Circadian Variation in Human Milk Composition, a Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Study Selection and Data Extraction
2.3. Methodological Quality Assessment
3. Results
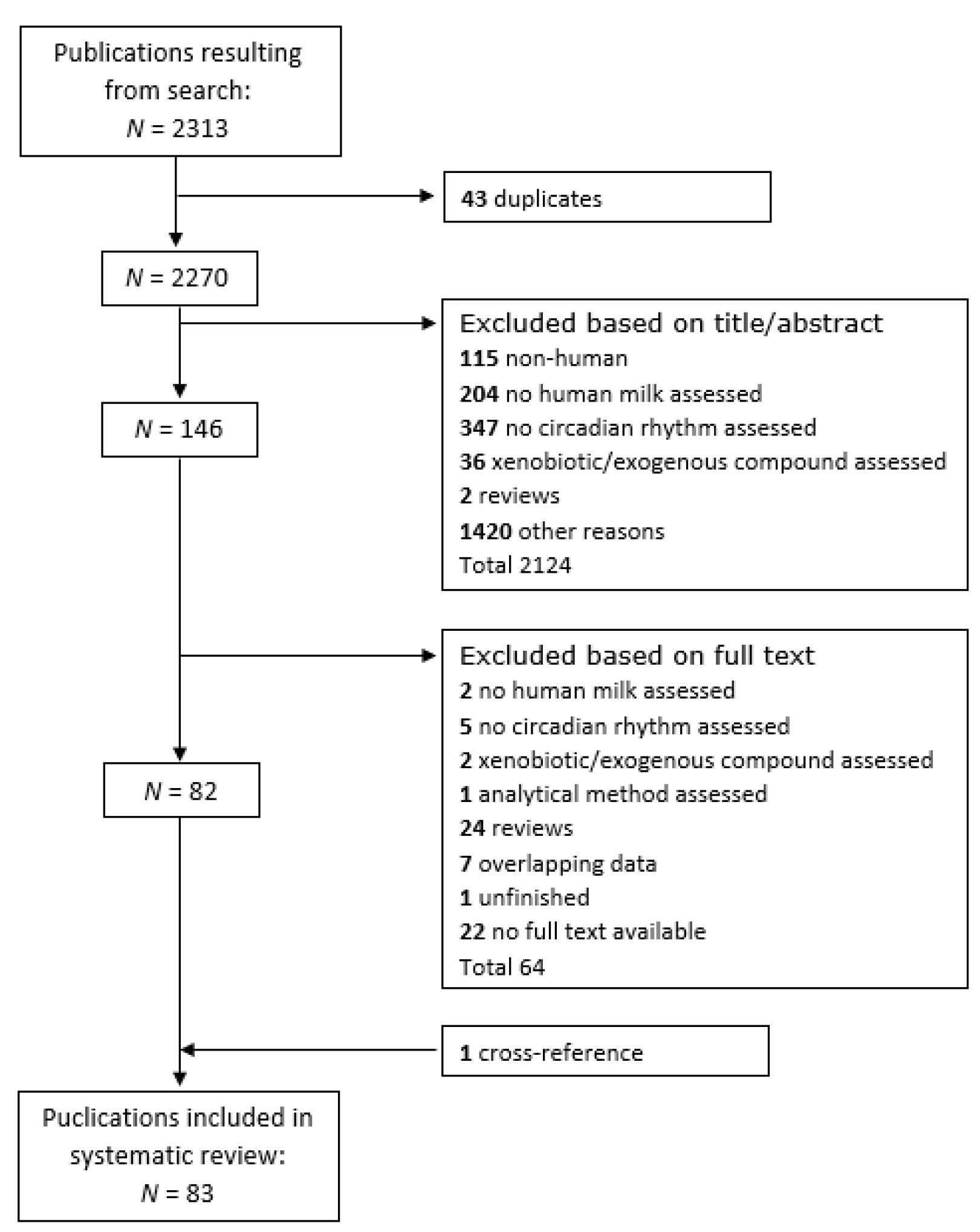
3.1. Study Selection
3.2. Study Characteristics
3.3. Methodological Quality Assessment
3.4. Circadian Variation in Human Milk Components
3.4.1. Macronutrients
Carbohydrates
Proteins
Fats
3.4.2. Micronutrients
Vitamins
Trace Elements and Electrolytes
3.4.3. Bioactive Factors
Hormones
Immune Factors
Other Bioactive Factors
4. Discussion
4.1. Strengths and Limitations
4.2. Physiological Role of Circadian Rhythmicity in Human Milk
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Victora, C.G.; Bahl, R.; Barros, A.J.; França, G.V.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization; UNICEF. Global Strategy for Infant and Young Child. Feeding; WHO: Geneva, Switzerland, 2003. [Google Scholar]
- Hennet, T.; Borsig, L. Breastfed at Tiffany’s. Trends. Biochem. Sci. 2016, 41, 508–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galante, L.; Milan, A.M.; Reynolds, C.M.; Cameron-Smith, D.; Vickers, M.H.; Pundir, S. Sex-Specific Human Milk Composition: The Role of Infant Sex in Determining Early Life Nutrition. Nutrients 2018, 10, 1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseini, M.; Valizadeh, E.; Hosseini, N.; Khatibshahidi, S.; Raeisi, S. The Role of Infant Sex on Human Milk Composition. Breastfeed. Med. 2020, 15, 341–346. [Google Scholar] [CrossRef]
- McKenna, H.; Reiss, I.K.M. The case for a chronobiological approach to neonatal care. Early Hum. Dev. 2018, 126, 1–5. [Google Scholar] [CrossRef]
- Fuhr, L.; Abreu, M.; Pett, J.P.; Relógio, A. Circadian systems biology: When time matters. Comput. Struct. Biotechnol. J. 2015, 13, 417–426. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2016, 18, 164–179. [Google Scholar] [CrossRef] [Green Version]
- Roenneberg, T.; Merrow, M. The Circadian Clock and Human Health. Curr. Biol. 2016, 26, R432–R443. [Google Scholar] [CrossRef]
- Buhr, E.D.; Takahashi, J.S. Molecular components of the Mammalian circadian clock. Handb. Exp. Pharmacol. 2013, 217, 3–27. [Google Scholar] [CrossRef] [Green Version]
- Asher, G.; Sassone-Corsi, P. Time for Food: The Intimate Interplay between Nutrition, Metabolism, and the Circadian Clock. Cell 2015, 161, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Ardura, J.; Gutierrez, R.; Andres, J.; Agapito, T. Emergence and evolution of the circadian rhythm of melatonin in children. Horm. Res. 2003, 59, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Kennaway, D.J.; Stamp, G.E.; Goble, F.C. Development of melatonin production in infants and the impact of prematurity. J. Clin. Endocrinol. Metab. 1992, 75, 367–369. [Google Scholar] [PubMed]
- Ivars, K.; Nelson, N.; Theodorsson, A.; Theodorsson, E.; Ström, J.O.; Morelius, E. Development of Salivary Cortisol Circadian Rhythm and Reference Intervals in Full-Term Infants. PLoS ONE 2015, 10, e0129502. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, M.; Maas, Y.G.H.; Ariagno, R.L. Development of fetal and neonatal sleep and circadian rhythms. Sleep Med. Rev. 2003, 7, 321–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christ, E.; Korf, H.-W.; Von Gall, C. When does it start ticking? Ontogenetic development of the mammalian circadian system. Prog. Brain Res. 2012, 199, 105–118. [Google Scholar]
