High-Dose Vitamin D Supplementation Improves Microcirculation and Reduces Inflammation in Diabetic Neuropathy Patients
Abstract
1. Introduction
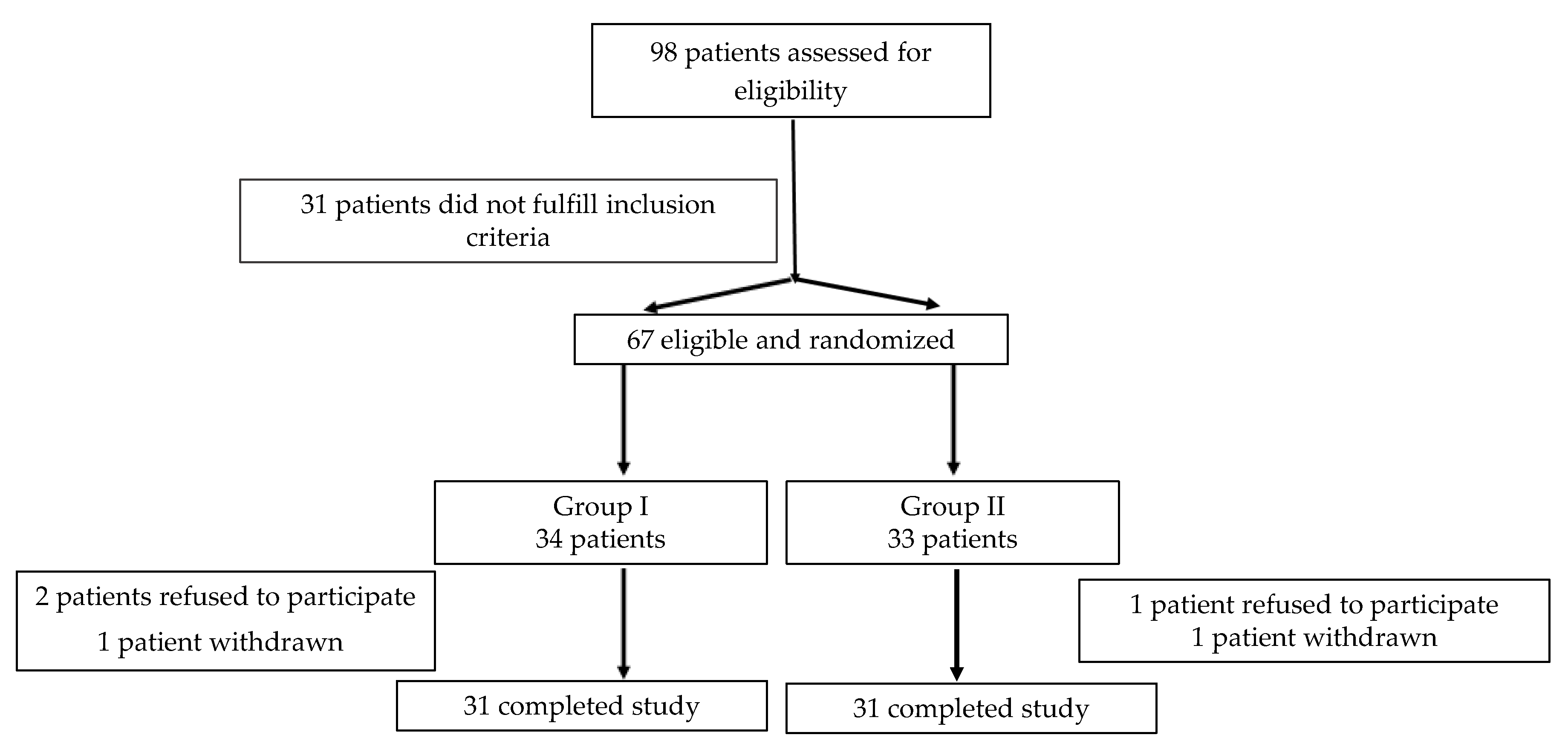
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Study Objective
2.4. Statistical Analysis
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pop-Busui, R.; Boulton, A.J.; Feldman, E.L.; Bril, V.; Freeman, R.; Malik, R.A.; Sosenko, J.M.; Ziegler, D. Diabetic neuropathy: A position statement by the American Diabetes Association. Diabetes Care 2017, 40, 136–154. [Google Scholar] [CrossRef]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Christian, M.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef]
- Qu, G.B.; Wang, L.L.; Tang, X.; Wu, W.; Sun, Y.H. The association between vitamin D level and diabetic peripheral neuropathy in patients with type 2 diabetes mellitus: An update systematic review and meta-analysis. J. Clin. Transl. Endocrinol. 2017, 9, 25–31. [Google Scholar] [CrossRef]
- Liu, E.; Meigs, J.B.; Pittas, A.G.; Economos, C.D.; McKeown, N.M.; Booth, S.L.; Jacques, P.F. Predicted 25-hydroxyvitamin D score and incident type 2 diabetes in the Framingham Offspring Study. Am. J. Clin. Nutr. 2010, 91, 1627–1633. [Google Scholar] [CrossRef]
- Gagnon, C.; Lu, Z.X.; Magliano, D.J.; Dunstan, D.W.; Shaw, J.E.; Zimmet, P.Z.; Sikaris, K.; Grantham, N.; Ebeling, P.R.; Daly, R.M. Serum 25-hydroxyvitamin D, calcium intake, and risk of type 2 diabetes after 5 years: Results from a national, population-based prospective study (the Australian Diabetes, Obesity and Lifestyle study). Diabetes Care 2011, 34, 1133–1138. [Google Scholar] [CrossRef]
- Moreira-Lucas, T.S.; Duncan, A.M.; Rabasa-Lhoret, R.; Vieth, R.; Gibbs, A.L.; Badawi, A.; Wolever, T.M. Effect of vitamin D supplementation on oral glucose tolerance in individuals with low vitamin D status and increased risk for developing type 2 diabetes (EVIDENCE): A double-blind, randomized, placebo-controlled clinical trial. Diabetes Obes. Metab. 2017, 19, 133–141. [Google Scholar] [CrossRef]
- Pittas, A.G.; Dawson-Hughes, B.; Sheehan, P.; Ware, J.H.; Knowler, W.C.; Aroda, V.R.; Brodsky, I.; Ceglia, L.; Chadha, C.; Chatterjeeet, R.; et al. Vitamin D Supplementation and Prevention of Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 520–530. [Google Scholar] [CrossRef]
- Davidson, M.B.; Duran, P.; Lee, M.L.; Friedman, T.C. High-Dose Vitamin D Supplementation in People with Prediabetes and Hypovitaminosis D. Diabetes Care 2012, 36, 260–266. [Google Scholar] [CrossRef]
