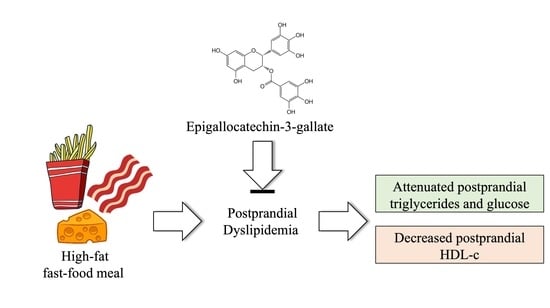
Acute Epigallocatechin-3-Gallate Supplementation Alters Postprandial Lipids after a Fast-Food Meal in Healthy Young Women: A Randomized, Double-Blind, Placebo-Controlled Crossover Study
Abstract
:1. Introduction
2. Materials and Methods
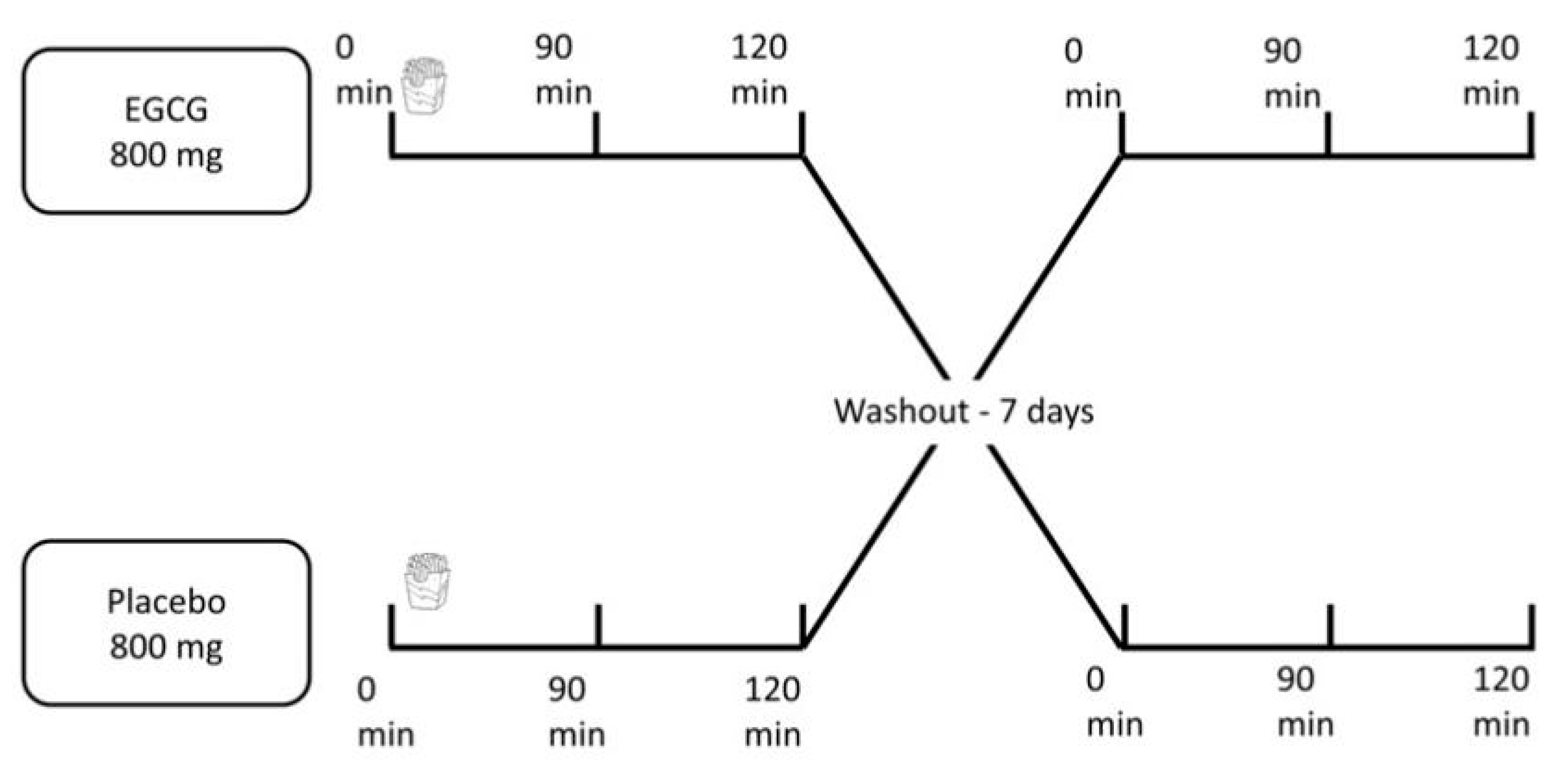
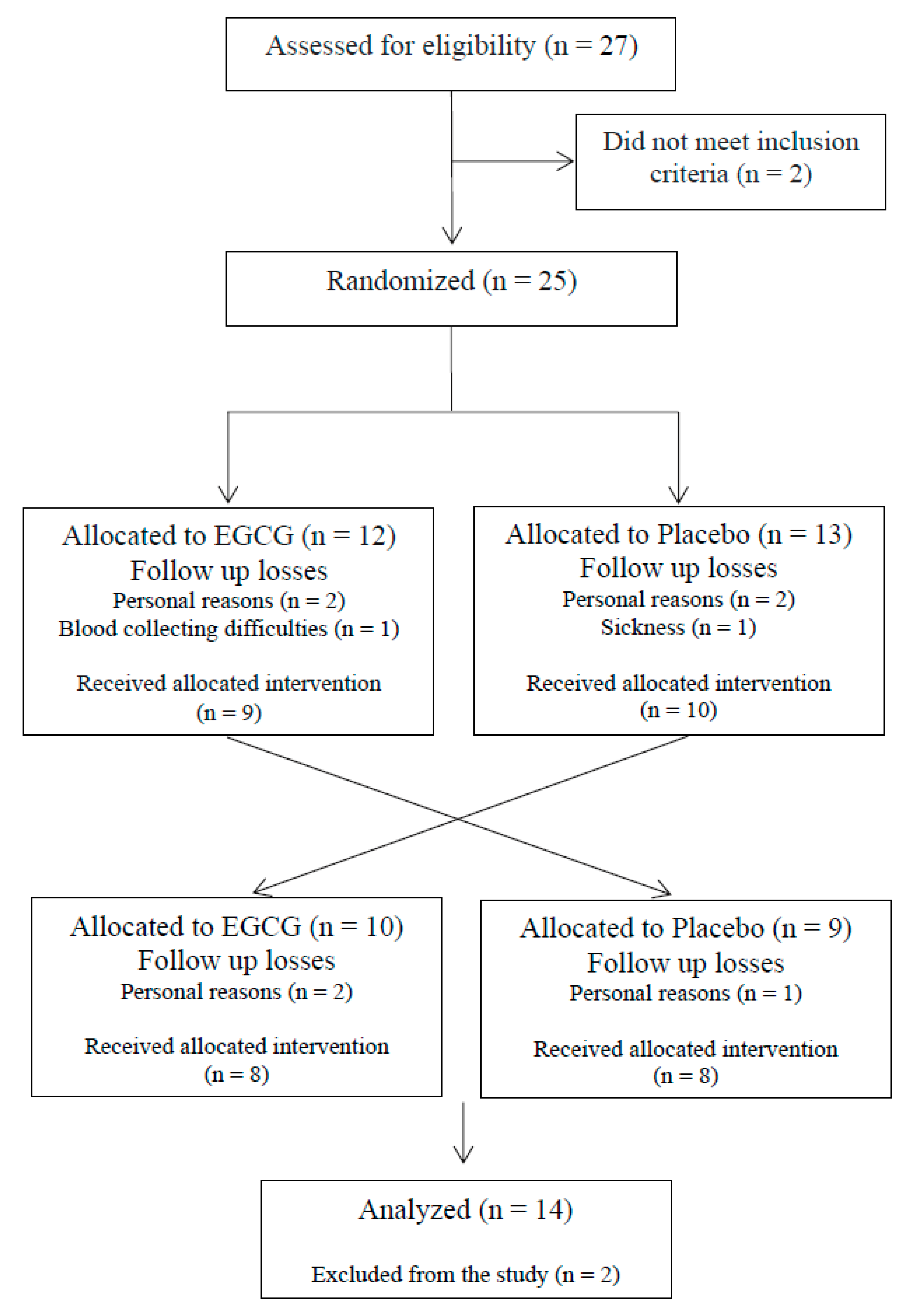
2.1. Study Design and Participants
2.2. Experimental Protocol
2.3. Anthropometric and Body Composition Assessments
2.4. Analyses of Samples
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bahadoran, Z.; Mirmiran, P.; Azizi, F. Fast Food Pattern and Cardiometabolic Disorders: A Review of Current Studies. Heal. Promot. Perspect. 2015, 5, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Janssen, H.G.; Davies, I.G.; Richardson, L.D.; Stevenson, L. Determinants of takeaway and fast food consumption: A narrative review. Nutr. Res. Rev. 2018, 31, 16–34. [Google Scholar] [CrossRef]
- Devaraj, S.; Wang-Polagruto, J.; Polagruto, J.; Keen, C.L.; Jialal, I. High-fat, energy-dense, fast-food-style breakfast results in an increase in oxidative stress in metabolic syndrome. Metabolism 2008, 57, 867–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudolph, T.K.; Ruempler, K.; Schwedhelm, E.; Tan-Andresen, J.; Riederer, U.; Böger, R.H.; Maas, R. Acute effects of various fast-food meals on vascular function and cardiovascular disease risk markers: The Hamburg Burger Trial 1-3. Am. J. Clin. Nutr. 2007, 86, 334–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.-J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1025–1032. [Google Scholar]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef]
- Chen, I.-J.; Liu, C.-Y.; Chiu, J.-P.; Hsu, C.-H. Therapeutic effect of high-dose green tea extract on weight reduction: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. 2015, 35, 592–599. [Google Scholar] [CrossRef]
