Association between the Phytochemical Index and Lower Prevalence of Obesity/Abdominal Obesity in Korean Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Study Population
2.2. Demographic and Lifestyle Data
2.3. Anthropometric Measurement and Diagnosis of Obesity
2.4. Nutritional Survey Data and PI
2.5. Statistical Analysis
3. Results
3.1. General Participant Characteristics According to PI
3.2. Association between PI and Obesity/Abdominal Obesity
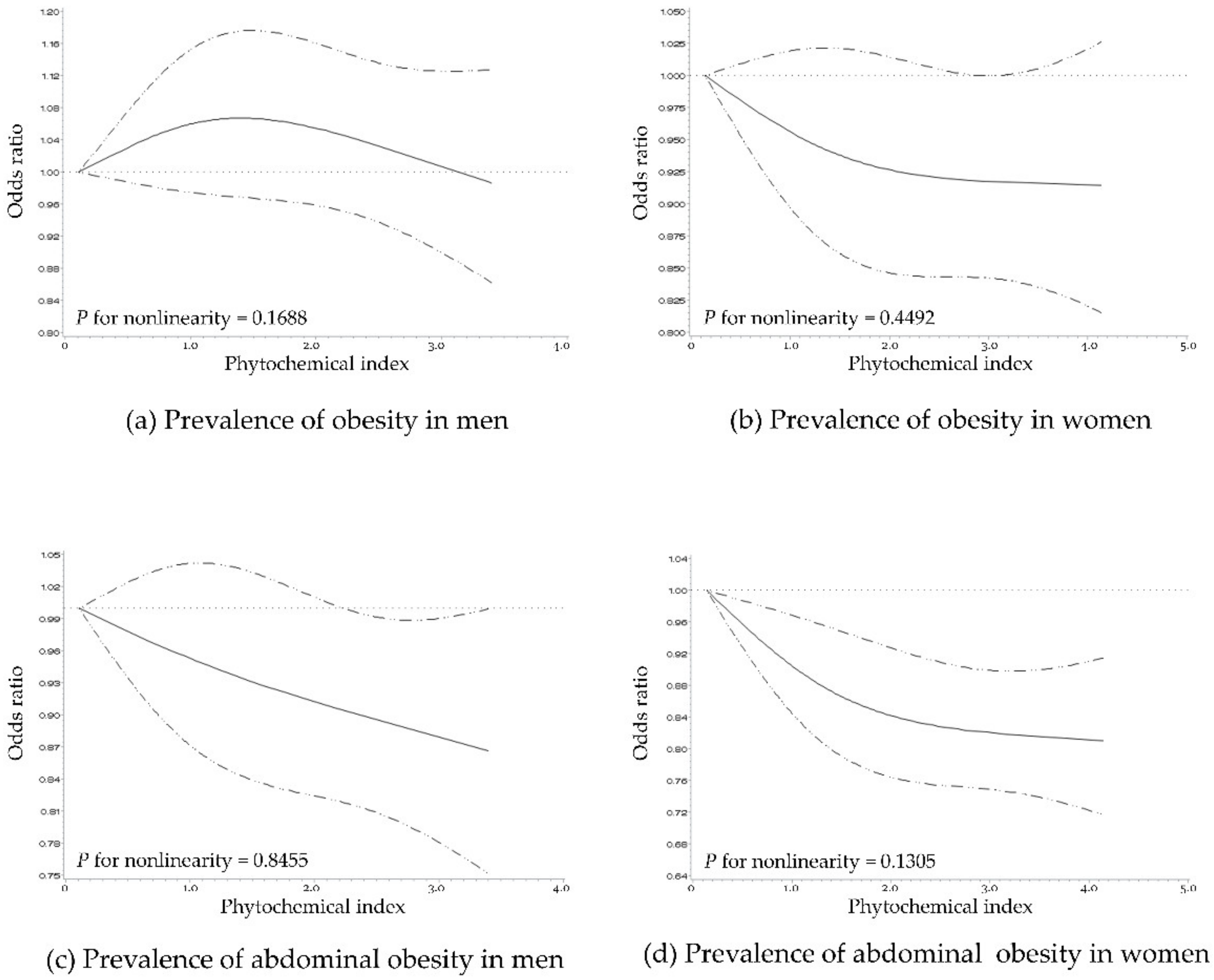
3.3. Dose–Response Association between PI and Obesity/Abdominal Obesity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Korea Centers for Disease Control and Prevention. Korea Health Statistics 2018: Korea National Health and Nutrition Examination Survey (KNHANES Ⅶ-3); Korea Centers for Disease Control and Prevention: Osong, Korea, 2020.
