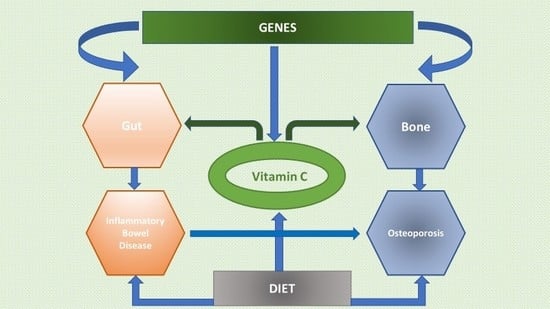
Vitamin C Deficiency and the Risk of Osteoporosis in Patients with an Inflammatory Bowel Disease
Abstract
1. Introduction
2. Vitamin C Deficiency in IBD Patients and the Risk of Osteoporosis
3. Pharmacological Treatment in IBD
4. Genetic Background for Vitamin C Deficiency
4.1. Genes Encoded Transport Proteins
4.2. Genes Encoded Enzymes
5. Vitamin C and Bone Tissue in IBD Patients
| Gene Symbol, (MIM) Full Name | Variant | MAF in World Population * | Impact on Vitamin C Level | Study Group | References | |
|---|---|---|---|---|---|---|
| Rs Number (Minor Allele) | Location | |||||
| SLC23A1 (MIM:603790) solute carrier family 23 member 1 | rs10063949 (T) | Intron | 42% | Allele C association with an elevated circulating ascorbic acid in BWHHS, but an effect does not appear in a meta-analysis | 15 087 participants from 5 independent studies | [45] |
| No association with higher vitamin C plasma level | 300 subjects (150 POAG cases and 150 controls) from a Mediterranean population | [74] | ||||
| AG and GG genotypes increase the risk of Crohn’s disease | 311 patients from the Manitoba IBD cohort | [46] | ||||
| rs33972313 (T) | Exon (p.V264M) | 4% | Rare allele A associated with a reduction in circulating concentrations of ascorbic acid | 15 087 participants from 5 independent studies | [45] | |
| G allele was associated with 11% higher plasma vitamin C | 97 203 white individuals including 10 123 subjects with ischemic heart disease | [75] | ||||
| GG genotype was associated with a 9% higher plasma vitamin C compared with AA and AG combined | 106 147 individuals from the Copenhagen General Population Study | [76] | ||||
| GA heterozygotes were associated with a 24% lower concentration | 365 cases and 1 284 controls from the EPIC cohort | [77] | ||||
| rs11950646 (A) | Intron | 38% | GG or AG genotypes (compared with AA) were associated with a 13% lower plasma vitamin C concentration | 365 cases and 1 284 controls from the EPIC cohort | [77] | |
| SLC23A2 (MIM:603791) solute carrier family 23 member 2 | rs6133175 (G) | Intron | 33% | GG homozygotes associated with 24% higher plasma vitamin C concentrations | 365 cases and 1 284 controls from the EPIC cohort | [77] |
| C allele have a reduced risk of heart disease (implied that it is due to an increased vitamin C transport) | 97 203 white individuals including 10 123 subjects with ischemic heart disease | [75] | ||||
| rs6053005 (T) | Intron | 31% | TT homozygotes associated with 24% higher plasma vitamin C concentrations | 365 cases and 1 284 controls from the EPIC cohort | [77] | |
| rs1279683 (T) | Intron | 45% | GG subjects had a significantly lower plasma vitamin C concentrations than the other genotypes | 300 subjects (150 POAG cases and 150 controls) from a Mediterranean population | [74] | |
| GSTM1 (MIM:138350) glutathione S-transferase mu 1 | GSTM1*0 (null) | Whole gene deletion | 47.4% # | Higher vitamin C concentration in plasma | 115 individuals from Slovakia (44 survivors of myocardial infarction, 44 clinically normal controls and 67 population subjects) | [58] |
| 4-fold increased risk of Vit. C deficiency for homozygote GSTM1*0/*0 and 2-fold for carriers of the GSTM1*1 allele | 905 nonsmoking Canadian | [59] | ||||
| A significantly lower level of vitamin C in plasma compared with subjects carrying functional gene. | 388 volunteers from Slovakia | [60] | ||||
| GSTT1 (MIM:600436) glutathione S-transferase theta 1 | GSTT1*0 (null) | Whole gene deletion | 25% # | Lower vitamin C concentration in plasma | 115 individuals from Slovakia (44 survivors of myocardial infarction, 44 clinically normal controls and 67 population subjects) | [58] |
| 12-fold increased risk of serum ascorbic acid deficiency for the GSTT1*0/*0 genotype and only 2-fold for carriers of the GSTT1*1 allele | 905 nonsmoking Canadian | [59] | ||||
| Significantly lower level of vitamin C in plasma as compared with subjects carrying functional gene | 388 volunteers from Slovakia | [60] | ||||
| GSTP1 (MIM:134660) glutathione S-transferase pi 1 | rs1695 (G) | Exon (p.Ile105Val) | 35% | Heterozygous individuals showed a significantly lower circulating vitamin C levels than homozygous GG | 115 individuals from Slovakia (44 survivors of myocardial infarction, 44 clinically normal controls and 67 population subjects) | [58] |
| No effect on serum ascorbic acid | 905 nonsmoking Canadian | [59] | ||||
| No association with vitamin C in plasma | 388 volunteers from Slovakia | [60] | ||||
6. Vitamin C in Various Diets
6.1. Mediterranean Diet
6.2. Vegetarian Diets
6.3. Low-Carbohydrates Diets
6.4. Low-FODMAP Diet
7. Gut Microbiota and Vitamin C in IBD Patients
8. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Padayatty, S.J.; Levine, M. Vitamin C physiology: The known and the unknown and Goldilocks. Oral Dis. 2016, 22, 463–493. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J.; Michels, A.J.; Frei, B. Vitamin C. Adv. Nutr. Bethesda Md 2014, 5, 16–18. [Google Scholar] [CrossRef]
- Travica, N.; Ried, K.; Sali, A.; Scholey, A.; Hudson, I.; Pipingas, A. Vitamin C Status and Cognitive Function: A Systematic Review. Nutrients 2017, 9, 960. [Google Scholar] [CrossRef]
- Jarmakiewicz, S.; Piątek, D.; Filip, R. Macro and micronutrient deficiency in inflammatory bowel diseases. Eur. J. Clin. Exp. Med. 2017, 15, 342–348. [Google Scholar] [CrossRef]
- Ioannidis, O.; Varnalidis, I.; Paraskevas, G.; Botsios, D. Nutritional Modulation of the Inflammatory Bowel Response. Digestion 2011, 84, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Office of Dietary Supplements—Vitamin C. Available online: https://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/ (accessed on 24 June 2020).