- Hahn-Holbrook, J.; Saxbe, D.; Bixby, C.; Steele, C.; Glynn, L. Human milk as “chrononutrition”: Implications for child health and development. Pediatr. Res. 2019, 85, 936–942. [Google Scholar] [CrossRef]
- Cubero, J.; Valero, V.; Sánchez, J.; Rivero, M.; Parvez, H.; Rodríguez, A.B.; Barriga, C. The circadian rhythm of tryptophan in breast milk affects the rhythms of 6-sulfatoxymelatonin and sleep in newborn. Neuroendocr. Lett. 2005, 26, 657–661. [Google Scholar]
- van der Voorn, B.; Heijboer, A.; de Waard, M.; Verheijen, H.; Rotteveel, J.; Finken, M. Circadian variation in cortisol concentration in mother’s milk. Horm. Res. Paediatr. 2015, 84, 513–514. [Google Scholar]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef] [Green Version]
- Modesti, P.A.; Reboldi, G. Panethnic differences in blood pressure in europe: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0147601. [Google Scholar] [CrossRef] [Green Version]
- Neu, J. Gastroenterology and Nutrition: Neonatology Questions and Controversies: Expert Consult—Online and Print; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Dizdar, E.; Yarcı, E.; Sari, F.N.; Oguz, S.S.; Uras, N.; Canpolat, F.E.; Çetinkaya, A.K. Does Circadian Variation of Mothers Affect Macronutrients of Breast Milk? Am. J. Perinatol. 2016, 34, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Hollanders, J.J.; Kouwenhoven, S.M.P.; van der Voorn, B.; van Goudoever, J.B.; Rotteveel, J.; Finken, M.J. The Association between Breastmilk Glucocorticoid Concentrations and Macronutrient Contents Throughout the Day. Nutrients 2019, 11, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moran-Lev, H.; Mimouni, F.; Ovental, A.; Mangel, L.; Mandel, D.; Lubetzky, R. Circadian Macronutrients Variations over the First 7 Weeks of Human Milk Feeding of Preterm Infants. Breastfeed. Med. 2015, 10, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Arthur, P.; Kent, J.C.; Hartmann, P. Metabolites of Lactose Synthesis in Milk from Women During Established Lactation. J. Pediatr. Gastroenterol. Nutr. 1991, 13, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Lammi-Keefe, C.J.; Ferris, A.M.; Jensen, R.G. Changes in Human Milk at 0600, 1000, 1400, 1800, and 2200 h. J. Pediatr. Gastroenterol. Nutr. 1990, 11, 83–88. [Google Scholar] [CrossRef]
- Cannon, A.M.; Kakulas, F.; Hepworth, A.R.; Lai, C.T.; Hartmann, P.; Geddes, D.T. The Effects of Leptin on Breastfeeding Behaviour. Int. J. Environ. Res. Public Health 2015, 12, 12340–12355. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Hepworth, A.R.; Prime, D.K.; Lai, C.T.; Trengove, N.J.; Hartmann, P.E. Variation in fat, lactose, and protein composition in breast milk over 24 hours: Associations with infant feeding patterns. J. Hum. Lact. 2013, 29, 81–89. [Google Scholar] [CrossRef]
- Viverge, D.; Grimmonprez, L.; Cassanas, G.; Bardet, L.; Solère, M. Diurnal Variations and within the Feed in Lactose and Oligosaccharides of Human Milk. Ann. Nutr. Metab. 1986, 30, 196–209. [Google Scholar] [CrossRef]
- Suryawan, A.; Davis, T.A. Regulation of protein synthesis by amino acids in muscle of neonates. Front. Biosci. 2011, 16, 1445–1460. [Google Scholar] [CrossRef] [Green Version]
- Hall, B. Uniformity of human milk. Am. J. Clin. Nutr. 1979, 32, 304–312. [Google Scholar] [CrossRef] [Green Version]