- Fernandes, R.; Araújo, V.; Giglio, B.; Marini, A.; Mota, J.; Teixeira, K.-I.-S.; Monteiro, P.; Lira, F.; Pimentel, G. Acute Epigallocatechin 3 Gallate (EGCG) Supplementation Delays Gastric Emptying in Healthy Women: A Randomized, Double-Blind, Placebo-Controlled Crossover Study. Nutrients 2018, 10, 1122. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-K.; Cheung, C.; Reuhl, K.R.; Liu, A.B.; Lee, M.-J.; Lu, Y.-P.; Yang, C.S. Effects of Green Tea Polyphenol (-)-Epigallocatechin-3-gallate on a Newly Developed High-fat/Western-style Diet-induced Obesity and Metabolic Syndrome in Mice. J. Agric. Food Chem. 2011, 59, 11862–11871. [Google Scholar] [CrossRef] [Green Version]
- Bhagwat, S.; Haytowitz, D.B. USDA Database for the Flavonoid Content of Selected Foods Release 3.2; Agricultural Research Service: Beltsville, MD, USA, 2015. [Google Scholar]
- Ferreira, M.A.; Silva, D.M.; de Morais, A.C.; Mota, J.F.; Botelho, P.B. Therapeutic potential of green tea on risk factors for type 2 diabetes in obese adults—A review. Obes. Rev. 2016, 17, 1316–1328. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.H.S.; Cai, Y.; Alberts, D.S.; Hakim, I.; Dorr, R.; Shahi, F.; Crowell, J.A.; Yang, C.S.; Hara, Y. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and Polyphenon E. Cancer Epidemiol. Biomark. Prev. 2001, 10, 53–58. [Google Scholar]
- Brown, A.L.; Lane, J.; Coverly, J.; Stocks, J.; Jackson, S.; Stephen, A.; Bluck, L.; Coward, A.; Hendrickx, H. Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: Randomized controlled trial. Br. J. Nutr. 2017, 101, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Lohman, T.G.; Roche, A.F.; Martorell, R. (Eds.) Anthropometric Standardization Reference Manual, 1st ed.; Human Kinetics Books: Champaign, IL, USA, 1988. [Google Scholar]
- Martin, S.S.; Blaha, M.J.; Elshazly, M.B.; Toth, P.P.; Kwiterovich, P.O.; Blumenthal, R.S.; Jones, S.R. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA J. Am. Med. Assoc. 2013, 310, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Kriketos, A.D.; Sam, W.; Schubert, T.; Maclean, E.; Campbell, L.V. Postprandial triglycerides in response to high fat: Role of dietary carbohydrate. Eur. J. Clin. Investig. 2003, 33, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Rosner, B. Fundamentals of Biostatistics, 7th ed.; Molly, T., Daniel, S., Walsh, S., Eds.; Brooks/Cole: Boston, MA, USA, 2011. [Google Scholar]