- Korean Society for the Study of Obesity. Diagnosis of Obesity. Available online: http://general.kosso.or.kr/html/?pmode=obesityDiagnosis (accessed on 21 April 2020).
- Sassi, F.; Devaux, M.; Cecchini, M.; Rusticelli, E. The Obesity Epidemic: Analysis of Past and Projected Future Trend in Selected OECD Countries; OECD: Paris, France, 2009. [Google Scholar]
- Park, C. Obesity and cardiovascular disease. Korean Circ. J. 1997, 27, 130–134. [Google Scholar] [CrossRef] [Green Version]
- Korean Society for the Study of Obesity. Comorbidities of Obesity. Available online: http://general.kosso.or.kr/html/?pmode=obesityDisease (accessed on 6 May 2020).
- Di Angelantonio, E.; Bhupathiraju, S.N.; Wormser, D.; Gao, P.; Kaptoge, S.; de Gonzalez, A.B.; Cairns, B.J.; Huxley, R.; Jackson, C.L.; Joshy, G.; et al. Body-mass index and all-cause mortality: Individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016, 388, 776–786. [Google Scholar] [CrossRef] [Green Version]
- He, K.; Hu, F.B.; Colditz, G.A.; Manson, J.E.; Willett, W.C.; Liu, S. Changes in intake of fruits and vegetables in relation to risk of obesity and weight gain among middle-aged women. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2004, 28, 1569–1574. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Willett, W.C.; Manson, J.E.; Hu, F.B.; Rosner, B.; Colditz, G. Relation between changes in intakes of dietary fiber and grain products and changes in weight and development of obesity among middle-aged women. Am. Soc. Clin. Nutr. 2003, 78, 920–927. [Google Scholar] [CrossRef] [Green Version]
- Serdula, M.K.; Byers, T.; Mokdad, A.H.; Simoes, E.; Mendlein, J.M.; Coates, R.J. The Association between Fruit and Vegetable Intake and Chronic Disease Risk Factors. Epidemiology 1996, 7, 161–165. [Google Scholar] [CrossRef]
- Holubková, A.; Penesová, A.; Šturdík, E.; Mošovská, S.; Mikušová, L. Phytochemicals with potential effects in metabolic syndrome prevention and therapy. Acta Chim. Slovaca 2012, 5, 186–199. [Google Scholar] [CrossRef] [Green Version]
- McCarty, M.F. Proposal for a dietary “phytochemical index”. Med. Hypotheses 2004, 63, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Eslami, O.; Khoshgoo, M.; Shidfar, F. Dietary phytochemical index and overweight/obesity in children: A cross-sectional study. BMC Res. Notes 2020, 13, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rupasinghe, H.P.; Sekhon-Loodu, S.; Mantso, T.; Panayiotidis, M.I. Phytochemicals in regulating fatty acid beta-oxidation: Potential underlying mechanisms and their involvement in obesity and weight loss. Pharmacol. Ther. 2016, 165, 153–163. [Google Scholar] [CrossRef]
- Vincent, H.K.; Bourguignon, C.M.; Taylor, A.G. Relationship of the dietary phytochemical index to weight gain, oxidative stress and inflammation in overweight young adults. J. Hum. Nutr. Diet. 2010, 23, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Carnauba, R.A.; Chaves, D.F.; Baptistella, A.B.; Paschoal, V.; Naves, A.; Buehler, A.M. Association between high consumption of phytochemical-rich foods and anthropometric measures: A systematic review. Int. J. Food Sci. Nutr. 2016, 68, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Golzarand, M.; Mirmiran, P.; Saadati, N.; Azizi, F. The association of dietary phytochemical index and cardiometabolic risk factors in adults: Tehran Lipid and Glucose Study. J. Hum. Nutr. Diet. 2013, 26 (Suppl. S1), 145–153. [Google Scholar] [CrossRef] [PubMed]
- OECD Indicators. Health at A Glance 2017; Organisation for Economic Cooperation and Development: Paris, France, 2017; p. 76. [Google Scholar]
- Ainsworth, B.E.; Haskell, W.L.; Leon, A.S.; Jacobs, D.R.; Montoye, H.J.; Sallis, J.F.; Paffenbarger, R.S. Compendium of Physical Activities: Classification of energy costs of human physical activities. Med. Sci. Sports Exerc. 1993, 25, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Korea Centers for Disease Control and Prevention. Guidelines for Examination and Inspection (2016–2018); Korea Centers for Disease Control and Prevention: Osong, Korea, 28 June 2016; pp. 64–67.