- West, K.D.; Kirksey, A. Influence of vitamin B6 intake on the content of the vitamin in human milk. Am. J. Clin. Nutr. 1976, 29, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Casadio, Y.S.; Lai, C.T.; Prime, D.K.; Hepworth, A.R.; Trengove, N.J.; Hartmann, P. Investigation of Short-term Variations in Casein and Whey Proteins in Breast Milk of Term Mothers. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Massmann, P.F.; França, E.L.; de Souza, E.G.; Souza, M.S.; Brune, M.F.S.S.; Honorio-França, A.C. Maternal hypertension induces alterations in immunological factors of colostrum and human milk. Front. Life Sci. 2013, 7, 155–163. [Google Scholar] [CrossRef] [Green Version]
- López, C.L.S.; Hernández, A.; Rodríguez, A.B.; Rivero, M.; Barriga, C.; Cubero, J. Nitrogen and protein content analysis of human milk, diurnality vs nocturnality. Nutr. Hosp. 2011, 26, 511–514. [Google Scholar]
- Clark, R.; Ross, S.; Hill, D.; Ferris, A. Within-Day Variation of Taurine and Other Nitrogen Substances in Human Milk. J. Dairy Sci. 1987, 70, 776–780. [Google Scholar] [CrossRef]
- LaVine, M.; Clark, R.; Hundrieser, K.; Ferris, A. Within-Day Variation of Lipolytic Activity in Human Milk. J. Dairy Sci. 1986, 69, 1784–1786. [Google Scholar] [CrossRef]
- Graf, M.V.; Hunter, C.A.; Kastin, A.J. Presence of delta-sleep-inducing peptide-like material in human milk. J. Clin. Endocrinol. Metab. 1984, 59, 127–132. [Google Scholar] [CrossRef]
- Freed, L.M.; Neville, M.C.; Hamosh, P.; Hamosh, M. Diurnal and within-feed variations in lipase activity and triglyceride content of human milk. J. Pediatr. Gastroenterol. Nutr. 1986, 5, 938–942. [Google Scholar] [CrossRef]
- Katzer, D.; Pauli, L.; Mueller, A.; Reutter, H.; Reinsberg, J.; Fimmers, R.; Bartmann, P.; Bağci, S. Melatonin concentrations and antioxidative capacity of human breast milk according to gestational age and the time of day. J. Hum. Lact. 2016, 32, NP105–NP110. [Google Scholar] [CrossRef]
- Hegardt, P.; Lindberg, T.; Börjesson, J.; Skude, G. Amylase in human milk from mothers of preterm and term infants. J. Pediatr. Gastroenterol. Nutr. 1984, 3, 563–566. [Google Scholar] [CrossRef]
- Sánchez, C.L.; Barriga, C.; Rodríguez, A.B. Human milk nucleotides improve sleep: A focus on circadian profiles. In Handbook of Nutrition, Diet and Sleep; Wageningen Academic Publishers: Wageningen, The Netherlands, 2013. [Google Scholar]
- França, E.L.; Nicomedes, T.D.R.; Calderon, I.M.; Honorio-França, A.C. Time-dependent alterations of soluble and cellular components in human milk. Biol. Rhythm. Res. 2010, 41, 333–347. [Google Scholar] [CrossRef]
- Spencer, S.A.; Hull, D. Fat content of expressed breast milk: A case for quality control. BMJ 1981, 282, 99–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunther, M.; Stanier, J. Diurnal variation in the fat content of breast-milk. Lancet 1949, 254, 235–237. [Google Scholar] [CrossRef]
- Jackson, D.A.; Imong, S.M.; Silprasert, A.; Ruckphaopunt, S.; Woolridge, M.W.; Baum, J.D.; Amatayakul, K. Circadian variation in fat concentration of breast-milk in a rural northern Thai population. Br. J. Nutr. 1988, 59, 349–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, R.G.; Lammi-Keefe, C.J.; Ferris, A.M.; Jackson, M.B.; Couch, S.C.; Capacchione, C.M.; Ahn, H.S.; Murtaugh, M. Human milk total lipid and cholesterol are dependent on interval of sampling during 24 hours. J. Pediatr. Gastroenterol. Nutr. 1995, 20, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Kociszewska-Najman, B.; Borek-Dzieciol, B.; Szpotanska-Sikorska, M.; Wilkos, E.; Pietrzak, B.; Wielgos, M. The creamatocrit, fat and energy concentration in human milk produced by mothers of preterm and term infants. J. Matern. Neonatal Med. 2012, 25, 1599–1602. [Google Scholar] [CrossRef]