- Muñoz-Garach, A.; García-Fontana, B.; Muñoz-Torres, M. Vitamin D Status, Calcium Intake and Risk of Developing Type 2 Diabetes: An Unresolved Issue. Nutrients 2019, 11, 642. [Google Scholar] [CrossRef]
- Jamwal, S.; Sharma, S. Vascular endothelium dysfunction: A conservative target in metabolic disorders. Inflamm. Res. 2018, 67, 391–405. [Google Scholar] [CrossRef]
- Magrinelli, F.; Briani, C.; Romano, M.; Ruggero, S.; Toffanin, E.; Triolo, G.; Peter, G.C.; Praitano, M.; Lauriola, F.; Zanette, G.; et al. The Association between Serum Cytokines and Damage to Large and Small Nerve Fibers in Diabetic Peripheral Neuropathy. J. Diabetes Res. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Fuchs, D.; Dupon, P.P.; Schaap, L.A.; Draijer, R. The association between diabetes and dermal microvascular dysfunction non-invasively assessed by laser Doppler with local thermal hyperemia: A systematic review with meta-analysis. Cardiovasc. Diabetol. 2017, 16. [Google Scholar] [CrossRef]
- Strain, W.D.; Paldanius, P.M. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc. Diabetol. 2018, 17, 57. [Google Scholar] [CrossRef]
- Pop-Busui, R.; Ang, L.; Holmes, C.; Gallagher, K.; Feldman, E.L. Inflammation as a Therapeutic Target for Diabetic Neuropathies. Curr. Diabetes Rep. 2016, 16, 29. [Google Scholar] [CrossRef]
- Alamdari, A.; Mozafari, R.; Tafakhori, A.; Faghihi-Kashani, S.; Hafezi-Nejad, N.; Sheikhbahaei, S.; Naderi, N.; Ebadi, M.; Esteghamati, A. An inverse association between serum vitamin D levels with the presence and severity of impaired nerve conduction velocity and large fiber peripheral neuropathy in diabetic subjects. Neurol. Sci. 2015, 36, 1121–1126. [Google Scholar] [CrossRef]
- He, R.; Hu, Y.; Zeng, H.; Zhao, J.; Zhao, J.; Chai, Y.; Lu, F.; Liu, F.; Jia, W. Vitamin D deficiency increases the risk of peripheral neuropathy in Chinese patients with Type 2 diabetes. Diabetes Metab. Res. Rev. 2017, 33, 2820. [Google Scholar] [CrossRef]
- Celikbilek, A.; Gocmen, A.Y.; Tanik, N.; Borekci, E.; Adam, M.; Celikbilek, M.; Suher, M.; Delibas, N. Decreased serum vitamin D levels are associated with diabetic peripheral neuropathy in a rural area of Turkey. Acta Neurol. Belg. 2015, 115, 47–52. [Google Scholar] [CrossRef]
- Straube, S.; Derry, S.; Straube, C.; Moore, R.A. Vitamin D for the treatment of chronic painful conditions in adults. Cochrane Database Syst. Rev. 2015, 5. [Google Scholar] [CrossRef]
- Bilir, B.; Tulubas, F.; Bilir, B.E.; Atile, N.S.; Kara, S.P.; Yildirim, T.; Gumustas, S.A.; Topcu, B.; Kaymaz, O.; Aydin, M. The association of vitamin D with inflammatory cytokines in diabetic peripheral neuropathy. J. Phys. Ther. Sci. 2016, 28, 2159–2163. [Google Scholar] [CrossRef]
