dohm, G.L. Peroxisome Proliferator-Activated Receptor-α Regulates Fatty Acid Utilization in Primary Human Skeletal Muscle Cells. Diabetes 2002, 51, 901–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frisard, M.I.; McMillan, R.P.; Marchand, J.; Wahlberg, K.A.; Wu, Y.; Voelker, K.A.; Heilbronn, L.; Haynie, K.; Muoio, B.; Li, L.; et al. Toll-like receptor 4 modulates skeletal muscle substrate metabolism. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E988–E998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carroll, M.F.; Izard, A.; Riboni, K.; Burge, M.R.; Schade, D.S. Fasting Hyperglycemia Predicts the Magnitude of Postprandial Hyperglycemia. Implic. Diabetes Ther. 2002, 25, 1247–1248. [Google Scholar] [CrossRef] [Green Version]
- Guillet-Deniau, I.; Leturque, A.; Girard, J. Expression and cellular localization of glucose transporters (GLUT1, GLUT3, GLUT4) during differentiation of myogenic cells isolated from rat foetuses. J. Cell Sci. 1994, 107 Pt 3, 487–496. [Google Scholar]
- Palmada, M.; Boehmer, C.; Akel, A.; Rajamanickam, J.; Jeyaraj, S.; Keller, K.; Lang, F. SGK1 Kinase Upregulates GLUT1 Activity and Plasma Membrane Expression. Diabetes 2006, 55, 421–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javaheri, B.; Sunters, A.; Zaman, G.; Suswillo, R.F.L.; Saxon, L.K.; Lanyon, L.E.; Price, J.S. Lrp5 Is Not Required for the Proliferative Response of Osteoblasts to Strain but Regulates Proliferation and Apoptosis in a Cell Autonomous Manner. PLOS ONE 2012, 7, e35726. [Google Scholar] [CrossRef] [PubMed]
- Kamli, M.R.; Kim, J.; Pokharel, S.; Jan, A.T.; Lee, E.J.; Choi, I. Expressional studies of the aldehyde oxidase (AOX1) gene during myogenic differentiation in C2C12 cells. Biochem. Biophys. Res. Commun. 2014, 450, 1291–1296. [Google Scholar] [CrossRef]
- Frisard, M.I.; Wu, Y.; McMillan, R.P.; Voelker, K.A.; Wahlberg, K.A.; Anderson, A.S.; Boutagy, N.; Resendes, K.; Ravussin, E.; Hulver, M.W. Low Levels of Lipopolysaccharide Modulate Mitochondrial Oxygen Consumption in Skeletal Muscle. Metab.-Clin. Exp. 2015, 64, 416–427. [Google Scholar] [CrossRef] [Green Version]
- Shintaku, J.; Peterson, J.M.; Talbert, E.E.; Gu, J.-M.; Ladner, K.J.; Williams, D.R.; Mousavi, K.; Wang, R.; Sartorelli, V.; Guttridge, D.C. MyoD Regulates Skeletal Muscle Oxidative Metabolism Cooperatively with Alternative NF-κB. Cell Rep. 2016, 17, 514–526. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, R.Z.; Scadden, D.T. Fate through Fat: Lipid Metabolism Determines Stem Cell Division Outcome. Cell Metab. 2012, 16, 411–413. [Google Scholar] [CrossRef] [Green Version]
- Shavlakadze, T.; McGeachie, J.; Grounds, M.D. Delayed but excellent myogenic stem cell response of regenerating geriatric skeletal muscles in mice. Biogerontology 2010, 11, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Schultz, E.; Lipton, B.H. Skeletal muscle satellite cells: Changes in proliferation potential as a function of age. Mech. Ageing Dev. 1982, 20, 377–383. [Google Scholar] [CrossRef]