- Lubetzky, R.; Littner, Y.; Mimouni, F.B.; Dollberg, S.; Mandel, D. Circadian variations in fat content of expressed breast milk from mothers of preterm infants. J. Am. Coll. Nutr. 2006, 25, 151–154. [Google Scholar] [CrossRef]
- Lubetzky, R.; Mimouni, F.; Dollberg, S.; Salomon, M.; Mandel, D. Consistent circadian variations in creamatocrit over the first 7 weeks of lactation: A longitudinal study. Breastfeed. Med. 2007, 2, 15–18. [Google Scholar] [CrossRef]
- Mes, J.; Davies, D.J. Variation in the polychlorinated biphenyl and organochlorine pesticide residues during human breastfeeding and its diurnal pattern. Chemosphere 1978, 7, 699–706. [Google Scholar] [CrossRef]
- Picciano, M.F.; Guthrie, H.A. Copper, iron, and zinc contents of mature human milk. Am. J. Clin. Nutr. 1976, 29, 242–254. [Google Scholar] [CrossRef] [Green Version]
- Stafford, J.; Villalpando, S.; Aguila, B.U. Circadian variation and changes after a meal in volume and lipid production of human milk from rural mexican women. Ann. Nutr. Metab. 1994, 38, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Kent, J.C.; Mitoulas, L.R.; Cregan, M.D.; Geddes, D.T.; Doherty, R.A.; Hartmann, P. Volume and frequency of breastfeedings and fat content of breast milk throughout the day. Pediatrics 2006, 117, 387–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harzer, G.; Haug, M.; Dieterich, I.; Gentner, P.R. Changing patterns of human milk lipids in the course of the lactation and during the day. Am. J. Clin. Nutr. 1983, 37, 612–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delplanque, B.; Gibson, R.; Koletzko, B.; Lapillonne, A.; Strandvik, B. Lipid quality in infant nutrition: Current knowledge and future opportunities. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 8–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruel, M.T.; Dewey, K.G.; Martinez, C.; Flores, R.; Brown, K.H. Validation of single daytime samples of human milk to estimate the 24-h concentration of lipids in urban Guatemalan mothers. Am. J. Clin. Nutr. 1997, 65, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Picciano, M.F.; Guthrie, H.A.; Sheehe, D.M. The cholesterol content of human milk. A variable constituent among women and within the same woman. Clin. Pediatr. 1978, 17, 359–362. [Google Scholar] [CrossRef]
- Guthrie, H.A.; Picciano, M.F.; Sheehe, D. Fatty acid patterns of human milk. J. Pediatr. 1977, 90, 39–41. [Google Scholar] [CrossRef]
- Mitoulas, L.R.; Gurrin, L.C.; Doherty, D.A.; Sherriff, J.L.; Hartmann, P.E. Infant intake of fatty acids from human milk over the first year of lactation. Br. J. Nutr. 2003, 90, 979–986. [Google Scholar] [CrossRef] [Green Version]
- Mitoulas, L.R.; Kent, J.C.; Cox, D.B.; Owens, R.A.; Sherriff, J.L.; Hartmann, P. Variation in fat, lactose and protein in human milk over 24h and throughout the first year of lactation. Br. J. Nutr. 2002, 88, 29–37. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Char, D.; Sheard, N.F. Choline, phosphatidylcholine and sphingomyelin in human and bovine milk and infant formulas. J. Nutr. 1986, 116, 50–58. [Google Scholar] [CrossRef]
