The Effect of Isoleucine Supplementation on Body Weight Gain and Blood Glucose Response in Lean and Obese Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Study Design
2.3. Oral Glucose Tolerance Test
2.4. Gastric Emptying Breath Test
2.5. Metabolic Monitoring
2.6. Tissue Collection
2.7. Plasma Metabolites
2.8. Liver Lipid Content
2.9. Statistical Tests
3. Results
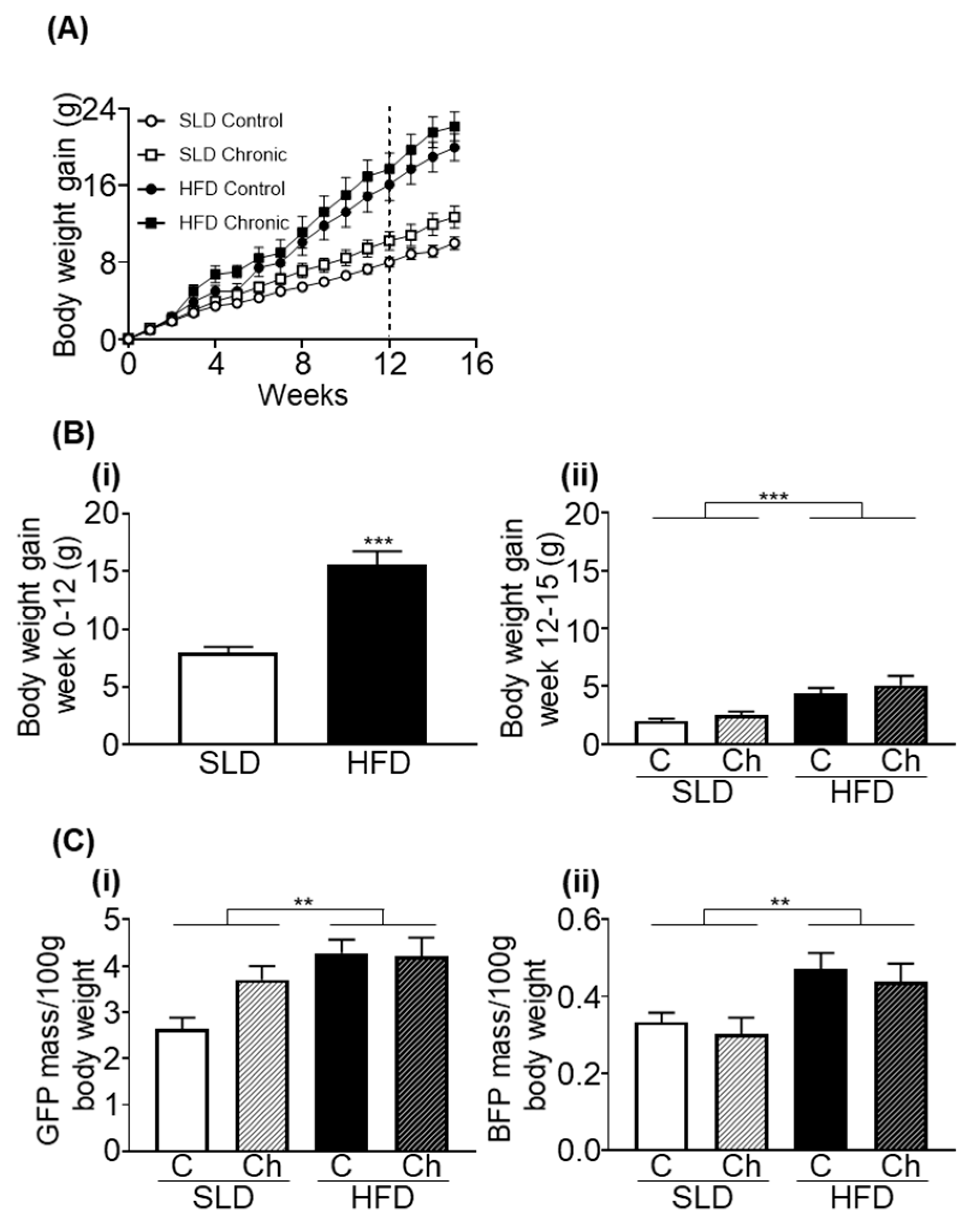
3.1. Chronic Isoleucine Treatment Does Not Affect Weight Gain and Adiposity
3.2. Chronic Isoleucine Treatment Does Not Affect Liver Lipid Content
3.3. Chronic Isoleucine Treatment Does Not Alter Energy Intake, Energy Expenditure, Activity, and Respiratory Quotient
3.4. Chronic Isoleucine Treatment Does Not Affect Plasma Lipid Metabolites
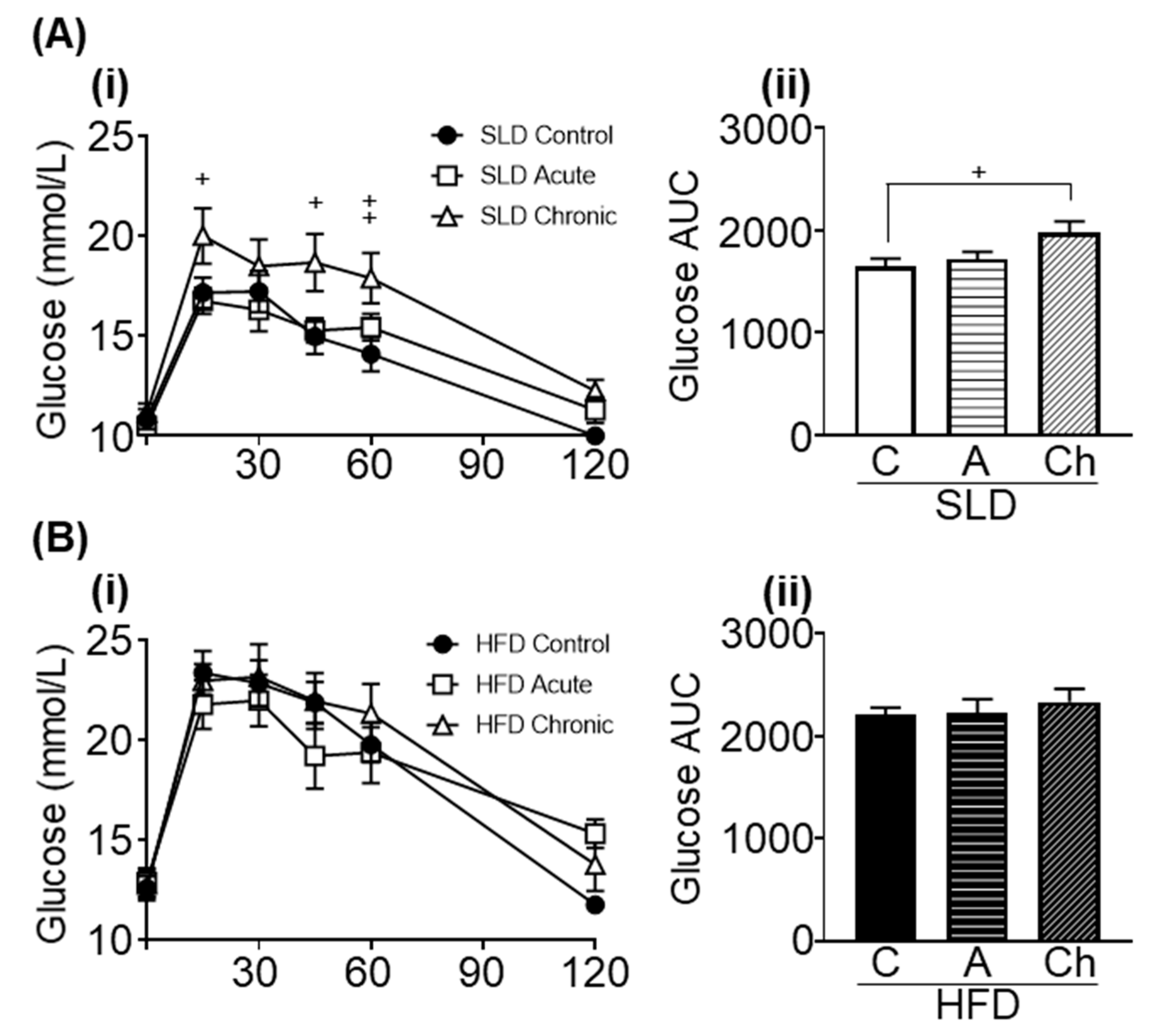
3.5. Acute and Chronic Isoleucine Treatment Differentially Affect Glucose Tolerance in SLD- and HFD-Mice
3.6. Acute and Chronic Isoleucine Treatment Do Not Affect Gastric Emptying
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holecek, M. Branched-Chain Amino Acids in Health and Disease: Metabolism, Alterations in Blood Plasma, and as Supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, L.; Xun, P.; Bujnowski, D.; Daviglus, M.L.; Van Horn, L.; Stamler, J.; He, K.; Intermap Cooperative Research Group. Higher Branched-Chain Amino Acid Intake Is Associated with a Lower Prevalence of Being Overweight or Obese in Middle-Aged East Asian and Western Adults. J. Nutr. 2011, 141, 249–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Li, Y.; Liu, L.; Chen, Y.; Zi, T.-Q.; Du, S.; Jiang, Y.-S.; Feng, R.-N.; Sun, C.H. The Ratio of Dietary Branched-Chain Amino Acids is Associated with a Lower Prevalence of Obesity in Young Northern Chinese Adults: An Internet-Based Cross-Sectional Study. Nutrients 2015, 7, 9573–9589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemon, P.W. Protein and Amino Acid Needs of the Strength Athlete. Int. J. Sport Nutr. 1991, 1, 127–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mero, A. Leucine supplementation and intensive training. Sports Med. 1999, 27, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Novin, Z.S.; Ghavamzadeh, S.; Mehdizadeh, A. The Weight Loss Effects of Branched Chain Amino Acids and Vitamin B6: A Randomized Controlled Trial on Obese and Overweight Women. Int. J. Vitam. Nutr. Res. 2018, 88, 80–89. [Google Scholar] [CrossRef]