- Knight, J.; Madduma-Liyanage, K.; Mobley, J.A.; Assimos, D.G.; Holmes, R.P. Ascorbic acid intake and oxalate synthesis. Urolithiasis 2016, 44, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Jarmakiewicz-Czaja, S.; Piątek, D.; Filip, R. The Influence of Nutrients on Inflammatory Bowel Diseases. J. Nutr. Metab. 2020, 2020, 2894169. [Google Scholar] [CrossRef]
- Li, Y.; Schellhorn, H.E. New developments and novel therapeutic perspectives for vitamin C. J. Nutr. 2007, 137, 2171–2184. [Google Scholar] [CrossRef]
- De Tullio, M.C. Beyond the antioxidant: The double life of vitamin C. Subcell. Biochem. 2012, 56, 49–65. [Google Scholar] [CrossRef]
- Maxfield, L.; Crane, J.S. Vitamin C Deficiency (Scurvy). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Spooren, C.E.G.M.; Wintjens, D.S.J.; de Jong, M.J.; van der Meulen-de Jong, A.E.; Romberg-Camps, M.J.; Becx, M.C.; Maljaars, J.P.; van Bodegraven, A.A.; Mahmmod, N.; Markus, T.; et al. Risk of impaired nutritional status and flare occurrence in IBD outpatients. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2019, 51, 1265–1269. [Google Scholar] [CrossRef]
- Subramanian, V.S.; Sabui, S.; Subramenium, G.A.; Marchant, J.S.; Said, H.M. Tumor necrosis factor alpha reduces intestinal vitamin C uptake: A role for NF-κB-mediated signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G241–G248. [Google Scholar] [CrossRef] [PubMed]
- Hartman, C.; Marderfeld, L.; Davidson, K.; Mozer-Glassberg, Y.; Poraz, I.; Silbermintz, A.; Zevit, N.; Shamir, R. Food Intake Adequacy in Children and Adolescents with Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 437–444. [Google Scholar] [CrossRef]
- Lim, H.; Kim, H.J.; Hong, S.J.; Kim, S. Nutrient Intake and Bone Mineral Density by Nutritional Status in Patients with Inflammatory Bowel Disease. J. Bone Metab. 2014, 21, 195–203. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Głąbska, D.; Guzek, D.; Lech, G. Analysis of the Nutrients and Food Products Intake of Polish Males with Ulcerative Colitis in Remission. Nutrients 2019, 11, 2333. [Google Scholar] [CrossRef]
- Vagianos, K.; Bector, S.; McConnell, J.; Bernstein, C.N. Nutrition assessment of patients with inflammatory bowel disease. JPEN J. Parenter. Enteral Nutr. 2007, 31, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Reif, S.; Klein, I.; Lubin, F.; Farbstein, M.; Hallak, A.; Gilat, T. Pre-illness dietary factors in inflammatory bowel disease. Gut 1997, 40, 754–760. [Google Scholar] [CrossRef]
- Moura, F.A.; de Andrade, K.Q.; Dos Santos, J.C.F.; Araújo, O.R.P.; Goulart, M.O.F. Antioxidant therapy for treatment of inflammatory bowel disease: Does it work? Redox Biol. 2015, 6, 617–639. [Google Scholar] [CrossRef]
- Iwakawa, H.; Fukui, T.; Fukuwatari, T.; Bamba, S.; Sasaki, M.; Tsujikawa, T.; Doi, Y.; Shibata, K. Blood concentrations and renal clearance of water-soluble vitamins in outpatients with ulcerative colitis. Biomed. Rep. 2019, 10, 202–210. [Google Scholar] [CrossRef]
- Geerling, B.J.; Badart-Smook, A.; Stockbrügger, R.W.; Brummer, R.J. Comprehensive Nutritional Status in Patients with Long-Standing Crohn Disease Currently in Remission. Available online: https://pubmed-1ncbi-1nlm-1nih-1gov-198kzp49o0025.han.ump.edu.pl/9583850/?dopt=Abstract (accessed on 8 July 2020).
- Linaker, B.D. Scurvy and vitamin C deficiency in Crohn’s disease. Postgrad. Med. J. 1979, 55, 26–29. [Google Scholar] [CrossRef][Green Version]
- Aghajanian, P.; Hall, S.; Wongworawat, M.D.; Mohan, S. The Roles and Mechanisms of Actions of Vitamin C in Bone: New Developments. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2015, 30, 1945–1955. [Google Scholar] [CrossRef]
- Malmir, H.; Shab-Bidar, S.; Djafarian, K. Vitamin C intake in relation to bone mineral density and risk of hip fracture and osteoporosis: A systematic review and meta-analysis of observational studies. Br. J. Nutr. 2018, 119, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Kim, K.M.; Lim, S.; Choi, S.H.; Moon, J.H.; Kim, J.H.; Kim, S.W.; Jang, H.C.; Shin, C.S. Favorable effect of dietary vitamin C on bone mineral density in postmenopausal women (KNHANES IV, 2009): Discrepancies regarding skeletal sites, age, and vitamin D status. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2015, 26, 2329–2337. [Google Scholar] [CrossRef] [PubMed]
- Leveille, S.G.; LaCroix, A.Z.; Koepsell, T.D.; Beresford, S.A.; Van Belle, G.; Buchner, D.M. Dietary vitamin C and bone mineral density in postmenopausal women in Washington State, USA. J. Epidemiol. Community Health 1997, 51, 479–485. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Curhan, G.C.; Gambaro, G.; Taylor, E.N. Total, Dietary, and Supplemental Vitamin C Intake and Risk of Incident Kidney Stones. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2016, 67, 400–407. [Google Scholar] [CrossRef]
- Uchwała nr 5/2019 Zespołu do Spraw Suplementów Diety z dnia 11.06.2019 w sprawie wyrażania opinii dotyczącej maksymalnej dawki witaminy C w zalecanej dziennej porcji w suplementach diety. 2019.