- World Health Organization. The Asia Pacific Perspective: Redefining Obesity and Its Treatment. Available online: www.wpro.who.int/nutrition/documents/.../Redefiningobesity.pdf (accessed on 26 March 2020).
- Korean Society for the Study of Obesity. Diagnosis and Evaluation of Obesity. Available online: http://general.kosso.or.kr/html/?pmode=BBBS0001300003&page=2&smode=view&seq=86&searchValue=&searchTitle=strTitle&setRowCount=undefined (accessed on 24 March 2020).
- Korea Centers for Disease Control and Prevention; Ministry of Health and Welfare. Guidelines for Nutrition Investigation (2015); Korea Centers for Disease Control and Prevention, Ministry of Health and Welfare: Osong, Korea, 2016; p. 39.
- Kim, M.; Park, K. Association between phytochemical index and metabolic syndrome. Nutr. Res. Pract. 2020, 14, 252. [Google Scholar] [CrossRef] [PubMed]
- Han, M.R.; Ju, D.L.; Park, Y.J.; Paik, H.Y.; Song, Y. An Iodine Database for Common Korean Foods and the Association between Iodine Intake and Thyroid Disease in Korean Adults. Int. J. Thyroidol. 2015, 8, 170–182. [Google Scholar] [CrossRef]
- Ham, D.; Kim, S.; Jun, S.; Kang, M.; Joung, H. Association between antioxidant vitamin intake and obesity among Korean women: Using the Korea National Health and Nutrition Examination Survey 2007 ~ 2016. J. Nutr. Health 2018, 51, 400–413. [Google Scholar] [CrossRef]
- Harrell, F.E., Jr. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis; Springer: Berlin, Germany, 2015. [Google Scholar]
- Kim, S.A.; Kim, J.; Jun, S.; Wie, G.A.; Shin, S.; Joung, H. Association between dietary flavonoid intake and obesity among adults in Korea. Appl. Physiol. Nutr. Metab. 2020, 45, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, K.; Inoue, T.; Hioki, R.; Ochiai, J.; Kusuhara, Y.; Ichino, N.; Osakabe, K.; Hamajima, N.; Ito, Y. Association of abdominal obesity with decreased serum levels of carotenoids in a healthy Japanese population. Clin. Nutr. 2006, 25, 780–789. [Google Scholar] [CrossRef]
- Feillet-Coudray, C.; Sutra, T.; Fouret, G.; Ramos, J.; Wrutniak-Cabello, C.; Cabello, G.; Cristol, J.P.; Coudray, C. Oxidative stress in rats fed a high-fat high-sucrose diet and preventive effect of polyphenols: Involvement of mitochondrial and NAD(P)H oxidase systems. Free Radic. Biol. Med. 2009, 46, 624–632. [Google Scholar] [CrossRef]
- SHimoda, H.; Tanaka, J.; Kikuchi, M.; Fukuda, T.; Ito, H.; Hatano, T.; Yoshida, T. Effect of polyphenol-rich extract from walnut on diet-induced hypertriglyceridemia in mice via enhancement of fatty acid oxidation in the liver. J. Agric. Food Chem. 2009, 57, 1786–1792. [Google Scholar] [CrossRef]
- Wu, T.; Tang, Q.; Gao, Z.; Yu, Z.; Song, H.; Zheng, X.; Chen, W. Blueberry and Mulberry Juice Prevent Obesity Development in C57BL/6 Mice. PLoS ONE 2013, 8, e77585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera, L.; Moron, R.; Zarzuelo, A.; Galisteo, M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem. Pharmacol. 2009, 77, 1053–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, L.; Fernandez-Quintela, A.; Arias, N.; Portillo, M.P. Resveratrol: Anti-obesity mechanisms of action. Molecules 2014, 19, 18632–18655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemieux, S.; Prud’homme, D.; Bouchard, C.; Tremblay, A.; Després, J.P. Sex differences in the relation of visceral adipose tissue accumulation to total body fatness. Am. J. Clin. Nutr. 1993, 58, 463–467. [Google Scholar] [CrossRef]