- Nishimura, J.; Masaki, T.; Arakawa, M.; Seike, M.; Yoshimatsu, H. Isoleucine Prevents the Accumulation of Tissue Triglycerides and Upregulates the Expression of Paralpha and Uncoupling Protein in Diet-Induced Obese Mice. J. Nutr. 2010, 140, 496–500. [Google Scholar] [CrossRef] [Green Version]
- Doi, M.; Yamaoka, I.; Fukunaga, T.; Nakayama, M. Isoleucine, a potent plasma glucose-lowering amino acid, stimulates glucose uptake in C2C12 myotubes. Biochem. Biophys. Res. Commun. 2003, 312, 1111–1117. [Google Scholar] [CrossRef]
- Nishitani, S.; Takehana, K.; Fujitani, S.; Sonaka, I. Branched-chain amino acids improve glucose metabolism in rats with liver cirrhosis. Am. J. Physiol. Liver Physiol. 2005, 288, G1292–G1300. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Q.; Ren, M.; Qiao, S.; He, P.; Li, D.; Zeng, X. Effects of Isoleucine on Glucose Uptake through the Enhancement of Muscular Membrane Concentrations of Glut1 and Glut4 and Intestinal Membrane Concentrations of Na+/Glucose Co-Transporter 1 (Sglt-1) and Glut2. Br. J. Nutr. 2016, 116, 593–602. [Google Scholar] [CrossRef] [Green Version]
- Watson, R.T.; Pessin, J.E. Bridging the GAP between Insulin Signaling and GLUT4 Translocation. Trends Biochem. Sci. 2006, 31, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Ikehara, O.; Kawasaki, N.; Maezono, K.; Komatsu, M.; Konishi, A. Acute and Chronic Treatment of L-isoleucine Ameliorates Glucose Metabolism in Glucose-Intolerant and Diabetic Mice. Biol. Pharm. Bull. 2008, 31, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Marathe, C.S.; Rayner, C.K.; Jones, K.L.; Horowitz, M. Relationships Between Gastric Emptying, Postprandial Glycemia, and Incretin Hormones. Diabetes Care 2013, 36, 1396–1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Jesudason, D.R.; Stevens, J.E.; Keogh, J.B.; Jones, K.L.; Clifton, P.M.; Horowitz, M.; Rayner, C.K. Sustained Effects of a Protein ‘Preload’ on Glycaemia and Gastric Emptying over 4 Weeks in Patients with Type 2 Diabetes: A Randomized Clinical Trial. Diabetes Res. Clin. Pract. 