- Pruessner, J.C.; Kirschbaum, C.; Meinlschmid, G.; Hellhammer, D.H. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 2003, 28, 916–931. [Google Scholar] [CrossRef]
- Larson, N.; Neumark-Sztainer, D.; Laska, M.N.; Story, M. Young adults and eating away from home: Associations with dietary intake patterns and weight status differ by choice of restaurant. J. Am. Diet. Assoc. 2011, 111, 1696–1703. [Google Scholar] [CrossRef] [Green Version]
- Rivellese, A.A.; Bozzetto, L.; Annuzzi, G. Postprandial lipemia, diet, and cardiovascular risk. Curr. Cardiovasc. Risk Rep. 2009, 3, 5–11. [Google Scholar] [CrossRef]
- Kolovou, G.D.; Mikhailidis, D.P.; Kovar, J.; Lairon, D.; Nordestgaard, B.G.; Chye Ooi, T.; Perez-Martinez, P.; Bilianou, H.; Anagnostopoulou, K.; Panotopoulos, G. Assessment and Clinical Relevance of Non-Fasting and Postprandial Triglycerides: An Expert Panel Statement. Curr. Vasc. Pharmacol. 2011, 9, 258–270. [Google Scholar] [CrossRef]
- Unno, T.; Tago, M.; Suzuki, Y.; Nozawa, A.; Sagesaka, Y.M.; Kakuda, T.; Egawa, K.; Kondo, K. Effect of tea catechins on postprandial plasma lipid responses in human subjects. Br. J. Nutr. 2005, 93, 543–547. [Google Scholar] [CrossRef] [Green Version]
- Ebenbichler, C.F.; Kirchmair, R.; Egger, C.; Patsch, J.R. Postprandial state and atherosclerosis. Curr. Opin. Lipidol. 1995, 6, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Stampfer, M.J. A Prospective Study of Triglyceride Level, Low-Density Lipoprotein Particle Diameter, and Risk of Myocardial Infarction. JAMA J. Am. Med. Assoc. 1996, 276, 882. [Google Scholar] [CrossRef]
- Friedrich, M.; Petzke, K.; Raederstorff, D.; Wolfram, S.; Klaus, S. Acute effects of epigallocatechin gallate from green tea on oxidation and tissue incorporation of dietary lipids in mice fed a high-fat diet. Int. J. Obes. 2012, 36136, 735–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suliburska, J.; Bogdanski, P.; Szulinska, M.; Stepien, M.; Pupek-Musialik, D.; Jablecka, A. Effects