- Lee, J.; Heo, J.; Park, Y.; Park, H. Survey on the Consumption of the Phytoestrogen Isoflavone in Postmenopausal Korean Women. J. Korean Soc. Menopause 2012, 18, 163–173. [Google Scholar] [CrossRef]
- Kim, M.; Choi, M.; Sung, C. The Study of Pytoestrogen Intake and Bone Mineral Density of Vegetarian and Nonvegetarian Postmenopausal Women. Korean J. Commun. Nutr. 2004, 9, 66–72. [Google Scholar]
- Kim, B.-J. Obesity and Sex Hormones. J. Korean Soc. Study Obes. 2010, 4, 113–118. [Google Scholar]
- Choi, H.M. Nutrition in the 21st Century; Gyomoon: Paju, Korea, 2017. [Google Scholar]
- Son, S.M.; Im, H.S.; Kim, J.H.; Lee, J.H.; Seo, J.S.; Son, J.M. Clinical Nutrition; Gyomoon: Paju, Korea, 2018. [Google Scholar]
| Phytochemical Index Quintiles | |||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | |
| Number of Participants | 11,588 | 11,588 | 11,588 | 11,588 | 11,588 |
| Phytochemical Index, Median (range) | 2.32 (0.00–4.46) | 6.60 (4.46–8.86) | 11.40 (8.86–14.31) | 17.90 (14.31–22.74) | 30.85 (22.74–98.91) |
| Sex | |||||
| Men | 5801 (50.06) | 5412 (46.70) | 4913 (42.40) | 4260 (36.76) | 3515 (30.33) |
| Women | 5787 (49.94) | 6176 (53.30) | 6675 (57.60) | 7328 (63.24) | 8073 (69.67) |
| Age (years) | |||||
| 19–29 | 2279 (19.67) | 1527 (13.18) | 1141 (9.85) | 839 (7.24) | 583 (5.03) |
| 30–39 | 2372 (20.47) | 2452 (21.16) | 1966 (16.97) | 1528 (13.19) | 1125 (9.71) |
| 40–49 | 1984 (17.12) | 2339 (20.18) | 2251 (19.43) | 2186 (18.86) | 1848 (15.95) |
| 50–59 | 1567 (13.52) | 1898 (16.38) | 2154 (18.59) | 2427 (20.94) | 2889 (24.93) |
| ≥60 | 3386 (29.22) | 3372 (29.10) | 4076 (35.17) | 4608 (39.77) | 5143 (44.38) |
| Household Income | |||||
| Mid-low or lower | 6499 (56.62) | 5960 (51.95) | 5677 (49.48) | 5313 (46.29) | 5129 (44.68) |
| Mid-high or higher | 4980 (43.38) | 5513 (48.05) | 5797 (50.52) | 6164 (53.71) | 6351 (55.32) |
| Education Level | |||||
| Lower than high school education | 3597 (32.54) | 3678 (33.17) | 4148 (37.39) | 4394 (39.72) | 4681 (42.16) |
| High school educated or higher | 7456 (67.46) | 7410 (66.83) | 6947 (62.61) | 6669 (60.28) | 6421 (57.84) |
| Smoking Status | |||||
| Non-smoker | 8185 (72.24) | 8644 (76.57) | 9277 (82.34) | 9753 (86.79) | 10,242 (90.87) |
| Current smoker | 3146 (27.76) | 2645 (23.43) | 1990 (17.66) | 1485 (13.21) | 1029 (9.13) |
| Alcohol Consumption | |||||
| No | 2685 (23.71) | 2723 (24.14) | 3178 (28.23) | 3668 (32.67) | 4387 (38.97) |
| Yes | 8640 (76.29) | 8557 (75.86) | 8078 (71.77) | 7561 (67.33) | 6869 (61.03) |
| Body Mass Index (kg/m2) | |||||
| <23 | 5259 (45.38) | 5153 (44.47) | 5098 (43.99) | 5065 (43.71) | 4866 (41.99) |
