Macronutrient Intake from Human Milk, Infant Growth, and Body Composition at Term Equivalent Age: A Longitudinal Study of Hospitalized Very Preterm Infants
Abstract
:1. Introduction
2. Materials and Method
2.1. Population
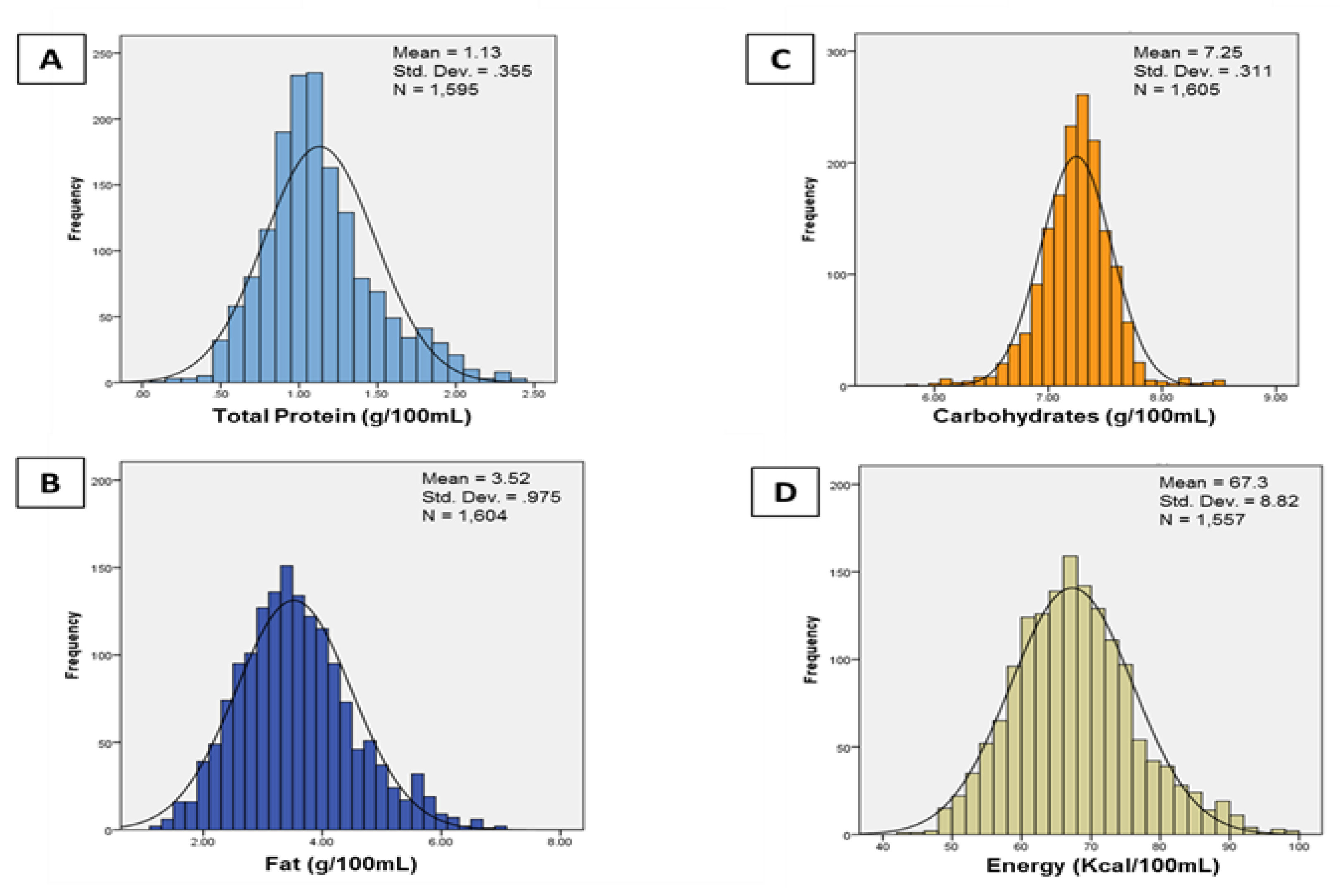
2.2. Milk Collection and Analysis
2.3. Anthropometry
2.4. Body Composition
2.5. Covariates
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Available online: https://www.marchofdimes.org/peristats/Peristats.aspx (accessed on 3 November 2017).