of green tea supplementation on elements, total antioxidants, lipids, and glucose values in the serum of obese patients. Biol. Trace Elem. Res. 2012, 149, 315–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves Ferreira, M.; Oliveira Gomes, A.P.; Guimarães de Moraes, A.P.; Ferreira Stringhini, M.L.; Mota, J.F.; Siqueira Guedes Coelho, A.; Borges Botelho, P. Green tea extract outperforms metformin in lipid profile and glycaemic control in overweight women: A double-blind, placebo-controlled, randomized trial. Clin. Nutr. ESPEN 2017, 22, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Skoczyńska, A.; Turczyn, B.; Wojakowska, A.; Kreczyńska, B.; Skoczyńska, M.; Wojtas, K. Postprandial decrease in LDL-cholesterol in men with metabolic syndrome. Open Med. 2015, 10, 138–151. [Google Scholar] [CrossRef]
- Huang, J.; Feng, S.; Liu, A.; Dai, Z.; Wang, H.; Reuhl, K.; Lu, W.; Yang, C.S. Green Tea Polyphenol EGCG Alleviates Metabolic Abnormality and Fatty Liver by Decreasing Bile Acid and Lipid Absorption in Mice. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef]
- Molinaro, A.; Wahlström, A.; Marschall, H.U. Role of Bile Acids in Metabolic Control. Trends Endocrinol. Metab. 2017, 29, 31–41. [Google Scholar] [CrossRef]
- Patsch, J.R.; Miesenböck, G.; Hopferwieser, T.; Mühlberger, V.; Knapp, E.; Kay Dunn, J.; Gotto, A.M.; Patsch, W. Relation of triglyceride metabolism and coronary artery disease—Studies in the postprandial state. Arterioscler. Thromb. Vasc. Biol. 1992, 12, 1336–1345. [Google Scholar] [CrossRef] [Green Version]
- Livesey, G.; Taylor, R.; Hulshof, T.; Howlett, J. Glycemic response and health-a systematic review and meta-analysis: Relations between dietary glycemic properties and health outcomes 1–5. Am. J. Clin. Nutr. 2008, 87, 223S–236S. [Google Scholar] [CrossRef] [Green Version]