- Randhawa, F.A.; Mustafa, S.; Khan, D.M.; Hamid, S. Effect of Vitamin D supplementation on reduction in levels of HbA1 in patients recently diagnosed with type 2 Diabetes Mellitus having asymptomatic Vitamin D deficiency. Pak. J. Med. Sci. 2017, 33, 881–885. [Google Scholar] [CrossRef]
- Basit, A.; Basit, K.A.; Fawwad, A.; Shaheen, F.; Fatima, N.; Petropoulos, I.N.; Alam, U.; Malik, R.A. Vitamin D for the treatment of painful diabetic neuropathy. BMJ Open Diabetes Res. Care 2016, 4, e000148. [Google Scholar] [CrossRef] [PubMed]
- Grant, I.A.; O’Brien, P.; Dyck, P.J. Neuropathy tests and normative results. In Diabetic Neuropathy, 2nd ed.; Dyck, P.J., Thomas, P.K., Eds.; Saunders: Philadelphia, PA, USA, 1999; pp. 123–141. [Google Scholar]
- Gries, A.; Cameron, N.E. Severity and Staging of Diabetic Polyneuropathy. Textb. Diabet. Neuropathy 2003. [CrossRef]
- Hawker, G.A.; Mian, S.; Kendzerska, T.; French, M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011, 63, 240–252. [Google Scholar] [CrossRef]
- Pigarova, E.A.; Rozhinskaia, L.Y.; Belaia, J.E.; Dzeranova, L.K.; Karonova, T.L.; Ilyin, A.V.; Melnichenko, G.A.; Dedov, I.I. Russian Association of Endocrinologists recommendations for diagnosis, treatment and prevention of vitamin D deficiency in adults. Probl. Endocrinol. 2016, 62, 60–84. [Google Scholar] [CrossRef]
- Karnafel, W.; Juskowa, J.; Maniewski, R.; Liebert, A.; Jasik, M.; Zbieć, A. Microcirculation in the diabetic foot as measured by a multichannel laser Doppler instrument. Med. Sci. Monit. 2002, 8, MT137–MT144. [Google Scholar]
- Karonova, T.; Andreeva, A.; Nikitina, I.; Belyaeva, O.; Mokhova, E.; Galkina, O.; Vasilyeva, E.; Grineva, E. Prevalence of Vitamin D deficiency in the North-West region of Russia: A cross-sectional study. J. Steroid Biochem. Mol. Biol. 2016, 230–234. [Google Scholar] [CrossRef]
- Maddaloni, E.; Cavallari, I.; Napoli, N.; Conte, C. Vitamin D and Diabetes Mellitus. Vitam. D Clin. Med. 2018, 50, 161–176. [Google Scholar] [CrossRef]
- Lips, P.; Eekhoff, M.; van Schoor, N.; Oosterwerff, M.; de Jongh, R.; Krul-Poel, Y.; Simsek, S. Vitamin D and type 2 diabetes. J. Steroid Biochem. Mol. Biol. 2017, 173, 280–285. [Google Scholar] [CrossRef]
- Pludowski, P.; Karczmarewicz, E.; Bayer, M.; Carter, G.; Chlebna-Sokół, D.; Czech-Kowalska, J.; Dębski, R.; Decsi, T.; Dobrzańska, A.; Franek, E. Practical guidelines for the supplementation of vitamin D and the treatment of deficits in Central Europe-recommended vitamin D intakes in the general population and groups at risk of vitamin D deficiency. Endokrynol. Pol. 2013, 64, 319–327. [Google Scholar] [CrossRef]