2015, 108, e31–e34. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Stevens, J.E.; Cukier, K.; Maddox, A.F.; Wishart, J.M.; Jones, K.L.; Clifton, P.M.; Horowitz, M.; Rayner, C.K. Effects of a Protein Preload on Gastric Emptying, Glycemia, and Gut Hormones After a Carbohydrate Meal in Diet-Controlled Type 2 Diabetes. Diabetes Care 2009, 32, 1600–1602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullrich, S.; Fitzgerald, P.C.; Schober, G.; Steinert, R.E.; Horowitz, M.C.; Feinle-Bisset, C. Intragastric Administration of Leucine or Isoleucine Lowers the Blood Glucose Response to a Mixed-Nutrient Drink by Different Mechanisms in Healthy, Lean Volunteers. Am. J. Clin. Nutr. 2016, 104, 1274–1284. [Google Scholar] [CrossRef] [Green Version]
- Lang, P.; Hasselwander, S.; Li, H.; Xia, N. Effects of Different Diets Used in Diet-Induced Obesity Models on Insulin Resistance and Vascular Dysfunction in C57bl/6 Mice. Sci. Rep. 2019, 9, 19556. [Google Scholar] [CrossRef] [Green Version]
- Winzell, M.S.; Ahrén, B. The High-Fat Diet–Fed Mouse: A Model for Studying Mechanisms and Treatment of Impaired Glucose Tolerance and Type 2. Diabetes 2004, 53, S215–S219. [Google Scholar] [CrossRef] [Green Version]
- Andrikopoulos, S.; Blair, A.R.; DeLuca, N.; Fam, B.C.; Proietto, J. Evaluating the glucose tolerance test in mice. Am. J. Physiol. Metab. 2008, 295, E1323–E1332. [Google Scholar] [CrossRef] [Green Version]
- Nagy, C.; Einwallner, E. Study of In Vivo Glucose Metabolism in High-fat Diet-fed Mice Using Oral Glucose Tolerance Test (OGTT) and Insulin Tolerance Test (ITT). J. Vis. Exp. 2018, 131, e56672. [Google Scholar] [CrossRef]
- Symonds, E.L.; Butler, R.; Omari, T.I. Noninvasive breath tests can detect alterations in gastric emptying in the mouse. Eur. J. Clin. Investig. 2002, 32, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Symonds, E.L.; Butler, R.N.; Omari, T.I. Assessment of gastric emptying in the mouse using the [13C]-octanoic acid breath test. Clin. Exp. Pharmacol. Physiol. 2000, 27, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Ghoos, Y.F.; Maes, B.D.; Geypens, B.J.; Mys, G.; Hiele, M.I.; Rutgeerts, P.J.; Vantrappen, G. Measurement of gastric emptying rate of solids by means of a carbon-labeled octanoic acid breath test. Gastroenterology 1993, 104, 1640–1647. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Mehlem, A.; Hagberg, C.; Muhl, L.; Eriksson, U.; Falkevall, A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat. Protoc. 2013, 8, 1149–1154. [Google Scholar] [CrossRef] [Green Version]
- Ciapaite, J.; van den Broek, N.M.; Brinke, H.T.; Nicolay, K.; Jeneson, J.A.; Houten, S.M.; Prompers, J.J. Differential Effects of Short- and Long-Term High-Fat Diet Feeding on Hepatic Fatty Acid Metabolism in Rats. Biochim. Biophys. Acta 2011, 1811, 441–451. [Google Scholar] [CrossRef]
- Christie, S.; Vincent, A.D.; Li, H.; Frisby, C.L.; Kentish, S.J.; O’Rielly, R.; Wittert, G.A.; Page, A.J. A Rotating Light Cycle Promotes Weight Gain and Hepatic Lipid Storage in Mice. Am. J. Physiol. Liver Physiol. 2018, 315, G932–G942. [Google Scholar] [CrossRef] [Green Version]
- Kentish, S.J.; Vincent, A.D.; Kennaway, D.J.; Wittert, G.A.; Page, A.J. High-Fat Diet-Induced Obesity Ablates Gastric Vagal Afferent Circadian Rhythms. J. Neurosci. Off. J. Soc. Neurosci. 2016, 36, 3199–3207. [Google Scholar] [CrossRef] [Green Version]
- Dokken, B.B.; Tsao, T.S. The Physiology of Body Weight Regulation: Are We Too Efficient for Our Own Good? Diabetes Spectr. 2007, 20, 166–170. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Guo, K.; Leblanc, R.E.; Loh, D.; Schwartz, G.J.; Yu, Y.H. Increasing Dietary Leucine Intake Reduces Diet-Induced Obesity and Improves Glucose and Cholesterol Metabolism in Mice via Multimechanisms. Diabetes 2007, 56, 1647–1654. [Google Scholar] [CrossRef] [Green Version]
- Doi, M.; Yamaoka, I.; Nakayama, M.; Sugahara, K.; Yoshizawa, F. Hypoglycemic Effect of Isoleucine Involves Increased Muscle Glucose Uptake and Whole Body Glucose Oxidation and Decreased Hepatic Gluconeogenesis. Am. J. Physiol. Metab. 2007, 292, E1683–E1693. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, F. New Therapeutic Strategy for Amino Acid Medicine: Notable Functions of Branched Chain Amino Acids as Biological Regulators. J. Pharmacol. Sci. 2012, 118, 149–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cummings, N.E.; Williams, E.M.; Kasza, I.; Konon, E.N.; Schaid, M.D.; Schmidt, B.A.; Poudel, C.; Sherman, D.S.; Yu, D.; Apelo, S.I.A.; et al. Restoration of metabolic health by decreased consumption of branched-chain amino acids. J. Physiol. 2017, 596, 623–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef] [Green Version]