| 23–25 | 2487 (21.46) | 2636 (22.75) | 2709 (23.38) | 2816 (24.30) | 2894 (24.97) |
| ≥25 | 3842 (33.15) | 3799 (32.78) | 3781 (32.63) | 3707 (31.99) | 3828 (33.03) |
| Physical Activity 1 | |||||
| Low | 4229 (38.21) | 3673 (33.09) | 3555 (32.02) | 3432 (31.00) | 3237 (29.12) |
| Mid | 3723 (33.64) | 3735 (33.65) | 3798 (34.21) | 3736 (33.75) | 3817 (34.34) |
| High | 3115 (28.15) | 3692 (33.26) | 3748 (33.76) | 3902 (35.25) | 4062 (36.54) |
| Phytochemical Index Quintiles | p for Trend | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| Obesity 1 | ||||||
| Men | ||||||
| N | 4780 | 4780 | 4781 | 4780 | 4780 | |
| No. of cases (%) | 1708 (35.73) | 1847 (38.64) | 1771 (37.04) | 1731 (36.21) | 1744 (36.49) | |
| Model 1 | 1 | 1.14 (1.03–1.26) | 1.06 (0.96–1.17) | 1.02 (0.92–1.13) | 1.04 (0.94–1.15) | 0.73 |
| Model 2 | 1 | 1.10 (1.00–1.22) | 1.04 (0.94–1.15) | 1.02 (0.92–1.14) | 1.07 (0.96–1.19) | 0.64 |
| Model 3 | 1 | 1.09 (0.99–1.21) | 1.05 (0.95–1.17) | 1.03 (0.92–1.14) | 1.05 (0.94–1.17) | 0.85 |
| Women | ||||||
| N | 6807 | 6808 | 6808 | 6808 | 6808 | |
| No. of cases (%) | 2010 (29.53) | 1929 (28.33) | 1989 (29.22) | 2075 (30.48) | 2153 (31.62) | |
| Model 1 | 1 | 0.95 (0.87–1.03) | 1.01 (0.92–1.10) | 1.06 (0.97–1.16) | 1.15 (1.05–1.26) | <0.001 |
| Model 2 | 1 | 0.86 (0.79–0.94) | 0.85 (0.77–0.93) | 0.84 (0.77–0.92) | 0.83 (0.76–0.91) | 0.001 |
| Model 3 | 1 | 0.87 (0.79–0.95) | 0.87 (0.79–0.96) | 0.87 (0.79–0.96) | 0.86 (0.78–0.94) | 0.01 |
| Abdominal Obesity 2 | ||||||
| Men | ||||||
| N | 4780 | 4780 | 4781 | 4780 | 4780 | |
| No. of cases (%) | 1377 (28.81) | 1393 (29.14) | 1366 (28.57) | 1388 (29.04) | 1408 (29.46) | |
| Model 1 | 1 | 1.04 (0.94–1.16) | 0.98 (0.88–1.10) | 0.99 (0.89–1.10) | 1.03 (0.93–1.15) | 0.81 |
| Model 2 | 1 | 1.00 (0.90–1.10) | 0.91 (0.82–1.02) | 0.88 (0.79–0.98) | 0.90 (0.81–1.00) | 0.01 |
| Model 3 | 1 | 0.99 (0.89–1.10) | 0.93 (0.83–1.04) | 0.89 (0.79–0.99) | 0.90 (0.81–1.01) | 0.03 |
| Women | ||||||
| N | 6807 | 6808 | 6808 | 6808 | 6808 | |
| No. of cases (%) | 1783 (26.19) | 1730 (25.41) | 1758 (25.82) | 1836 (26.97) | 1926 (28.29) | |
| Model 1 | 1 | 1.00 (0.91–1.09) | 1.04 (0.95–1.14) | 1.09 (0.99–1.19) | 1.20 (1.09–1.32) | <0.001 |
| Model 2 | 1 | 0.88 (0.80–0.97) | 0.82 (0.74–0.91) | 0.79 (0.72–0.87) | 0.77 (0.70–0.86) | <0.001 |
| Model 3 | 1 | 0.89 (0.80–0.98) | 0.84 (0.76–0.93) | 0.84 (0.76–0.93) | 0.81 (0.73–0.90) | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Im, J.; Kim, M.; Park, K. Association between the Phytochemical Index and Lower Prevalence of Obesity/Abdominal Obesity in Korean Adults. Nutrients 2020, 12, 2312. https://doi.org/10.3390/nu12082312
Im J, Kim M, Park K. Association between the Phytochemical Index and Lower Prevalence of Obesity/Abdominal Obesity in Korean Adults. Nutrients. 2020; 12(8):2312. https://doi.org/10.3390/nu12082312
Chicago/Turabian StyleIm, Jihyun, Minkyeong Kim, and Kyong Park. 2020. "Association between the Phytochemical Index and Lower Prevalence of Obesity/Abdominal Obesity in Korean Adults" Nutrients 12, no. 8: 2312. https://doi.org/10.3390/nu12082312