- Bischoff, S.C.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN practical guideline: Clinical Nutrition in inflammatory bowel disease. Clin. Nutr. Edinb. Scotl. 2020, 39, 632–653. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohns Colitis 2020, 14, 4–22. [Google Scholar] [CrossRef]
- Leszczyński, P.; Łącki, J.K.; Mackiewicz, S.H. Glucocorticosteroid—induced osteoporosis—patogenesis, prevention and treatment options. Postępy Nauk Med. 2000, 2, 3–7. [Google Scholar]
- Van Staa, T.P. The pathogenesis, epidemiology and management of glucocorticoid-induced osteoporosis. Calcif. Tissue Int. 2006, 79, 129–137. [Google Scholar] [CrossRef]
- Lima, C.A.; Lyra, A.C.; Rocha, R.; Santana, G.O. Risk factors for osteoporosis in inflammatory bowel disease patients. World J. Gastrointest. Pathophysiol. 2015, 6, 210–218. [Google Scholar] [CrossRef]
- Veerappan, S.G.; O’Morain, C.A.; Daly, J.S.; Ryan, B.M. Review article: The effects of antitumour necrosis factor-α on bone metabolism in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2011, 33, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Chung, W.J.; Kwak, H.B.; Chung, C.H.; Kwack, K.B.; Lee, Z.H.; Kim, H.H. Tumor necrosis factor-alpha supports the survival of osteoclasts through the activation of Akt and ERK. J. Biol. Chem. 2001, 276, 49343–49349. [Google Scholar] [CrossRef]
- Zerbini, C.A.F.; Clark, P.; Mendez-Sanchez, L.; Pereira, R.M.R.; Messina, O.D.; Uña, C.R.; Adachi, J.D.; Lems, W.F.; Cooper, C.; Lane, N.E.; et al. Biologic therapies and bone loss in rheumatoid arthritis. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2017, 28, 429–446. [Google Scholar] [CrossRef] [PubMed]
- Ziolkowska, M.; Kurowska, M.; Radzikowska, A.; Luszczykiewicz, G.; Wiland, P.; Dziewczopolski, W.; Filipowicz-Sosnowska, A.; Pazdur, J.; Szechinski, J.; Kowalczewski, J.; et al. High levels of osteoprotegerin and soluble receptor activator of nuclear factor κB ligand in serum of rheumatoid arthritis patients and their normalization after anti–tumor necrosis factor α treatment. Arthritis Rheum. 2002, 46, 1744–1753. [Google Scholar] [CrossRef]
- Ratajczak, A.E.; Rychter, A.M.; Zawada, A.; Dobrowolska, A.; Krela-Kaźmierczak, I. Nutrients in the Prevention of Osteoporosis in Patients with Inflammatory Bowel Diseases. Nutrients 2020, 12, 1702. [Google Scholar] [CrossRef]
- Chang, Y.-L.; Rossetti, M.; Vlamakis, H.; Casero, D.; Sunga, G.; Harre, N.; Miller, S.; Humphries, R.; Stappenbeck, T.; Simpson, K.W.; et al. A screen of Crohn’s disease-associated microbial metabolites identifies ascorbate as a novel metabolic inhibitor of activated human T cells. Mucosal Immunol. 2019, 12, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J.; Tveden-Nyborg, P. The Pharmacokinetics of Vitamin C. Nutrients 2019, 11, 2412. [Google Scholar] [CrossRef] [PubMed]
- Savini, I.; Rossi, A.; Pierro, C.; Avigliano, L.; Catani, M.V. SVCT1 and SVCT2: Key proteins for vitamin C uptake. Amino Acids 2008, 34, 347–355. [Google Scholar] [CrossRef]
- MacDonald, L.; Thumser, A.E.; Sharp, P. Decreased expression of the vitamin C transporter SVCT1 by ascorbic acid in a human intestinal epithelial cell line. Br. J. Nutr. 2002, 87, 97–100. [Google Scholar] [CrossRef]
- Boyer, J.C.; Campbell, C.E.; Sigurdson, W.J.; Kuo, S.-M. Polarized localization of vitamin C transporters, SVCT1 and SVCT2, in epithelial cells. Biochem. Biophys. Res. Commun. 2005, 334, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Michels, A.J.; Hagen, T.M.; Frei, B. Human genetic variation influences vitamin C homeostasis by altering vitamin C transport and antioxidant enzyme function. Annu. Rev. Nutr. 2013, 33, 45–70. [Google Scholar] [CrossRef] [PubMed]
- Timpson, N.J.; Forouhi, N.G.; Brion, M.-J.; Harbord, R.M.; Cook, D.G.; Johnson, P.; McConnachie, A.; Morris, R.W.; Rodriguez, S.; Luan, J.; et al. Genetic variation at the SLC23A1 locus is associated with circulating concentrations of L-ascorbic acid (vitamin C): Evidence from 5 independent studies with >15,000 participants. Am. J. Clin. Nutr. 2010, 92, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Amir Shaghaghi, M.; Bernstein, C.N.; Serrano León, A.; El-Gabalawy, H.; Eck, P. Polymorphisms in the sodium-dependent ascorbate transporter gene SLC23A1 are associated with susceptibility to Crohn disease. Am. J. Clin. Nutr. 2014, 99, 378–383. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Michels, A.J.; Hagen, T.M. Hepatocyte nuclear factor 1 is essential for transcription of sodium-dependent vitamin C transporter protein 1. Am. J. Physiol. Cell Physiol. 2009, 297, C1220–C1227. [Google Scholar] [CrossRef] [PubMed]
- Reidling, J.C.; Rubin, S.A. Promoter analysis of the human ascorbic acid transporters SVCT1 and 2: Mechanisms of adaptive regulation in liver epithelial cells. J. Nutr. Biochem. 2011, 22, 344–350. [Google Scholar] [CrossRef]
- Subramanian, V.S.; Sabui, S.; Marchant, J.S.; Said, H.M. MicroRNA-103a regulates sodium-dependent vitamin C transporter-1 expression in intestinal epithelial cells. J. Nutr. Biochem. 2019, 65, 46–53. [Google Scholar] [CrossRef]
- Rumsey, S.C.; Kwon, O.; Xu, G.W.; Burant, C.F.; Simpson, I.; Levine, M. Glucose transporter isoforms GLUT1 and GLUT3 transport dehydroascorbic acid. J. Biol. Chem. 1997, 272, 18982–18989. [Google Scholar] [CrossRef]