- Kent, A.L. Developmental origins of health and adult disease: What should neonatologists/paediatricians be considering about the long-term health of their patients? J. Paediatr. Child Health 2012, 48, 730–734. [Google Scholar] [CrossRef]
- Belfort, M.B.; Edwards, E.M.; Greenberg, L.T.; Parker, M.G.; Ehret, D.Y.; Horbar, J.D. Diet, weight gain, and head growth in hospitalized US very preterm infants: A 10-year observational study. Am. J. Clin. Nutr. 2019, 109, 1373–1379. [Google Scholar] [CrossRef]
- Horbar, J.D.; Ehrenkranz, R.A.; Badger, G.J.; Edwards, E.M.; Morrow, K.A.; Soll, R.F.; Buzas, J.S.; Bertino, E.; Gagliardi, L.; Bellu, R. Weight Growth Velocity and Postnatal Growth Failure in Infants 501 to 1500 Grams: 2000–2013. Pediatrics 2015, 136, e84–e92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Committee on Nutrition; Section on Breastfeeding; Committee on Fetus and Newbornf. Donor human milk for the high-risk infant: Preparation, safety, and usage options in the United States. Pediatrics 2017, 139. [Google Scholar] [CrossRef] [Green Version]
- American Academy of Pediatrics Committee on Nutrition. Pediatric Nutrition, 7th ed.; American Academy of Pediatrics: Elk Grove, IL, USA, 2014. [Google Scholar]
- Ziegler, E.E.; O’Donnell, A.M.; Nelson, S.E.; Fomon, S.J. Body composition of the reference fetus. Growth 1976, 40, 329–341. [Google Scholar]
- Demerath, E.W.; Johnson, W.; Davern, B.A.; Anderson, C.G.; Shenberger, J.S.; Misra, S.; Ramel, S.E. New body composition reference charts for preterm infants. Am. J. Clin. Nutr. 2017, 105, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Perrin, M.T. Donor human milk and fortifier use in united states level 2, 3, and 4 neonatal care hospitals. J. Pediatr. Gastroenterol. Nutr. 2017. [Google Scholar] [CrossRef]
- Koletzko, B.; Poindexter, B.; Uauy, R. Nutritional Care of Preterm Infants: Scientific Basis and Practical Considerations, 3rd ed.; Karger: Basel, Switzerland, 2014. [Google Scholar]
- Bloom, B.T.; Mulligan, J.; Arnold, C.; Ellis, S.; Moffitt, S.; Rivera, A.; Kunamneni, S.; Thomas, P.; Clark, R.H.; Peabody, J. Improving growth of very low birth weight infants in the first 28 days. Pediatrics 2003, 112, 8–14. [Google Scholar] [CrossRef]
- Colaizy, T.T.; Carlson, S.; Saftlas, A.F.; Morriss, F.H., Jr. Growth in VLBW infants fed predominantly fortified maternal and donor human milk diets: A retrospective cohort study. BMC Pediatr. 2012, 12, 124. [Google Scholar] [CrossRef] [Green Version]
- Chowning, R.; Radmacher, P.; Lewis, S.; Serke, L.; Pettit, N.; Adamkin, D.H. A retrospective analysis of the effect of human milk on prevention of necrotizing enterocolitis and postnatal growth. J. Perinatol. 2016, 36, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Roze, J.C.; Darmaun, D.; Boquien, C.Y.; Flamant, C.; Picaud, J.C.; Savagner, C.; Claris, O.; Lapillonne, A.; Mitanchez, D.; Branger, B.; et al. The apparent breastfeeding paradox in very preterm infants: Relationship between breast feeding, early weight gain and neurodevelopment based on results from two cohorts, EPIPAGE and LIFT. BMJ Open 2012, 2, e000834. [Google Scholar] [CrossRef] [PubMed]