- Patton, S.; Huston, G.E. Incidence and characteristics of cell pieces on human milk fat globules. Biochim. Biophys. Acta 1988, 965, 146–153. [Google Scholar] [CrossRef]
- Hampel, D.; Shahab-Ferdows, S.; Islam, M.M.; Peerson, J.M.; Allen, L.H. Vitamin Concentrations in Human Milk Vary with Time within Feed, Circadian Rhythm, and Single-Dose Supplementation. J. Nutr. 2017, 147, 603–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mock, D.M.; Mock, N.I.; Dankle, J.A. Secretory Patterns of Biotin in Human Milk. J. Nutr. 1992, 122, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Barkova, E.N.; Nazarenko, E.V.; Zhdanova, E.V. Diurnal variations in qualitative composition of breast milk in women with iron deficiency. Bull. Exp. Biol. Med. 2005, 140, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Trugo, N.M.; Sardinha, F. Cobalamin and cobalamin-binding capacity in human milk. Nutr. Res. 1994, 14, 23–33. [Google Scholar] [CrossRef]
- Udipi, S.A.; Kirksey, A.; Roepke, J.L. Diurnal variations in folacin levels of human milk: Use of a single sample to represent folacin concentration in milk during a 24-h period. Am. J. Clin. Nutr. 1987, 45, 770–779. [Google Scholar] [CrossRef]
- Feeley, R.M.; Eitenmiller, R.R.; Jones, J.B.; Barnhart, H. Copper, iron, and zinc contents of human milk at early stages of lactation. Am. J. Clin. Nutr. 1983, 37, 443–448. [Google Scholar] [CrossRef]
- Silvestre, M.D.; Lagarda, M.J.; Farré, R.; Martínez-Costa, C.; Brines, J.; Molina, A.; Clemente, G. A Study of Factors that May Influence the Determination of Copper, Iron, and Zinc in Human Milk During Sampling and in Sample Individuals. Biol. Trace Element Res. 2000, 76, 217–228. [Google Scholar] [CrossRef]
- Keenan, B.S.; Buzek, S.W.; Garza, C.; Potts, E.; Nichols, B.L. Diurnal and longitudinal variations in human milk sodium and potassium: Implication for nutrition and physiology. Am. J. Clin. Nutr. 1982, 35, 527–534. [Google Scholar] [CrossRef]
- Feeley, R.M.; Eitenmiller, R.R.; Jones, J.B., Jr.; Barnhart, H. Calcium, phosphorus, and magnesium contents of human milk during early lactation. J. Pediatr. Gastroenterol. Nutr. 1983, 2, 262–267. [Google Scholar] [CrossRef]
- Karra, M.V.; Kirksey, A. Variation in Zinc, Calcium, and Magnesium Concentrations of Human Milk within a 24-Hour Period from 1 to 6 Months of Lactation. J. Pediatr. Gastroenterol. Nutr. 1988, 7, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Stawarz, R.; Formicki, G.; Massányi, P. Daily fluctuations and distribution of xenobiotics, nutritional and biogenic elements in human milk in Southern Poland. J. Environ. Sci. Health Part A Toxic Hazard. Subst. Environ. Eng. 2007, 42, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, S.Y.; El-Hodhod, M.A.A.; Nassar, M.F.; Hegazy, A.E.-T.; El-Arab, S.E.; Shaheen, F.M. Zinc status of lactating Egyptian mothers and their infants: Effect of maternal zinc supplementation. Nutr. Res. 2005, 25, 45–53. [Google Scholar] [CrossRef]
- Krebs, N.F.; Hambidge, K.M.; Jacobs, M.A.; Mylet, S. Zinc in human milk: Diurnal and within-feed patterns. J. Pediatr. Gastroenterol. Nutr. 1985, 4, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Kirk, A.; Dyke, J.V.; Martin, C.F.; Dasgupta, P.K. Temporal patterns in perchlorate, thiocyanate, and iodide excretion in human milk. Environ. Health Perspect. 2006, 115, 182–186. [Google Scholar] [CrossRef]