- Rumsey, S.C.; Daruwala, R.; Al-Hasani, H.; Zarnowski, M.J.; Simpson, I.A.; Levine, M. Dehydroascorbic acid transport by GLUT4 in Xenopus oocytes and isolated rat adipocytes. J. Biol. Chem. 2000, 275, 28246–28253. [Google Scholar] [CrossRef]
- Corpe, C.P.; Eck, P.; Wang, J.; Al-Hasani, H.; Levine, M. Intestinal dehydroascorbic acid (DHA) transport mediated by the facilitative sugar transporters, GLUT2 and GLUT8. J. Biol. Chem. 2013, 288, 9092–9101. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Huang, H.-Y.; Chang, C.-J.; Cheng, C.-H.; Chen, Y.-T. Mitochondrial GLUT10 facilitates dehydroascorbic acid import and protects cells against oxidative stress: Mechanistic insight into arterial tortuosity syndrome. Hum. Mol. Genet. 2010, 19, 3721–3733. [Google Scholar] [CrossRef]
- Shaghaghi, M.A.; Kloss, O.; Eck, P. Genetic Variation in Human Vitamin C Transporter Genes in Common Complex Diseases. Adv. Nutr. Bethesda Md 2016, 7, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Amir Shaghaghi, M.; Zhouyao, H.; Tu, H.; El-Gabalawy, H.; Crow, G.H.; Levine, M.; Bernstein, C.N.; Eck, P. The SLC2A14 gene, encoding the novel glucose/dehydroascorbate transporter GLUT14, is associated with inflammatory bowel disease. Am. J. Clin. Nutr. 2017, 106, 1508–1513. [Google Scholar] [CrossRef]
- Tu, H.; Li, H.; Wang, Y.; Niyyati, M.; Wang, Y.; Leshin, J.; Levine, M. Low Red Blood Cell Vitamin C Concentrations Induce Red Blood Cell Fragility: A Link to Diabetes Via Glucose, Glucose Transporters, and Dehydroascorbic Acid. EBioMedicine 2015, 2, 1735–1750. [Google Scholar] [CrossRef]
- Strange, R.C.; Spiteri, M.A.; Ramachandran, S.; Fryer, A.A. Glutathione-S-transferase family of enzymes. Mutat. Res. 2001, 482, 21–26. [Google Scholar] [CrossRef]
- Dusinská, M.; Ficek, A.; Horská, A.; Raslová, K.; Petrovská, H.; Vallová, B.; Drlicková, M.; Wood, S.G.; Stupáková, A.; Gasparovic, J.; et al. Glutathione S-transferase polymorphisms influence the level of oxidative DNA damage and antioxidant protection in humans. Mutat. Res. 2001, 482, 47–55. [Google Scholar] [CrossRef]
- Cahill, L.E.; Fontaine-Bisson, B.; El-Sohemy, A. Functional genetic variants of glutathione S-transferase protect against serum ascorbic acid deficiency. Am. J. Clin. Nutr. 2009, 90, 1411–1417. [Google Scholar] [CrossRef]
- Horska, A.; Mislanova, C.; Bonassi, S.; Ceppi, M.; Volkovova, K.; Dusinska, M. Vitamin C levels in blood are influenced by polymorphisms in glutathione S-transferases. Eur. J. Nutr. 2011, 50, 437–446. [Google Scholar] [CrossRef]
- Ilich, J.Z.; Kerstetter, J.E. Nutrition in bone health revisited: A story beyond calcium. J. Am. Coll. Nutr. 2000, 19, 715–737. [Google Scholar] [CrossRef]
- Franceschi, R.T.; Iyer, B.S.; Cui, Y. Effects of ascorbic acid on collagen matrix formation and osteoblast differentiation in murine MC3T3-E1 cells. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 1994, 9, 843–854. [Google Scholar] [CrossRef]
- Marini, J.C.; Cabral, W.A.; Barnes, A.M.; Chang, W. Components of the collagen prolyl 3-hydroxylation complex are crucial for normal bone development. Cell Cycle Georget. Tex 2007, 6, 1675–1681. [Google Scholar] [CrossRef]
- Finck, H.; Hart, A.R.; Jennings, A.; Welch, A.A. Is there a role for vitamin C in preventing osteoporosis and fractures? A review of the potential underlying mechanisms and current epidemiological evidence. Nutr. Res. Rev. 2014, 27, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Ragab, A.A.; Lavish, S.A.; Banks, M.A.; Goldberg, V.M.; Greenfield, E.M. Osteoclast differentiation requires ascorbic acid. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 1998, 13, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, E.; Kato, Y.; Hirose, S.; Hagiwara, H. Role of ascorbic acid in the osteoclast formation: Induction of osteoclast differentiation factor with formation of the extracellular collagen matrix. Endocrinology 2000, 141, 3006–3011. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Le Nihouannen, D.; Barralet, J.E.; Fong, J.E.; Komarova, S.V. Ascorbic acid accelerates osteoclast formation and death. Bone 2010, 46, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-K.; Lee, E.-M.; Kim, A.-Y.; Lee, E.-J.; Min, C.-W.; Kang, K.-K.; Lee, M.-M.; Jeong, K.-S. Vitamin C deficiency accelerates bone loss inducing an increase in PPAR-γ expression in SMP30 knockout mice. Int. J. Exp. Pathol. 2012, 93, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, R.T.; Iyer, B.S. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 1992, 7, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Daniel, J.C.; Pauli, B.U.; Kuettner, K.E. Synthesis of cartilage matrix by mammalian chondrocytes in vitro. III. Effects of ascorbate. J. Cell Biol. 1984, 99, 1960–1969. [Google Scholar] [CrossRef]
- Temu, T.M.; Wu, K.-Y.; Gruppuso, P.A.; Phornphutkul, C. The mechanism of ascorbic acid-induced differentiation of ATDC5 chondrogenic cells. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E325–E334. [Google Scholar] [CrossRef]
- Iotsova, V.; Caamaño, J.; Loy, J.; Yang, Y.; Lewin, A.; Bravo, R. Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nat. Med. 1997, 3, 1285–1289. [Google Scholar] [CrossRef]