- Venables, M.C.; Hulston, C.J.; Cox, H.R.; Jeukendrup, A.E. Green tea extract ingestion, fat oxidation, and glucose tolerance in healthy humans. Am. J. Clin. Nutr. 2008, 87, 778–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuneki, H.; Ishizuka, M.; Terasawa, M.; Wu, J.-B.; Sasaoka, T.; Kimura, I. Effect of green tea on blood glucose levels and serum proteomic patterns in diabetic (db/db) mice and on glucose metabolism in healthy humans. BMC Pharmacol. 2004, 4, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balentine, D.A.; Wiseman, S.A.; Bouwens, L.C.M. The chemistry of tea flavonoids. Crit. Rev. Food Sci. Nutr. 1997, 37, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F. Antioxidants in food and food antioxidants. Food Nahr. 2000, 44, 158–163. [Google Scholar] [CrossRef]
| Variables | Mean ± SEM n = 14 |
|---|---|
| Anthropometry and Body Composition | |
| Height (m) | 1.6 ± 0.02 |
| Weight (kg) | 54.9 ± 1.21 |
| BMI (kg/m2) | 21.4 ± 0.41 |
| Waist circumference (cm) | 67.5 ± 0.91 |
| Lean mass (kg) | 34.4 ± 0.99 |
| Fat mass (kg) | 16.8 ± 0.87 |
| Fat mass (%) | 32.8 ± 1.42 |
| Food Intake | |
| Calories (kcal) | 1619 ± 138 |
| Carbohydrate (g) | 200 ± 22.8 |
| Carbohydrate (%) | 49.1 ± 2.33 |
| Protein (g) | 79.4 ± 7.97 |
| Protein (%) | 22.9 ± 2.83 |
| Fat (g) | 49.3 ± 4.55 |
| Fat (%) | 27.98 ± 2.02 |
| Cholesterol (mg) | 460 ± 57.7 |
| Total fiber (g) | 16.1 ± 2.52 |
| Biochemical analyzes | |
| Total cholesterol (mg/dL) | 152 ± 7.12 |
| HDL-c (mg/dL) | 59 ± 3.28 |
| LDL-c (mg/dL) | 76.8 ± 6.4 |
| Triglycerides (mg/dL) | 83.3 ± 7.17 |
| VLDL-c (mg/dL) | 16.6 ± 1.19 |
| Blood glucose (mg/dL) | 89.5 ± 0.89 |
| Insulin (µU/mL) | 7.89 ± 0.81 |
| HOMA-IR | 1.74 ± 0.18 |
| Placebo | EGCG | |
|---|---|---|
| Total cholesterol at baseline (mg/dL) | 152 ± 21.2 | 151 ± 5.88 |
| Δ 90 | −2.93 ± 1.34 | −4.07 ± 1.65 |
| Δ 120 | −7.86 ± 1.73 | −11.14 ± 1.69 |
| iAUC | −293 ± 99.2 | −411 ± 115 |
| HDL-c at baseline (mg/dL) | 59.3 ± 3.17 | 60 ± 3.44 |
| Δ 90 | −2.64 ± 0.47 | −3.64 ± 0.68 |
| Δ 120 | −4.50 ± 0.44 | −6.5 ± 0.72 * |
| iAUC | −226 ± 32.0 | −316 ± 48.3 † |
| LDL-c at baseline (mg/dL) | 76.2 ± 6.87 | 73.0 ± 6.14 |
| Δ 90 | −9.36 ± 1.37 | −8.77 ± 1.13 |
| Δ 120 | −12.5 ± 1.61 | −11.9 ± 1.40 |
| iAUC | −749 ± 103 | −704 ± 78.1 |
| Triglycerides at baseline (mg/dL) | 84.2 ± 7.57 | 89.9 ± 9.21 |