- Lahesmaa, P.; Vilkki, P. The iodine content of human milk in Finland. Acta Paediatr. 1960, 49, 371–376. [Google Scholar] [CrossRef]
- Bouglé, D.; Bureau, F.; Foucault, P.; Duhamel, J.F.; Müller, G.; Drosdowsky, M. Molybdenum content of term and preterm human milk during the first 2 months of lactation. Am. J. Clin. Nutr. 1988, 48, 652–654. [Google Scholar] [CrossRef]
- Anderson, G.; Vaillancourt, C.; Maes, M.; Reiter, R.J. Breastfeeding and the gut-brain axis: Is there a role for melatonin? Biomol. Concepts 2017, 8, 185–195. [Google Scholar] [CrossRef]
- Engler, A.C.; Hadash, A.; Shehadeh, N.; Pillar, G. Breastfeeding may improve nocturnal sleep and reduce infantile colic: Potential role of breast milk melatonin. Eur. J. Pediatr. 2012, 174, 729–732. [Google Scholar] [CrossRef]
- Honorio-França, A.C.; Hara, C.C.P.; Ormonde, J.V.S.; Nunes, G.T.; França, E.L. Human colostrum melatonin exhibits a day-night variation and modulates the activity of colostral phagocytes. J. Appl. Biomed. 2013, 11, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Illnerova, H.; Buresova, M.; Presl, J. Melatonin rhythm in human milk. J. Clin. Endocrinol. Metab. 1993, 77, 838–841. [Google Scholar] [PubMed] [Green Version]
- Molad, M.; Ashkenazi, L.; Gover, A.; Lavie-Nevo, K.; Zaltsberg-Barak, T.; Shaked-Mishan, P.; Soloveichik, M.; Kessel, I.; Rotschild, A.; Etzioni, T. Melatonin Stability in Human Milk. Breastfeed. Med. 2019, 14, 680–682. [Google Scholar] [CrossRef] [PubMed]
- Pontes, G.N.; Cardoso, E.C.; Carneiro-Sampaio, M.M.S.; Markus, R.P. Pineal melatonin and the innate immune response: The TNF-alpha increase after cesarean section suppresses nocturnal melatonin production. J. Pineal Res. 2007, 43, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Pontes, G.N.; Cardoso, E.C.; Carneiro-Sampaio, M.M.S.; Markus, R.P. Injury switches melatonin production source from endocrine (pineal) to paracrine (phagocytes)—Melatonin in human colostrum and colostrum phagocytes. J. Pineal Res. 2006, 41, 136–141. [Google Scholar] [CrossRef]
- Prentice, A.M.; Whitehead, R.G. Breast-milk fat concentrations of rural African women. 1. Short-term variations within individuals. Br. J. Nutr. 1981, 45, 483–494. [Google Scholar] [CrossRef] [Green Version]
- Silva, N.A.; Honorio-França, A.C.; Giachini, F.R.; Mores, L.; De Souza, E.G.; França, E.L. Bioactive factors of colostrum and human milk exhibits a day-night variation. Am. J. Immunol. 2013, 9, 68–74. [Google Scholar] [CrossRef]
- Hollanders, J.J.; Dijkstra, L.R.; van Der Voorn, B.; Kouwenhoven, S.M.; Toorop, A.A.; van Goudoever, J.B.; Rotteveel, J.; Finken, M.J. No Association between Glucocorticoid Diurnal Rhythm in Breastmilk and Infant Body Composition at 3 Months. Nutrients 2019, 11, 2351. [Google Scholar] [CrossRef] [Green Version]
- Keenan, B.S.; Buzek, S.W.; Garza, C. Cortisol and its possible role in regulation of sodium and potassium in human milk. Am. J. Physiol. Metab. 1983, 244, E253–E261. [Google Scholar] [CrossRef]
- Pundir, S.; Wall, C.; Mitchell, C.J.; Thorstensen, E.B.; Lai, C.T.; Geddes, D.T.; Cameron-Smith, D. Variation of Human Milk Glucocorticoids over 24 hour Period. J. Mammary Gland. Biol. Neoplasia 2017, 22, 85–92. [Google Scholar] [CrossRef]