- Schreck, R.; Albermann, K.; Baeuerle, P.A. Nuclear factor kappa B: An oxidative stress-responsive transcription factor of eukaryotic cells (a review). Free Radic. Res. Commun. 1992, 17, 221–237. [Google Scholar] [CrossRef]
- Zanon-Moreno, V.; Ciancotti-Olivares, L.; Asencio, J.; Sanz, P.; Ortega-Azorin, C.; Pinazo-Duran, M.D.; Corella, D. Association between a SLC23A2 gene variation, plasma vitamin C levels, and risk of glaucoma in a Mediterranean population. Mol. Vis. 2011, 17, 2997–3004. [Google Scholar] [PubMed]
- Kobylecki, C.J.; Afzal, S.; Davey Smith, G.; Nordestgaard, B.G. Genetically high plasma vitamin C, intake of fruit and vegetables, and risk of ischemic heart disease and all-cause mortality: A Mendelian randomization study. Am. J. Clin. Nutr. 2015, 101, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Kobylecki, C.J.; Afzal, S.; Nordestgaard, B.G. Genetically high plasma vitamin C and urate: A Mendelian randomization study in 106 147 individuals from the general population. Rheumatol. Oxf. Engl. 2018, 57, 1769–1776. [Google Scholar] [CrossRef] [PubMed]
- Duell, E.J.; Lujan-Barroso, L.; Llivina, C.; Muñoz, X.; Jenab, M.; Boutron-Ruault, M.-C.; Clavel-Chapelon, F.; Racine, A.; Boeing, H.; Buijsse, B.; et al. Vitamin C transporter gene (SLC23A1 and SLC23A2) polymorphisms, plasma vitamin C levels, and gastric cancer risk in the EPIC cohort. Genes Nutr. 2013, 8, 549–560. [Google Scholar] [CrossRef]
- Kasthurinaidu, S.P.; Ramasamy, T.; Ayyavoo, J.; Dave, D.K.; Adroja, D.A. GST M1-T1 null allele frequency patterns in geographically assorted human populations: A phylogenetic approach. PLoS ONE 2015, 10, e0118660. [Google Scholar] [CrossRef]
- Serra-Majem, L.; de la Cruz, J.N.; Ribas, L.; Salleras, L. Mediterranean diet and health: Is all the secret in olive oil? Pathophysiol. Haemost. Thromb. 2003, 33, 461–465. [Google Scholar] [CrossRef]
- Skouroliakou, M.; Grosomanidis, D.; Massara, P.; Kostara, C.; Papandreou, P.; Ntountaniotis, D.; Xepapadakis, G. Serum antioxidant capacity, biochemical profile and body composition of breast cancer survivors in a randomized Mediterranean dietary intervention study. Eur. J. Nutr. 2018, 57, 2133–2145. [Google Scholar] [CrossRef]
- Hagfors, L.; Leanderson, P.; Sköldstam, L.; Andersson, J.; Johansson, G. Antioxidant intake, plasma antioxidants and oxidative stress in a randomized, controlled, parallel, Mediterranean dietary intervention study on patients with rheumatoid arthritis. Nutr. J. 2003, 2, 5. [Google Scholar] [CrossRef]
- Kolomvotsou, A.I.; Rallidis, L.S.; Mountzouris, K.C.; Lekakis, J.; Koutelidakis, A.; Efstathiou, S.; Nana-Anastasiou, M.; Zampelas, A. Adherence to Mediterranean diet and close dietetic supervision increase total dietary antioxidant intake and plasma antioxidant capacity in subjects with abdominal obesity. Eur. J. Nutr. 2013, 52, 37–48. [Google Scholar] [CrossRef]
- Racine, A.; Carbonnel, F.; Chan, S.S.M.; Hart, A.R.; Bueno-de-Mesquita, H.B.; Oldenburg, B.; van Schaik, F.D.M.; Tjønneland, A.; Olsen, A.; Dahm, C.C.; et al. Dietary Patterns and Risk of Inflammatory Bowel Disease in Europe: Results from the EPIC Study. Inflamm. Bowel Dis. 2016, 22, 345–354. [Google Scholar] [CrossRef]
- Tomasello, G.; Mazzola, M.; Leone, A.; Sinagra, E.; Zummo, G.; Farina, F.; Damiani, P.; Cappello, F.; Gerges Geagea, A.; Jurjus, A.; et al. Nutrition, oxidative stress and intestinal dysbiosis: Influence of diet on gut microbiota in inflammatory bowel diseases. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czechoslov. 2016, 160, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, N.; Kono, S.; Wakai, K.; Fukuda, Y.; Satomi, M.; Shimoyama, T.; Inaba, Y.; Miyake, Y.; Sasaki, S.; Okamoto, K.; et al. Dietary risk factors for inflammatory bowel disease: A multicenter case-control study in Japan. Inflamm. Bowel Dis. 2005, 11, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Marlow, G.; Ellett, S.; Ferguson, I.R.; Zhu, S.; Karunasinghe, N.; Jesuthasan, A.C.; Han, D.Y.; Fraser, A.G.; Ferguson, L.R. Transcriptomics to study the effect of a Mediterranean-inspired diet on inflammation in Crohn’s disease patients. Hum. Genomics 2013, 7, 24. [Google Scholar] [CrossRef]
- Pérez-Rey, J.; Roncero-Martín, R.; Rico-Martín, S.; Rey-Sánchez, P.; Pedrera-Zamorano, J.D.; Pedrera-Canal, M.; López-Espuela, F.; Lavado-García, J.M. Adherence to a Mediterranean Diet and Bone Mineral Density in Spanish Premenopausal Women. Nutrients 2019, 11, 555. [Google Scholar] [CrossRef]
- Palomeras-Vilches, A.; Viñals-Mayolas, E.; Bou-Mias, C.; Jordà-Castro, M.; Agüero-Martínez, M.; Busquets-Barceló, M.; Pujol-Busquets, G.; Carrion, C.; Bosque-Prous, M.; Serra-Majem, L.; et al. Adherence to the Mediterranean Diet and Bone Fracture Risk in Middle-Aged Women: A Case Control Study. Nutrients 2019, 11, 2508. [Google Scholar] [CrossRef]
- Da Silva, T.R.; Martins, C.C.; Ferreira, L.L.; Spritzer, P.M. Mediterranean diet is associated with bone mineral density and muscle mass in postmenopausal women. Climacteric J. Int. Menopause Soc. 2019, 22, 162–168. [Google Scholar] [CrossRef]