- Earthman, C.P.; Beckman, L.M.; Masodkar, K.; Sibley, S.D. The link between obesity and low circulating 25-hydroxyvitamin D concentrations: Considerations and implications. Int. J. Obes. 2012, 36, 387–396. [Google Scholar] [CrossRef]
- Chapman, D.; Foxcroft, R.; Dale-Harris, L.; Ronte, H.; Bidgoli, F.; Bellary, S. Insights for Care: The Healthcare Utilisation and Cost Impact of Managing Type 2 Diabetes-Associated Microvascular Complications. Diabetes Ther. 2019, 10, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Calton, E.K.; Keane, K.N.; Newsholme, P.; Soares, M.J. The Impact of Vitamin D Levels on Inflammatory Status: A Systematic Review of Immune Cell Studies. PLoS ONE 2015, 10, e0141770. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, A.P.; Karonova, T.L.; Bystrova, A.A.; Bregovsky, V.B. Role of vitamin D deficiency in type 2 diabetes mellitus and diabetic neuropathy development. Diabetes Mellitus. 2018, 21, 301–306. (In Russian) [Google Scholar] [CrossRef]
- Zeng, J.; Xu, Y.; Shi, Y.; Jiang, C. Inflammation role in sensory neuropathy in Chinese patients with diabetes/prediabetes. Clin. Neurol. Neurosurg. 2018, 166, 136–140. [Google Scholar] [CrossRef]
- Mazidi, M.; Toth, P.P.; Banach, M. C-reactive Protein Is Associated with Prevalence of the Metabolic Syndrome, Hypertension, and Diabetes Mellitus in US Adults. Angiology 2017, 69, 438–442. [Google Scholar] [CrossRef]
- Salekzamani, S.; Bavil, A.S.; Mehralizadeh, H.; Jafarabadi, M.A.; Ghezel, A.; Gargari, B.P. The effects of vitamin D supplementation on proatherogenic inflammatory markers and carotid intima media thickness in subjects with metabolic syndrome: A randomized double-blind placebo-controlled clinical trial. Endocrine 2017, 57, 51–59. [Google Scholar] [CrossRef]
- Jackson, J.L.; Judd, S.E.; Panwar, B.; Howard, V.J.; Wadley, V.G.; Jenny, N.S.; Guti, O.M. Associations of 25-hydroxyvitamin D with markers of inflammation, insulin resistance and obesity in black and white community-dwelling adults. J. Clin. Transl. Endocrinol. 2016, 5, 21–25. [Google Scholar] [CrossRef][Green Version]
- Mousa, A.; Naderpoor, N.; Teede, H.; Scragg, R.; de Courten, B. Vitamin D supplementation for improvement of chronic low-grade inflammation in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2018, 76, 380–394. [Google Scholar] [CrossRef]