- Conboy, I.M.; Conboy, M.J.; Wagers, A.J.; Girma, E.R.; Weissman, I.L.; Rando, T.A. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005, 433, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Shefer, G.; Rauner, G.; Yablonka-Reuveni, Z.; Benayahu, D. Reduced Satellite Cell Numbers and Myogenic Capacity in Aging Can Be Alleviated by Endurance Exercise. PLoS ONE 2010, 5, e13307. [Google Scholar] [CrossRef] [Green Version]
- Scheele, C.; Nielsen, S.; Kelly, M.; Broholm, C.; Nielsen, A.R.; Taudorf, S.; Pedersen, M.; Fischer, C.P.; Pedersen, B.K. Satellite Cells Derived from Obese Humans with Type 2 Diabetes and Differentiated into Myocytes In Vitro Exhibit Abnormal Response to IL-6. PLoS ONE 2012, 7, e39657. [Google Scholar] [CrossRef]
- Brzeszczyńska, J.; Johns, N.; Schilb, A.; Degen, S.; Degen, M.; Langen, R.; Schols, A.; Glass, D.J.; Roubenoff, R.; Greig, C.A.; et al. Loss of oxidative defense and potential blockade of satellite cell maturation in the skeletal muscle of patients with cancer but not in the healthy elderly. Aging 2016, 8, 1690–1702. [Google Scholar] [CrossRef] [Green Version]
- Sacco, A.; Mourkioti, F.; Tran, R.; Choi, J.; Llewellyn, M.; Kraft, P.; Shkreli, M.; Delp, S.; Pomerantz, J.H.; Artandi, S.E.; et al. Short Telomeres and Stem Cell Exhaustion Model Duchenne Muscular Dystrophy in mdx/mTR Mice. Cell 2010, 143, 1059–1071. [Google Scholar] [CrossRef] [Green Version]
- Yablonka-Reuveni, Z.; Anderson, J.E. Satellite cells from dystrophic (mdx) mice display accelerated differentiation in primary cultures and in isolated myofibers. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2006, 235, 203–212. [Google Scholar] [CrossRef]
- Zhu, L.N.; Ren, Y.; Chen, J.Q.; Wang, Y.Z. Effects of myogenin on muscle fiber types and key metabolic enzymes in gene transfer mice and C2C12 myoblasts. Gene 2013, 532, 246–252. [Google Scholar] [CrossRef]
- Xu, J.; Liu, D.; Yin, H.; Tong, H.; Li, S.; Yan, Y. Fatty acids promote bovine skeletal muscle satellite cell differentiation by regulating ELOVL3 expression. Cell Tissue Res. 2018, 373, 499–508. [Google Scholar] [CrossRef]
- Sente, T.; Van Berendoncks, A.M.; Fransen, E.; Vrints, C.J.; Hoymans, V.Y. Tumor necrosis factor-alpha impairs adiponectin signalling, mitochondrial biogenesis, and myogenesis in primary human myotubes cultures. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H1164–H1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jørgensen, W.; Rud, K.A.; Mortensen, O.H.; Frandsen, L.; Grunnet, N.; Quistorff, B. Your mitochondria are what you eat: A high-fat or a high-sucrose diet eliminates metabolic flexibility in isolated mitochondria from rat skeletal muscle. Physiol. Rep. 2017, 5, e13207. [Google Scholar] [CrossRef] [PubMed]