- Maas, C.; Wiechers, C.; Bernhard, W.; Poets, C.F.; Franz, A.R. Early feeding of fortified breast milk and in-hospital-growth in very premature infants: A retrospective cohort analysis. BMC Pediatr. 2013, 13, 178. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.G.; Greenberg, L.T.; Edwards, E.M.; Ehret, D.; Belfort, M.B.; Horbar, J.D. National Trends in the provision of human milk at hospital discharge among very low-birth-weight infants. JAMA Pediatr. 2019. [Google Scholar] [CrossRef] [PubMed]
- Zachariassen, G.; Fenger-Gron, J.; Hviid, M.V.; Halken, S. The content of macronutrients in milk from mothers of very preterm infants is highly variable. Dan. Med. J. 2013, 60, A4631. [Google Scholar] [PubMed]
- Cooper, A.R.; Barnett, D.; Gentles, E.; Cairns, L.; Simpson, J.H. Macronutrient content of donor human breast milk. Arch. Dis. Child. Fetal Neonatal Ed. 2013, 98, F539–F541. [Google Scholar] [CrossRef]
- Gidrewicz, D.A.; Fenton, T.R. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatr. 2014, 14, 216. [Google Scholar] [CrossRef] [Green Version]
- Fusch, G.; Kwan, C.; Kotrri, G.; Fusch, C. “Bed side” human milk analysis in the neonatal intensive care unit: A systematic review. Clin. Perinatol. 2017, 44, 209–267. [Google Scholar] [CrossRef]
- FAO. Food Energy—Methods of Analysis and Conversion Factors; FAO Food and Nutriton Paper 77; Food and Agriculture Organization of the United Nations Technical Workshop: Rome, Italy, 2003. [Google Scholar]
- Available online: http://www.miris.se/media.ashx/manual-hma-287-en.pdf (accessed on 3 December 2019).
- Kwan, C.; Fusch, G.; Rochow, N.; Fusch, C.; Collaborators, M.S. Milk analysis using milk analyzers in a standardized setting (MAMAS) study: A multicentre quality initiative. Clin. Nutr. 2019. [Google Scholar] [CrossRef]
- Mills, B.L.; van de Voort, F.R.; Usborne, W.R. Mojonnier method as reference for infrared determination of fat in meat products. J. Assoc. Off. Anal. Chem. 1983, 66, 1048–1050. [Google Scholar] [CrossRef]
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roggero, P.; Gianni, M.L.; Amato, O.; Piemontese, P.; Morniroli, D.; Wong, W.W.; Mosca, F. Evaluation of air-displacement plethysmography for body composition assessment in preterm infants. Pediatr. Res. 2012, 72, 316–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demerath, E.W.; Fields, D.A. Body composition assessment in the infant. Am. J. Hum. Biol. 2014, 26, 291–304. [Google Scholar] [CrossRef] [Green Version]
- Norris, T.; Ramel, S.E.; Catalano, P.; Caoimh, C.N.; Roggero, P.; Murray, D.; Fields, D.A.; Demerath, E.W.; Johnson, W. New charts for the assessment of body composition, according to air-displacement plethysmography, at birth and across the first 6 mo of life. Am. J. Clin. Nutr. 2019, 109, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Committee on Obstetric Practice; the American Institute of Ultrasound in Medicine; the Society for Maternal-Fetal Medicine. Committee opinion No 700: Methods for estimating the due date. Obstet. Gynecol. 2017, 129, e150–e154. [Google Scholar] [CrossRef] [PubMed]
- Horbar, J.D.; Soll, R.F. 2015 Manual of Operations, Part 2, Release 19.0; Vermont Oxford Network: Burlington, VT, USA, 2016. [Google Scholar]
- Lin, Y.H.; Hsu, Y.C.; Lin, M.C.; Chen, C.H.; Wang, T.M. The association of macronutrients in human milk with the growth of preterm infants. PLoS ONE 2020, 15, e0230800. [Google Scholar] [CrossRef] [PubMed]
- Belfort, M.B.; Ramel, S.E. NICU diet, physical growth and nutrient accretion, and preterm infant neurodevelopment. NeoReviews 2020, in press. [Google Scholar]