- Colotta, F.; Jansson, B.; Bonelli, F. Modulation of inflammatory and immune responses by vitamin D. J. Autoimmun. 2017, 85, 78–97. [Google Scholar] [CrossRef]
- Vitamin D Attenuates Endothelial Dysfunction in Uremic Rats and Maintains Human Endothelial Stability. J. Am. Heart Assoc. 2018, 7, e02709. [CrossRef]
- Gil, Á.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and Novel Actions. Ann. Nutr. Metab. 2018, 72, 87–95. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | 5000 IU/Week, n = 31 (Group I) | 40,000 IU/Week, n = 31 (Group II) | p |
|---|---|---|---|
| Males, n (%)/Females, n (%) | 15 (48.4)/16 (51.6) | 16 (51.6)/15 (48.4) | 0.800 |
| Age, years | 57 (48; 62) | 55 (52; 60) | 0.756 |
| Body mass index, kg/m2 | 30 (28.3; 31.8) | 31 (29.5; 32.)7 | 0.155 |
| Obesity, n (%) | 21 (68) | 20 (65) | 0.789 |
| Duration of type 2 diabetes, years | 6 (5; 8.5) | 7 (5; 11) | 0.733 |
| Diabetic peripheral neuropathy, n (%) | 31 (100) | 31 (100) | 1.000 |
| Neuropathic symptomatic score, points | 5 (4; 6) | 5 (4; 6) | 0.799 |
| Neuropathic dysfunctional score, points | 8 (7; 9) | 8 (7; 9) | 0.857 |
| Visual analog scale, mm | 50 (40; 60) | 50 (42.5; 55) | 0.744 |
| Diabetic retinopathy, n (%) | 21(68) | 24(77) | 0.394 |
| Diabetic nephropathy, n (%) | 11(35) | 9(29) | 0.584 |
| Arterial hypertension, n (%) | 23 (74) | 25 (81) | 0.544 |
| Coronary heart disease, n (%) | 17 (55) | 15 (48) | 0.701 |
| Insulin, n (%) | 11 (35) | 9 (29) | 0.587 |
| Metformin, n (%) | 29 (94) | 25 (81) | 0.130 |
| Sulfonylureas, n (%) | 4 (13) | 5 (16) | 0.719 |
| DPP-4 inhibitors, n (%) | 5(16) | 5(16) | 1.000 |
| SGLT-2 inhibitors, n (%) | 1 (3) | 3(10) | 0.302 |
| GLP-1R agonists, n (%) | - | 1(3) | 0.314 |
| ACE inhibitors/ARB, n (%) | 23 (74) | 25 (81) | 0.544 |
| Calcium channel blockers, n (%) | 5 (16) | 7 (22) | 0.521 |
| β-adrenergic receptor blockers, n (%) | 21 (68) | 23 (74) | 0.576 |
| Diuretics, n (%) | 14 (45) | 11 (35) | 0.438 |
| Statins, n (%) | 15 (48) | 16 (52) | 0.800 |
| Parameters | 5000 IU/Week, n = 31 (Group I) | 40,000 IU/Week, n = 31 (Group II) | ||
|---|---|---|---|---|
| Baseline | After 24 Weeks | Baseline | After 24 Weeks | |
| BMI, kg/m2 | 30 (28.3; 31.8) | 30 (28.4; 31.8) | 31 (29.5; 32.7) | 28,7 (25.4; 30.4) **,# |
| 25(OH)D, ng/mL | 18.8 (10.7; 27.4) | 26.9 (20; 34.6) * | 16.2 (8.7; 25.3) | 71.6 (54.8; 88.3) **,## |
| HbA1c, % | 7.9 (7.1; 8.3) | 7.9 (7.2; 8.4) | 7.9 (7.1; 8.5) | 7.4 (6.5; 7.7) *,# |
| PTH, pg/mL | 34.5 (24.3; 45.7) | 28.6 (23.4; 40.4) | 32.8 (23.5; 45.2) | 26.6 (19.2; 34.6) |