- Rudloff, S.; Bührer, C.; Jochum, F.; Kauth, T.; Kersting, M.; Körner, A.; Koletzko, B.; Mihatsch, W.; Prell, C.; Reinehr, T.; et al. Vegetarian diets in childhood and adolescence. Mol. Cell. Pediatr. 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Hakala, P.; Karvetti, R.L. Weight reduction on lactovegetarian and mixed diets. Changes in weight, nutrient intake, skinfold thicknesses and blood pressure. Eur. J. Clin. Nutr. 1989, 43, 421–430. [Google Scholar]
- Janelle, K.C.; Barr, S.I. Nutrient intakes and eating behavior scores of vegetarian and nonvegetarian women. J. Am. Diet. Assoc. 1995, 95, 180–189. [Google Scholar] [CrossRef]
- Kahleova, H.; Matoulek, M.; Malinska, H.; Oliyarnik, O.; Kazdova, L.; Neskudla, T.; Skoch, A.; Hajek, M.; Hill, M.; Kahle, M.; et al. Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with Type 2 diabetes. Diabet. Med. J. Br. Diabet. Assoc. 2011, 28, 549–559. [Google Scholar] [CrossRef]
- Amarapurkar, A.D.; Amarapurkar, D.N.; Rathi, P.; Sawant, P.; Patel, N.; Kamani, P.; Rawal, K.; Baijal, R.; Sonawane, A.; Narawane, N.; et al. Risk factors for inflammatory bowel disease: A prospective multi-center study. Indian J. Gastroenterol. Off. J. Indian Soc. Gastroenterol. 2018, 37, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, P.; Yilmaz, B.; Rossel, J.-B.; Franc, Y.; Misselwitz, B.; Scharl, M.; Zeitz, J.; Frei, P.; Greuter, T.; Vavricka, S.R.; et al. Vegetarian or gluten-free diets in patients with inflammatory bowel disease are associated with lower psychological well-being and a different gut microbiota, but no beneficial effects on the course of the disease. United Eur. Gastroenterol. J. 2019, 7, 767–781. [Google Scholar] [CrossRef] [PubMed]
- Grosse, C.S.J.; Christophersen, C.T.; Devine, A.; Lawrance, I.C. The role of a plant-based diet in the pathogenesis, etiology and management of the inflammatory bowel diseases. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Chiba, M.; Tsuji, T.; Nakane, K.; Komatsu, M. High amount of dietary fiber not harmful but favorable for Crohn disease. Perm. J. 2015, 19, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Movassagh, E.Z.; Baxter-Jones, A.D.G.; Kontulainen, S.; Whiting, S.; Szafron, M.; Vatanparast, H. Vegetarian-style dietary pattern during adolescence has long-term positive impact on bone from adolescence to young adulthood: A longitudinal study. Nutr. J. 2018, 17, 36. [Google Scholar] [CrossRef]
- Ho-Pham, L.T.; Vu, B.Q.; Lai, T.Q.; Nguyen, N.D.; Nguyen, T.V. Vegetarianism, bone loss, fracture and vitamin D: A longitudinal study in Asian vegans and non-vegans. Eur. J. Clin. Nutr. 2012, 66, 75–82. [Google Scholar] [CrossRef]
- Thorpe, D.L.; Knulsen, S.F.; Beeson, W.L.; Rajaram, S.; Fraser, G.E. Effects of meat consumption and vegetarian diet on risk of wrist fracture over 25 years in a cohort of peri- and postmenopausal women. Public Health Nutr. 2008, 11, 564–572. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Chiu, J.-S.; Chuang, M.-H.; Chiu, J.-E.; Lin, C.-L. Bone mineral density of vegetarian and non-vegetarian adults in Taiwan. Asia Pac. J. Clin. Nutr. 2008, 17, 101–106. [Google Scholar]
- Ho-Pham, L.T.; Nguyen, P.L.T.; Le, T.T.T.; Doan, T.A.T.; Tran, N.T.; Le, T.A.; Nguyen, T.V. Veganism, bone mineral density, and body composition: A study in Buddhist nuns. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2009, 20, 2087–2093. [Google Scholar] [CrossRef]
- Chiu, J.F.; Lan, S.J.; Yang, C.Y.; Wang, P.W.; Yao, W.J.; Su, L.H.; Hsieh, C.C. Long-term vegetarian diet and bone mineral density in postmenopausal Taiwanese women. Calcif. Tissue Int. 1997, 60, 245–249. [Google Scholar] [CrossRef]
- Brouns, F. Overweight and diabetes prevention: Is a low-carbohydrate-high-fat diet recommendable? Eur. J. Nutr. 2018, 57, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Greene-Finestone, L.S.; Campbell, M.K.; Evers, S.E.; Gutmanis, I.A. Adolescents’ low-carbohydrate-density diets are related to poorer dietary intakes. J. Am. Diet. Assoc. 2005, 105, 1783–1788. [Google Scholar] [CrossRef] [PubMed]
- Wyka, J.; Malczyk, E.; Misiarz, M.; Zołoteńka-Synowiec, M.; Całyniuk, B.; Baczyńska, S. Assessment of food intakes for women adopting the high protein Dukan diet. Rocz. Panstw. Zakl. Hig. 2015, 66, 137–142. [Google Scholar] [PubMed]
- Gardner, C.D.; Kim, S.; Bersamin, A.; Dopler-Nelson, M.; Otten, J.; Oelrich, B.; Cherin, R. Micronutrient quality of weight-loss diets that focus on macronutrients: Results from the A TO Z study. Am. J. Clin. Nutr. 2010, 92, 304–312. [Google Scholar] [CrossRef]
- Shoda, R.; Matsueda, K.; Yamato, S.; Umeda, N. Epidemiologic analysis of Crohn disease in Japan: Increased dietary intake of n-6 polyunsaturated fatty acids and animal protein relates to the increased incidence of Crohn disease in Japan. Am. J. Clin. Nutr. 1996, 63, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Amre, D.K.; D’Souza, S.; Morgan, K.; Seidman, G.; Lambrette, P.; Grimard, G.; Israel, D.; Mack, D.; Ghadirian, P.; Deslandres, C.; et al. Imbalances in dietary consumption of fatty acids, vegetables, and fruits are associated with risk for Crohn’s disease in children. Am. J. Gastroenterol. 2007, 102, 2016–2025. [Google Scholar] [CrossRef] [PubMed]