- van Der Voorn, B.; De Waard, M.; van Goudoever, J.B.; Rotteveel, J.; Heijboer, A.C.; Finken, M.J. Breast-milk cortisol and cortisone concentrations follow the diurnal rhythm of maternal hypothalamus-pituitary-adrenal axis activity. J. Nutr. 2016, 146, 2174–2179. [Google Scholar] [CrossRef] [Green Version]
- Hollanders, J.J.; van Der Voorn, B.; De Goede, P.; Toorop, A.A.; Dijkstra, L.R.; Honig, A.; Rotteveel, J.; Dolman, K.M.; Kalsbeek, A.; Finken, M.J.J. Biphasic Glucocorticoid Rhythm in One-Month-Old Infants: Reflection of a Developing HPA-Axis? J. Clin. Endocrinol. Metab. 2019, 105, e544. [Google Scholar] [CrossRef] [PubMed]
- Cregan, M.D.; Mitoulas, L.R.; Hartmann, P. Milk prolactin, feed volume and duration between feeds in women breastfeeding their full-term infants over a 24 h period. Exp. Physiol. 2002, 87, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cross, N.A.; Hillman, L.S.; Forte, L.R. The effects of calcium supplementation, duration of lactation, and time of day on concentrations of parathyroid hormone-related protein in human milk: A pilot study. J. Hum. Lact. 1998, 14, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, S.J.; Dickson, J.S.; Barnhart, H.M.; Toledo, R.T.; Eiten-Miller, R.R. IgA, IgG, IgM and Lactoferrin Contents of Human Milk During Early Lactation and the Effect of Processing and Storage. J. Food Prot. 1983, 46, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Peitersen, B.; Bohn, L.; Andersen, H. Quantitative determination of immunoglobulins, lysozyme, and certain electrolytes in breast milk during the entire period of lactation, during a 24-hour period, and in milk from the individual mammary gland. Acta Paediatr. 1975, 64, 709–717. [Google Scholar] [CrossRef]
- Morais, T.C.; Honorio-França, A.C.; Silva, R.R.; Fujimori, M.; Fagundes, D.L.G.; França, E.L. Temporal fluctuations of cytokine concentrations in human milk. Biol. Rhythm. Res. 2015, 46, 811–821. [Google Scholar] [CrossRef]
- Houghton, M.R.; Gracey, M.; Burke, V.; Bottrell, C.; Spargo, R.M. Breast milk lactoferrin levels in relation to maternal nutritional status. J. Pediatr. Gastroenterol. Nutr. 1985, 4, 230–233. [Google Scholar] [CrossRef] [Green Version]
- Moran, J.R.; Courtney, M.E.; Orth, D.N.; Vaughan, R.; Coy, S.; Mount, C.D.; Sherrell, B.J.; Greene, H.L. Epidermal growth factor in human milk: Daily production and diurnal variation during early lactation in mothers delivering at term and at premature gestation. J. Pediatr. 1983, 103, 402–405. [Google Scholar] [CrossRef]
- Sánchez, C.L.; Cubero, J.; Sanchez, J.; Chanclón, B.; Rivero, M.; Rodríguez, A.B.; Barriga, C. The possible role of human milk nucleotides as sleep inducers. Nutr. Neurosci. 2009, 12, 2–8. [Google Scholar] [CrossRef]
- Skala, J.P.; Koldovský, O.; Hahn, P. Cyclic nucleotides in breast milk. Am. J. Clin. Nutr. 1981, 34, 343–350. [Google Scholar] [CrossRef] [Green Version]
- Cubero, J.; SĂĄnchez, C.L.; Bravo, R.; SĂĄnchez, J.; Rodriguez, A.B.; Rivero, M.; Barriga, C. Analysis of the antioxidant activity in human milk, day vs night. Cell Membr. Free Radic. Res. 2010, 2, 36–37. [Google Scholar]
- Jackson, J.G.; Lien, E.L.; White, S.J.; Bruns, N.J.; Kuhlman, C.F. Major Carotenoids in Mature human Milk: Longitudinal and Diurnal Patterns. J. Nutr. Biochem. 1998, 9, 2–7. [Google Scholar] [CrossRef]