| Δ 90 | 45.4 ± 5.92 | 41.7 ± 7.49 |
| Δ 120 | 45.9 ± 6.46 | 36.1 ± 6.25 * |
| iAUC | 3409 ± 446 | 3045 ± 518 |
| VLDL-c at baseline (mg/dL) | 16.8 ± 1.51 | 18.0 ± 1.84 |
| Δ 90 | 9.07 ± 1.18 | 8.34 ± 1.50 |
| Δ 120 | 9.17 ± 1.42 | 7.23 ± 1.16 * |
| iAUC | 681 ± 89.4 | 609 ± 103 |
| Blood glucose at baseline (mg/dL) | 88.4 ± 1.19 | 90.8 ± 1.27 |
| Δ 90 | −0.86 ± 1.31 | −5.78 ± 1.89 † |
| Δ 120 | 2.57 ± 1.59 | −0.07 ± 1.58 † |
| iAUC | −12.86 ± 94.6 | −348 ± 122 * |
| Insulin at baseline (µU/mL) | 6.59 ± 0.77 | 9.59 ± 0.99 * |
| Δ 90 | 15.4 ± 2.83 | 10.5 ± 2.63 |
| Δ 120 | 17.9 ± 3.45 | 13.6 ± 2.85 |
| iAUC | 1191 ± 214 | 835 ± 192 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Morais Junior, A.C.; Schincaglia, R.M.; Passarelli, M.; Pimentel, G.D.; Mota, J.F. Acute Epigallocatechin-3-Gallate Supplementation Alters Postprandial Lipids after a Fast-Food Meal in Healthy Young Women: A Randomized, Double-Blind, Placebo-Controlled Crossover Study. Nutrients 2020, 12, 2533. https://doi.org/10.3390/nu12092533
de Morais Junior AC, Schincaglia RM, Passarelli M, Pimentel GD, Mota JF. Acute Epigallocatechin-3-Gallate Supplementation Alters Postprandial Lipids after a Fast-Food Meal in Healthy Young Women: A Randomized, Double-Blind, Placebo-Controlled Crossover Study. Nutrients. 2020; 12(9):2533. https://doi.org/10.3390/nu12092533
Chicago/Turabian Stylede Morais Junior, Alcides C., Raquel M. Schincaglia, Marisa Passarelli, Gustavo D. Pimentel, and João F. Mota. 2020. "Acute Epigallocatechin-3-Gallate Supplementation Alters Postprandial Lipids after a Fast-Food Meal in Healthy Young Women: A Randomized, Double-Blind, Placebo-Controlled Crossover Study" Nutrients 12, no. 9: 2533. https://doi.org/10.3390/nu12092533
APA Stylede Morais Junior, A. C., Schincaglia, R. M., Passarelli, M., Pimentel, G. D., & Mota, J. F. (2020). Acute Epigallocatechin-3-Gallate Supplementation Alters Postprandial Lipids after a Fast-Food Meal in Healthy Young Women: A Randomized, Double-Blind, Placebo-Controlled Crossover Study. Nutrients, 12(9), 2533. https://doi.org/10.3390/nu12092533