| TC, mmol/L | 4.9 (4.1; 6.1) | 5.3 (4.1; 6.3) | 5.5 (4.5; 6.5) | 5.4 (4.7; 6.1) |
| TNFα pg/mL | 2.0 (2.0; 2.0) | 2.0 (2.0; 2.0) | 2.0 (2.0; 2.0) | 2.0 (2.0; 2.0) |
| CRP ml/L | 1.4 (0.7; 2.0) | 1.4 (0.8; 2.1) | 1.5 (1.1; 2.0) | 2.0 (0.8; 3.0) |
| IL-1β pg/mL | 1.0 (1.0; 1.0) | 1.0 (1.0; 1.0) | 1.0 (1.0; 1.0) | 1.0 (1.0; 1.0) |
| IL-6 pg/mL | 1.9 (1.3; 3.1) | 2.3 (1.3; 3.1) | 2.5 (1.5; 4.1) | 0.6 (0.5; 0.8) **,## |
| IL-10 pg/mL | 3.3 (2.5; 4.8) | 3.5 (2.5; 5.0) | 2.5 (2.5; 3.6) | 4.5 (3.5; 5.7) **,# |
| Parameters | 5000 IU/Week, n = 31 (Group I) | 40,000 IU/Week, n = 31 (Group II) | ||
|---|---|---|---|---|
| Baseline | After 24 Weeks | Baseline | After 24 Weeks | |
| M, pf units | 7.41 ± 3.97 | 7.16 ± 4.26 # | 6.01 ± 1.89 | 7.01 ± 2.46 *,# |
| σ, pf units | 1.11 ± 0.57 | 1.05 ± 0.56 # | 0.85 ± 0.57 | 1.81 ± 1.14 *,# |
| Kν *, % | 17.68 ± 10.14 | 18.89 ± 10.83 # | 16.65 ± 10.99 | 27.96 ± 16.38 *,# |
| Δ Kν, % | +6.8% | +68.3% | ||
| Postural Test | ||||
| Mbase, pf unit | 7.75 ± 1.8 | 7.78 ± 2.3 # | 6.69 ± 1.51 | 7.97 ± 2.13 *,# |
| Mmin, pf unit | 6.10 ± 1.52 | 6.13 ± 2.26 # | 5.36 ± 1.47 | 5.07 ± 1.72 *,# |
| DDB, % | 24.82 ± 9.27 | 23.87 ± 9.1 # | 23.4 ± 12.68 | 51.88 ± 36.71 **,# |
| Δ DDB, % | −3.8% | +121.7% | ||
| Occlusal Test | ||||
| Mbase pf unit | 7.10 ± 1.72 | 6.74 ± 1.75 # | 6.49 ± 2.10 | 7.54 ± 2.89 *,# |
| Mmax pf unit | 9.73 ± 2.25 | 8.97 ± 3.60 # | 9.59 ± 3.15 | 14.57 ± 3.63 *,# |
| RCB, % | 40.85 ± 20.31 | 35.79 ± 17.10 # | 48.57 ± 18.56 | 106.8 ± 44.8 **,# |
| Δ RCB, % | −12.4% | +120% | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karonova, T.; Stepanova, A.; Bystrova, A.; Jude, E.B. High-Dose Vitamin D Supplementation Improves Microcirculation and Reduces Inflammation in Diabetic Neuropathy Patients. Nutrients 2020, 12, 2518. https://doi.org/10.3390/nu12092518
Karonova T, Stepanova A, Bystrova A, Jude EB. High-Dose Vitamin D Supplementation Improves Microcirculation and Reduces Inflammation in Diabetic Neuropathy Patients. Nutrients. 2020; 12(9):2518. https://doi.org/10.3390/nu12092518
Chicago/Turabian StyleKaronova, Tatiana, Anna Stepanova, Anna Bystrova, and Edward B. Jude. 2020. "High-Dose Vitamin D Supplementation Improves Microcirculation and Reduces Inflammation in Diabetic Neuropathy Patients" Nutrients 12, no. 9: 2518. https://doi.org/10.3390/nu12092518
APA StyleKaronova, T., Stepanova, A., Bystrova, A., & Jude, E. B. (2020). High-Dose Vitamin D Supplementation Improves Microcirculation and Reduces Inflammation in Diabetic Neuropathy Patients. Nutrients, 12(9), 2518. https://doi.org/10.3390/nu12092518