- Jantchou, P.; Morois, S.; Clavel-Chapelon, F.; Boutron-Ruault, M.-C.; Carbonnel, F. Animal protein intake and risk of inflammatory bowel disease: The E3N prospective study. Am. J. Gastroenterol. 2010, 105, 2195–2201. [Google Scholar] [CrossRef]
- Hou, J.K.; Abraham, B.; El-Serag, H. Dietary intake and risk of developing inflammatory bowel disease: A systematic review of the literature. Am. J. Gastroenterol. 2011, 106, 563–573. [Google Scholar] [CrossRef]
- Zengin, A.; Kropp, B.; Chevalier, Y.; Junnila, R.; Sustarsic, E.; Herbach, N.; Fanelli, F.; Mezzullo, M.; Milz, S.; Bidlingmaier, M.; et al. Low-carbohydrate, high-fat diets have sex-specific effects on bone health in rats. Eur. J. Nutr. 2016, 55, 2307–2320. [Google Scholar] [CrossRef]
- Kerstetter, J.E.; O’Brien, K.O.; Caseria, D.M.; Wall, D.E.; Insogna, K.L. The impact of dietary protein on calcium absorption and kinetic measures of bone turnover in women. J. Clin. Endocrinol. Metab. 2005, 90, 26–31. [Google Scholar] [CrossRef]
- Bushinsky, D.A.; Smith, S.B.; Gavrilov, K.L.; Gavrilov, L.F.; Li, J.; Levi-Setti, R. Chronic acidosis-induced alteration in bone bicarbonate and phosphate. Am. J. Physiol. Renal Physiol. 2003, 285, F532–F539. [Google Scholar] [CrossRef] [PubMed]
- Draaisma, J.M.T.; Hampsink, B.M.; Janssen, M.; van Houdt, N.B.M.; Linders, E.T.A.M.; Willemsen, M.A. The Ketogenic Diet and Its Effect on Bone Mineral Density: A Retrospective Observational Cohort Study. Neuropediatrics 2019, 50, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Bergqvist, A.G.C.; Schall, J.I.; Stallings, V.A.; Zemel, B.S. Progressive bone mineral content loss in children with intractable epilepsy treated with the ketogenic diet. Am. J. Clin. Nutr. 2008, 88, 1678–1684. [Google Scholar] [CrossRef] [PubMed]
- Catassi, G.; Lionetti, E.; Gatti, S.; Catassi, C. The Low FODMAP Diet: Many Question Marks for a Catchy Acronym. Nutrients 2017, 9, 292. [Google Scholar] [CrossRef] [PubMed]
- Bascuñán, K.A.; Elli, L.; Pellegrini, N.; Scricciolo, A.; Lombardo, V.; Doneda, L.; Vecchi, M.; Scarpa, C.; Araya, M.; Roncoroni, L. Impact of FODMAP Content Restrictions on the Quality of Diet for Patients with Celiac Disease on a Gluten-Free Diet. Nutrients 2019, 11, 2220. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Ralph, F.S.E.; Irving, P.M.; Whelan, K.; Lomer, M.C.E. Nutrient Intake, Diet Quality, and Diet Diversity in Irritable Bowel Syndrome and the Impact of the Low FODMAP Diet. J. Acad. Nutr. Diet. 2020, 120, 535–547. [Google Scholar] [CrossRef]
- Eswaran, S.; Dolan, R.D.; Ball, S.C.; Jackson, K.; Chey, W. The Impact of a 4-Week Low-FODMAP and mNICE Diet on Nutrient Intake in a Sample of US Adults with Irritable Bowel Syndrome with Diarrhea. J. Acad. Nutr. Diet. 2020, 120, 641–649. [Google Scholar] [CrossRef]
- Pedersen, N.; Ankersen, D.V.; Felding, M.; Wachmann, H.; Végh, Z.; Molzen, L.; Burisch, J.; Andersen, J.R.; Munkholm, P. Low-FODMAP diet reduces irritable bowel symptoms in patients with inflammatory bowel disease. World J. Gastroenterol. 2017, 23, 3356–3366. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhan, Y.-A.; Dai, S.-X. Is a low FODMAP diet beneficial for patients with inflammatory bowel disease? A meta-analysis and systematic review. Clin. Nutr. Edinb. Scotl. 2018, 37, 123–129. [Google Scholar] [CrossRef]
- Colombel, J.-F.; Shin, A.; Gibson, P.R. AGA Clinical Practice Update on Functional Gastrointestinal Symptoms in Patients With Inflammatory Bowel Disease: Expert Review. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2019, 17, 380–390.e1. [Google Scholar] [CrossRef]
- Marum, A.P.; Moreira, C.; Tomas-Carus, P.; Saraiva, F.; Guerreiro, C.S. A low fermentable oligo-di-mono-saccharides and polyols (FODMAP) diet is a balanced therapy for fibromyalgia with nutritional and symptomatic benefits. Nutr. Hosp. 2017, 34, 667–674. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Franzosa, E.A.; Sirota-Madi, A.; Avila-Pacheco, J.; Fornelos, N.; Haiser, H.J.; Reinker, S.; Vatanen, T.; Hall, A.B.; Mallick, H.; McIver, L.J.; et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 2019, 4, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, C.; Romero, E.; Garrido-Sanchez, L.; Alcaín-Martínez, G.; Andrade, R.J.; Taminiau, B.; Daube, G.; García-Fuentes, E. Microbiota insights in Clostridium difficile infection and inflammatory bowel disease. Gut Microbes 2020, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Krause, L.; Somerset, S. Associations between micronutrient intakes and gut microbiota in a group of adults with cystic fibrosis. Clin. Nutr. 2017, 36, 1097–1104. [Google Scholar] [CrossRef]
- Xu, J.; Xu, C.; Chen, X.; Cai, X.; Yang, S.; Sheng, Y.; Wang, T. Regulation of an antioxidant blend on intestinal redox status and major microbiota in early weaned piglets. Nutrition 2014, 30, 584–589. [Google Scholar] [CrossRef]
- Wilson, R.; Willis, J.; Gearry, R.; Hughes, A.; Lawley, B.; Skidmore, P.; Frampton, C.; Fleming, E.; Anderson, A.; Jones, L.; et al. SunGold Kiwifruit Supplementation of Individuals with Prediabetes Alters Gut Microbiota and Improves Vitamin C Status, Anthropometric and Clinical Markers. Nutrients 2018, 10, 895. [Google Scholar] [CrossRef]