- Nyman, L.R.; Tian, L.; Hamm, D.A.; Schoeb, T.R.; Gower, B.A.; Nagy, T.R.; Wood, P.A. Long-term effects of high-fat or high-carbohydrate diets on glucose tolerance in mice with heterozygous carnitine palmitoyltransferase-1a deficiency. Nutr. Diabetes 2011, 1, e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, M.A.; Kauter, K.; Brown, L. Naringin Improves Diet-Induced Cardiovascular Dysfunction and Obesity in High Carbohydrate, High Fat Diet-Fed Rats. Nutrients 2013, 5, 637–650. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, T.; Church, C.; Baker, D.J.; Jones, S.W. The role of adipokines in skeletal muscle inflammation and insulin sensitivity. J. Inflamm. 2018, 15, 9. [Google Scholar] [CrossRef] [Green Version]
- Therkelsen, K.E.; Pedley, A.; Speliotes, E.K.; Massaro, J.M.; Murabito, J.; Hoffmann, U.; Fox, C.S. Intramuscular Fat and Associations With Metabolic Risk Factors in the Framingham Heart Study. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 863–870. [Google Scholar] [CrossRef] [Green Version]
- Addison, O.; Marcus, R.L.; Lastayo, P.C.; Ryan, A.S. Intermuscular fat: A review of the consequences and causes. Int. J. Endocrinol. 2014, 2014, 309570. [Google Scholar] [CrossRef] [Green Version]





| SedLn | SedOvOb | SedOld | SedLn vs. SedOvOb | SedLn vs. SedOld | |
|---|---|---|---|---|---|
| n = 18 | n = 5 | n = 20 | |||
| Age (years) | 23.6 ± 1 | 24.8 ± 1.9 | 66.3 ± 0.9 | 0.818 | <0.001 *** |
| BMI (kg/m2) | 23 ± 0.3 | 29.7 ± 1.8 | 26.5 ± 0.6 | <0.001 *** | <0.001 *** |
| Bodyfat % | 19.1 ± 1.5 | 32.8 ± 2.3 | 28.1 ± 1.3 | <0.001 *** | <0.001 *** |
| Plasma Glucose (nM) | 4.5 ± 0.3 | 5.1 ± 0.1 | 5.7 ± 0.3 | 0.634 | 0.022 * |
| Cholesterol (mg/dl) | 147.3 ± 7.6 | 175 ± 10.3 | 195.8 ± 9.7 | 0.331 | 0.001 *** |
| HDL (mg/dL) | 56.8 ± 3.1 | 45.3 ± 3.9 | 54.8 ± 2.9 | 0.186 | 0.849 |
| LDL (mg/dL) | 85.2 ± 6.1 | 120 ± 8.3 | 116.6 ± 8.8 | 0.125 | 0.014 * |
| Triglycerides (mg/dL) | 90.6 ± 5.9 | 119.3 ± 12.7 | 121.8 ± 11.1 | 0.349 | 0.045 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fausnacht, D.W.; McMillan, R.P.; Boutagy, N.E.; Lupi, R.A.; Harvey, M.M.; Davy, B.M.; Davy, K.P.; Rhoads, R.P.; Hulver, M.W. Overfeeding and Substrate Availability, But Not Age or BMI, Alter Human Satellite Cell Function. Nutrients 2020, 12, 2215. https://doi.org/10.3390/nu12082215
Fausnacht DW, McMillan RP, Boutagy NE, Lupi RA, Harvey MM, Davy BM, Davy KP, Rhoads RP, Hulver MW. Overfeeding and Substrate Availability, But Not Age or BMI, Alter Human Satellite Cell Function. Nutrients. 2020; 12(8):2215. https://doi.org/10.3390/nu12082215
Chicago/Turabian StyleFausnacht, Dane W., Ryan P. McMillan, Nabil E. Boutagy, Ryan A. Lupi, Mordecai M. Harvey, Brenda M. Davy, Kevin P. Davy, Robert P. Rhoads, and Matthew W. Hulver. 2020. "Overfeeding and Substrate Availability, But Not Age or BMI, Alter Human Satellite Cell Function" Nutrients 12, no. 8: 2215. https://doi.org/10.3390/nu12082215
APA StyleFausnacht, D. W., McMillan, R. P., Boutagy, N. E., Lupi, R. A., Harvey, M. M., Davy, B. M., Davy, K. P., Rhoads, R. P., & Hulver, M. W. (2020). Overfeeding and Substrate Availability, But Not Age or BMI, Alter Human Satellite Cell Function. Nutrients, 12(8), 2215. https://doi.org/10.3390/nu12082215