- Floris, I.; Billard, H.; Boquien, C.-Y.; Joram-Gauvard, E.; Simon, L.; Legrand, A.; Boscher, C.; Rozé, J.; Bolaños-Jiménez, F.; Kaeffer, B. MiRNA Analysis by Quantitative PCR in Preterm Human Breast Milk Reveals Daily Fluctuations of hsa-miR-16-5p. PLoS ONE 2015, 10, e0140488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Y.; Shi, W.; Zhuang, J.; Liu, Y.; Tang, L.; Bu, J.; Sun, J.; Bei, F. Variations in melatonin levels in preterm and term human breast milk during the first month after delivery. Sci. Rep. 2019, 9, 17984. [Google Scholar] [CrossRef]
- Finger, C. Caesarean section rates skyrocket in Brazil. Many women are opting for caesareans in the belief that it is a practical solution. Lancet 2003, 362, 628. [Google Scholar] [CrossRef]
- Lönnerdal, B. Trace Element Transport in the Mammary Gland. Annu. Rev. Nutr. 2007, 27, 165–177. [Google Scholar] [CrossRef]
- Sánchez, S.; Sánchez, C.L.; Paredes, S.D.; Rodriguez, A.B.; Barriga, C. The effect of tryptophan administration on the circadian rhythms of melatonin in plasma and the pineal gland of rats. J. Appl. Biomed. 2008, 6, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, S.; Nishihara, K.; Eto, H.; Horiuchi, S.; Hoshi, Y. The influence of feeding method on a mother’s daily rhythm and on the development of her infant’s circadian restactivity rhythm. J. Sleep Res. 2012, 21, 332. [Google Scholar]

| Compound Group | Circadian Variation Identified | No Circadian Variation Identified | Inconclusive |
|---|---|---|---|
| Carbohydrates | Carbohydrates | Lactose, glucose, glucose 6-phosphatase, glucose 1-phosphate, UDP-glucose, UDP-galactose | |
| Proteins | Individual amino acids i.e., tryptophan | Total protein, BSSL, total nitrogen | Urea, cobalamin, delta-sleep-inducing peptide, serum-stimulated lipase, lipoprotein lipase, superoxide dismutase, glutathion peroxidase 3, amylase |
| Fats | Fats, triacylglycerol, cholesterol | Fatty acids | Sphingomyelin and phospholipids |
| Vitamins | A, B1-3, B6, B8, B11, B12, E, choline | ||
| Trace elements and elektrolytes | iron | Ca, Cu | Zn, P, Mg, CuZn-SOD, I2, I-, molybdenum |
| Hormones | Melatonin, cortisol, cortison | Leptin, prolactin, PTHrP | |
| Immune factors | IgA, IgG, IgM, cytokines, interleukins, TNF-α, TGF-β, IFN-γ, lactoferrin, C3, C4, lysozyme, EGF | ||
| Other bioactive factors | Nucleotides, antioxidant activity, carotenoids, miRNA’s, citrate, malondialdehyde, perchlorate, thiocyanate, oligosaccharides |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Italianer, M.F.; Naninck, E.F.G.; Roelants, J.A.; van der Horst, G.T.J.; Reiss, I.K.M.; Goudoever, J.B.v.; Joosten, K.F.M.; Chaves, I.; Vermeulen, M.J. Circadian Variation in Human Milk Composition, a Systematic Review. Nutrients 2020, 12, 2328. https://doi.org/10.3390/nu12082328
Italianer MF, Naninck EFG, Roelants JA, van der Horst GTJ, Reiss IKM, Goudoever JBv, Joosten KFM, Chaves I, Vermeulen MJ. Circadian Variation in Human Milk Composition, a Systematic Review. Nutrients. 2020; 12(8):2328. https://doi.org/10.3390/nu12082328
Chicago/Turabian StyleItalianer, Merel F., Eva F. G. Naninck, Jorine A. Roelants, Gijsbertus T. J. van der Horst, Irwin K. M. Reiss, Johannes B. van Goudoever, Koen F. M. Joosten, Inês Chaves, and Marijn J. Vermeulen. 2020. "Circadian Variation in Human Milk Composition, a Systematic Review" Nutrients 12, no. 8: 2328. https://doi.org/10.3390/nu12082328