- McLeod, G.; Sherriff, J.; Nathan, E.; Hartmann, P.E.; Simmer, K. Four-week nutritional audit of preterm infants born <33 weeks gestation. J. Paediatr. Child Health 2013, 49, E332–E339. [Google Scholar] [CrossRef] [Green Version]
- McLeod, G.; Simmer, K.; Sherriff, J.; Nathan, E.; Geddes, D.; Hartmann, P. Feasibility study: Assessing the influence of macronutrient intakes on preterm body composition, using air displacement plethysmography. J. Paediatr. Child Health 2015, 51, 862–869. [Google Scholar] [CrossRef]
- Belfort, M.B.; Rifas-Shiman, S.L.; Sullivan, T.; Collins, C.T.; McPhee, A.J.; Ryan, P.; Kleinman, K.P.; Gillman, M.W.; Gibson, R.A.; Makrides, M. Infant growth before and after term: Effects on neurodevelopment in preterm infants. Pediatrics 2011, 128, e899–e906. [Google Scholar] [CrossRef]
- Ramel, S.E.; Demerath, E.W.; Gray, H.L.; Younge, N.; Boys, C.; Georgieff, M.K. The relationship of poor linear growth velocity with neonatal illness and two-year neurodevelopment in preterm infants. Neonatology 2012, 102, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Rochow, N.; Fusch, G.; Choi, A.; Chessell, L.; Elliott, L.; McDonald, K.; Kuiper, E.; Purcha, M.; Turner, S.; Chan, E.; et al. Target fortification of breast milk with fat, protein, and carbohydrates for preterm infants. J. Pediatr. 2013, 163, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Fusch, C. Avoiding Postnatal growth retardation by individualized fortification of breast milk: Implications for somatic and neurodevelopmental outcomes. Breastfeed. Med. 2019, 14, S15–S17. [Google Scholar] [CrossRef] [Green Version]
- McLeod, G.; Sherriff, J.; Hartmann, P.E.; Nathan, E.; Geddes, D.; Simmer, K. Comparing different methods of human breast milk fortification using measured v. assumed macronutrient composition to target reference growth: A randomised controlled trial. Br. J. Nutr. 2016, 115, 431–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maas, C.; Mathes, M.; Bleeker, C.; Vek, J.; Bernhard, W.; Wiechers, C.; Peter, A.; Poets, C.F.; Franz, A.R. Effect of Increased enteral protein intake on growth in human milk-fed preterm infants: A randomized clinical trial. JAMA Pediatr. 2017, 171, 16–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brion, L.P.; Rosenfeld, C.R.; Heyne, R.; Brown, L.S.; Lair, C.S.; Petrosyan, E.; Jacob, T.; Caraig, M.; Burchfield, P.J. Optimizing individual nutrition in preterm very low birth weight infants: Double-blinded randomized controlled trial. J. Perinatol. 2020, 40, 655. [Google Scholar] [CrossRef] [PubMed]
- Parat, S.; Raza, P.; Kamleh, M.; Super, D.; Groh-Wargo, S. Targeted breast milk fortification for very low birth weight (VLBW) Infants: Nutritional Intake, growth outcome and body composition. Nutrients 2020, 12, 1156. [Google Scholar] [CrossRef]
- John, A.; Sun, R.; Maillart, L.; Schaefer, A.; Hamilton Spence, E.; Perrin, M.T. Macronutrient variability in human milk from donors to a milk bank: Implications for feeding preterm infants. PLoS ONE 2019, 14, e0210610. [Google Scholar] [CrossRef]

| Mothers | |||
| Mean | SD | ||
| Age, years | 34 | 5 | |
| Number | Percent | ||
| Race | |||
| White | 22 | 60% | |
| Black | 10 | 27% | |
| Asian | 2 | 5% | |
| Other or missing | 3 | 8% | |
| Hispanic ethnicity | 2 | 5% | |