- Pierre, J.F.; Hinterleitner, R.; Bouziat, R.; Hubert, N.; Leone, V.; Miyoshi, J.; Jabri, B.; Chang, E.B. Data on changes to mucosal inflammation and the intestinal microbiota following dietary micronutrients in genetically susceptible hosts. Data Brief 2018, 20, 387–393. [Google Scholar] [CrossRef]
- Yan, H.; Wang, H.; Zhang, X.; Li, X.; Yu, J. Ascorbic acid ameliorates oxidative stress and inflammation in dextran sulfate sodium-induced ulcerative colitis in mice. Int. J. Clin. Exp. Med. 2015, 8, 20245. [Google Scholar]
- Huang, K.; Dong, W.; Liu, W.; Yan, Y.; Wan, P.; Peng, Y.; Xu, Y.; Zeng, X.; Cao, Y. 2-O-β-d-Glucopyranosyl-l-ascorbic Acid, an Ascorbic Acid Derivative Isolated from the Fruits of Lycium Barbarum L., Modulates Gut Microbiota and Palliates Colitis in Dextran Sodium Sulfate-Induced Colitis in Mice. J. Agric. Food Chem. 2019, 67, 11408–11419. [Google Scholar] [CrossRef]
- Qi, Y.; Lohman, J.; Bratlie, K.M.; Peroutka-Bigus, N.; Bellaire, B.; Wannemuehler, M.; Yoon, K.-J.; Barrett, T.A.; Wang, Q. Vitamin C and B3 as new biomaterials to alter intestinal stem cells. J. Biomed. Mater. Res. A 2019, 107, 1886–1897. [Google Scholar] [CrossRef]
- Hardy, M.A. The biology of scar formation. Phys. Ther. 1989, 69, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Hiramoto, K.; Yamate, Y.; Goto, K.; Sekijima, H.; Ooi, K. Ameliorative Effect of High-Dose Vitamin C Administration on Dextran Sulfate Sodium-Induced Colitis Mouse Model. Biol. Pharm. Bull. 2019, 42, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Britton, R.A.; Irwin, R.; Quach, D.; Schaefer, L.; Zhang, J.; Lee, T.; Parameswaran, N.; McCabe, L.R. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J. Cell. Physiol. 2014, 229, 1822–1830. [Google Scholar] [CrossRef]
- Li, J.-Y.; Chassaing, B.; Tyagi, A.M.; Vaccaro, C.; Luo, T.; Adams, J.; Darby, T.M.; Weitzmann, M.N.; Mulle, J.G.; Gewirtz, A.T.; et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J. Clin. Invest. 2016, 126, 2049–2063. [Google Scholar] [CrossRef]
- Narva, M.; Nevala, R.; Poussa, T.; Korpela, R. The effect of Lactobacillus helveticus fermented milk on acute changes in calcium metabolism in postmenopausal women. Eur. J. Nutr. 2004, 43, 61–68. [Google Scholar] [CrossRef]
- Parvaneh, K.; Ebrahimi, M.; Sabran, M.R.; Karimi, G.; Hwei, A.N.M.; Abdul-Majeed, S.; Ahmad, Z.; Ibrahim, Z.; Jamaluddin, R. Probiotics (Bifidobacterium longum) Increase Bone Mass Density and Upregulate Sparc and Bmp-2 Genes in Rats with Bone Loss Resulting from Ovariectomy. BioMed Res. Int. 2015, 2015, 897639. [Google Scholar] [CrossRef]
- Takimoto, T.; Hatanaka, M.; Hoshino, T.; Takara, T.; Tanaka, K.; Shimizu, A.; Morita, H.; Nakamura, T. Effect of Bacillus subtilis C-3102 on bone mineral density in healthy postmenopausal Japanese women: A randomized, placebo-controlled, double-blind clinical trial. Biosci. Microbiota Food Health 2018, 37, 87–96. [Google Scholar] [CrossRef]
| Concentration of Vitamin C | Value |
|---|---|
| Ulcerative colitis [20] | 39.1 ± 18.1 mmol/L |
| Crohn disease [21] | 35.3 ± 25.8 mmol/L |
| Healthy individuals [20] | 64.5 ± 12.5 mmol/L |
| Intake of Vitamin C | Value |
|---|---|
| Ulcerative colitis in active phase [20] | 43.2 ± 27.6 mg/1000 kcal/day |
| Ulcerative colitis in remission [20] | 67.5 ± 34.5 mg/1000 kcal/day |
| Crohn’s disease patients [22] | 50.8 ± 40.3 mg/day |
| Healthy individuals [20] | 49.5 ± 6.2 mg/1000 kcal/day |
| Diet | Vitamin C Content (Compared to Control) | Importance for IBD | Importance for Osteoporosis |
|---|---|---|---|
| Mediterranean diet | Higher | Protects | Prevents |
| Vegetarian diets | Higher | An increased risk of CD protects from UC | Contradictory data |
| Low-carbohydrates diets | Lower | Possibly an increased risk of IBD, because of a high intake of animal protein | Possibly an increased risk |
| Low-FODMAP diet | Higher | Appropriate for IBD patients with symptoms of IBS | Contradictory data |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratajczak, A.E.; Szymczak-Tomczak, A.; Skrzypczak-Zielińska, M.; Rychter, A.M.; Zawada, A.; Dobrowolska, A.; Krela-Kaźmierczak, I. Vitamin C Deficiency and the Risk of Osteoporosis in Patients with an Inflammatory Bowel Disease. Nutrients 2020, 12, 2263. https://doi.org/10.3390/nu12082263
Ratajczak AE, Szymczak-Tomczak A, Skrzypczak-Zielińska M, Rychter AM, Zawada A, Dobrowolska A, Krela-Kaźmierczak I. Vitamin C Deficiency and the Risk of Osteoporosis in Patients with an Inflammatory Bowel Disease. Nutrients. 2020; 12(8):2263. https://doi.org/10.3390/nu12082263
Chicago/Turabian StyleRatajczak, Alicja Ewa, Aleksandra Szymczak-Tomczak, Marzena Skrzypczak-Zielińska, Anna Maria Rychter, Agnieszka Zawada, Agnieszka Dobrowolska, and Iwona Krela-Kaźmierczak. 2020. "Vitamin C Deficiency and the Risk of Osteoporosis in Patients with an Inflammatory Bowel Disease" Nutrients 12, no. 8: 2263. https://doi.org/10.3390/nu12082263
APA StyleRatajczak, A. E., Szymczak-Tomczak, A., Skrzypczak-Zielińska, M., Rychter, A. M., Zawada, A., Dobrowolska, A., & Krela-Kaźmierczak, I. (2020). Vitamin C Deficiency and the Risk of Osteoporosis in Patients with an Inflammatory Bowel Disease. Nutrients, 12(8), 2263. https://doi.org/10.3390/nu12082263