- Nie, C.; He, T.; Zhang, W.-J.; Zhang, G.; Ma, X. Branched Chain Amino Acids: Beyond Nutrition Metabolism. Int. J. Mol. Sci. 2018, 19, 954. [Google Scholar] [CrossRef] [Green Version]
- Cummings, D.E.; Overduin, J. Gastrointestinal regulation of food intake. J. Clin. Investig. 2007, 117, 13–23. [Google Scholar] [CrossRef]
- Mihai, B.M.; Mihai, C.; Cijevschi-Prelipcean, C.; Grigorescu, E.D.; Dranga, M.; Drug, V.; Sporea, I.; Lăcătușu, C.M. Bidirectional Relationship between Gastric Emptying and Plasma Glucose Control in Normoglycemic Individuals and Diabetic Patients. J. Diabetes Res. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef] [Green Version]
- Uchida, M.; Kobayashi, O.; Iwasawa, K.; Shimizu, K. Effects of Straight Alkyl Chain, Extra Hydroxylated Alkyl Chain and Branched Chain Amino Acids on Gastric Emptying Evaluated Using a Non-Invasive Breath Test in Conscious Rats. J. Smooth Muscle Res. 2016, 52, 36–44. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Rielly, R.; Li, H.; Lim, S.M.; Yazbeck, R.; Kritas, S.; Ullrich, S.S.; Feinle-Bisset, C.; Heilbronn, L.; Page, A.J. The Effect of Isoleucine Supplementation on Body Weight Gain and Blood Glucose Response in Lean and Obese Mice. Nutrients 2020, 12, 2446. https://doi.org/10.3390/nu12082446
O’Rielly R, Li H, Lim SM, Yazbeck R, Kritas S, Ullrich SS, Feinle-Bisset C, Heilbronn L, Page AJ. The Effect of Isoleucine Supplementation on Body Weight Gain and Blood Glucose Response in Lean and Obese Mice. Nutrients. 2020; 12(8):2446. https://doi.org/10.3390/nu12082446
Chicago/Turabian StyleO’Rielly, Rebecca, Hui Li, See Meng Lim, Roger Yazbeck, Stamatiki Kritas, Sina S. Ullrich, Christine Feinle-Bisset, Leonie Heilbronn, and Amanda J. Page. 2020. "The Effect of Isoleucine Supplementation on Body Weight Gain and Blood Glucose Response in Lean and Obese Mice" Nutrients 12, no. 8: 2446. https://doi.org/10.3390/nu12082446