| Primiparous | 17 | 46% | |
| Antenatal steroids given | 36 | 97% | |
| Delivery mode | |||
| Vaginal | 16 | 43% | |
| Cesarean | 21 | 57% | |
| Infants | |||
| Mean | SD | Range | |
| Gestational age, weeks | 28 | 2.4 | 23.6, 31.9 |
| Birth weight, grams | 1104 | 434 | 410, 2065 |
| Birth z-scores | |||
| Weight | −0.08 | 1.02 | −2.36, 1.83 |
| Length | 0.03 | 1.20 | −4.00, 1.87 |
| Head circumference | −0.37 | 0.95 | −2.26, 1.87 |
| Number | Percent | ||
| Small for gestational age | 5 | 14% | |
| Sex | |||
| Male | 21 | 57% | |
| Female | 16 | 43% | |
| Gestation number | |||
| Singleton | 28 | ||
| Twins | 9 | ||
| NEC, Stage 2 or higher | 0 | 0% | |
| IVH, Grade 3 or 4 | 1 | 3% | |
| Culture proven sepsis | 2 | 5% | |
| Retinopathy treatment | 1 | 3% | |
| Postnatal steroids | 5 | 14% |
| Mean | SD | |
| Raw measurements | ||
| Weight, kg | 2.86 | 0.55 |
| Length, cm | 47.2 | 2.8 |
| Head circumference, cm | 33.4 | 1.6 |
| Fat mass, kg | 0.58 | 0.20 |
| Fat-free mass, kg | 2.38 | 0.40 |
| Percent body fat | 19.4 | 4.8 |
| Z-scores | ||
| Weight | −0.80 | 1.30 |
| Length | −0.95 | 1.43 |
| Head circumference | −0.36 | 1.23 |
| Fat mass | 1.99 | 1.23 |
| Fat-free mass | −1.31 | 1.52 |
| Percent body fat | 2.5 | 1.2 |
| Mean | Range | |
| PMA at outcome, weeks | 38.11 | 34, 42 |
| Weight z-Score | Length z-Score | Head Circumference z-Score | ||||
|---|---|---|---|---|---|---|
| Beta | 95% CI | Beta | 95% CI | Beta | 95% CI | |
| Protein, g/kg/day | 0.65 | −1.15, 2.44 | 0.84 | 0.09, 1.58 | −0.05 | −0.72, 0.62 |
| Fat, g/kg/day | 0.61 | 0.21, 1.01 | 0.34 | −0.20, 0.88 | 0.12 | −0.31, 0.55 |
| Carbohydrate, g/kg/day | −0.41 | −1.63, 0.80 | −0.39 | −1.09, 0.31 | −0.02 | −0.67, 0.63 |
| Energy, 10 kcal/kg/day | 0.69 | 0.28, 1.10 | 0.41 | −0.08, 0.90 | 0.13 | −0.28, 0.55 |
| Fat Mass z-Score | Fat-Free Mass z-Score | |||
|---|---|---|---|---|
| Beta | 95% CI | Beta | 95% CI | |
| Protein, g/kg/day | 0.72 | −2.19, 3.63 | 0.09 | −1.29, 1.46 |
| Fat, g/kg/day | 0.82 | 0.13, 1.51 | 0.54 | 0.25, 0.83 |
| Carbohydrate, g/kg/day | 0.04 | −2.32, 2.40 | −0.95 | −2.04, 0.13 |
| Energy, 10 kcal/kg/day | 0.93 | −0.05, 1.92 | 0.49 | −0.002, 0.98 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belfort, M.; Cherkerzian, S.; Bell, K.; Soldateli, B.; Cordova Ramos, E.; Palmer, C.; Steele, T.; Pepin, H.; Ellard, D.; Drouin, K.; et al. Macronutrient Intake from Human Milk, Infant Growth, and Body Composition at Term Equivalent Age: A Longitudinal Study of Hospitalized Very Preterm Infants. Nutrients 2020, 12, 2249. https://doi.org/10.3390/nu12082249
Belfort M, Cherkerzian S, Bell K, Soldateli B, Cordova Ramos E, Palmer C, Steele T, Pepin H, Ellard D, Drouin K, et al. Macronutrient Intake from Human Milk, Infant Growth, and Body Composition at Term Equivalent Age: A Longitudinal Study of Hospitalized Very Preterm Infants. Nutrients. 2020; 12(8):2249. https://doi.org/10.3390/nu12082249
Chicago/Turabian StyleBelfort, Mandy, Sara Cherkerzian, Katherine Bell, Betina Soldateli, Erika Cordova Ramos, Caroline Palmer, Tina Steele, Hunter Pepin, Deirdre Ellard, Kaitlin Drouin, and et al. 2020. "Macronutrient Intake from Human Milk, Infant Growth, and Body Composition at Term Equivalent Age: A Longitudinal Study of Hospitalized Very Preterm Infants" Nutrients 12, no. 8: 2249. https://doi.org/10.3390/nu12082249





