Nutrient Intake through Childhood and Early Menarche Onset in Girls: Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.1.1. Definitions of EMO and Non-EMO (NEMO)
2.1.2. Inclusion and Exclusion Criteria
2.2. Search Strategy
2.3. Data Extraction
2.4. Quality Assessment of Evidence
2.5. Data Analysis and Statistical Methods
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Quality Assessment
3.4. Meta-Analysis, Publication Bias, and Sensitivity Analysis
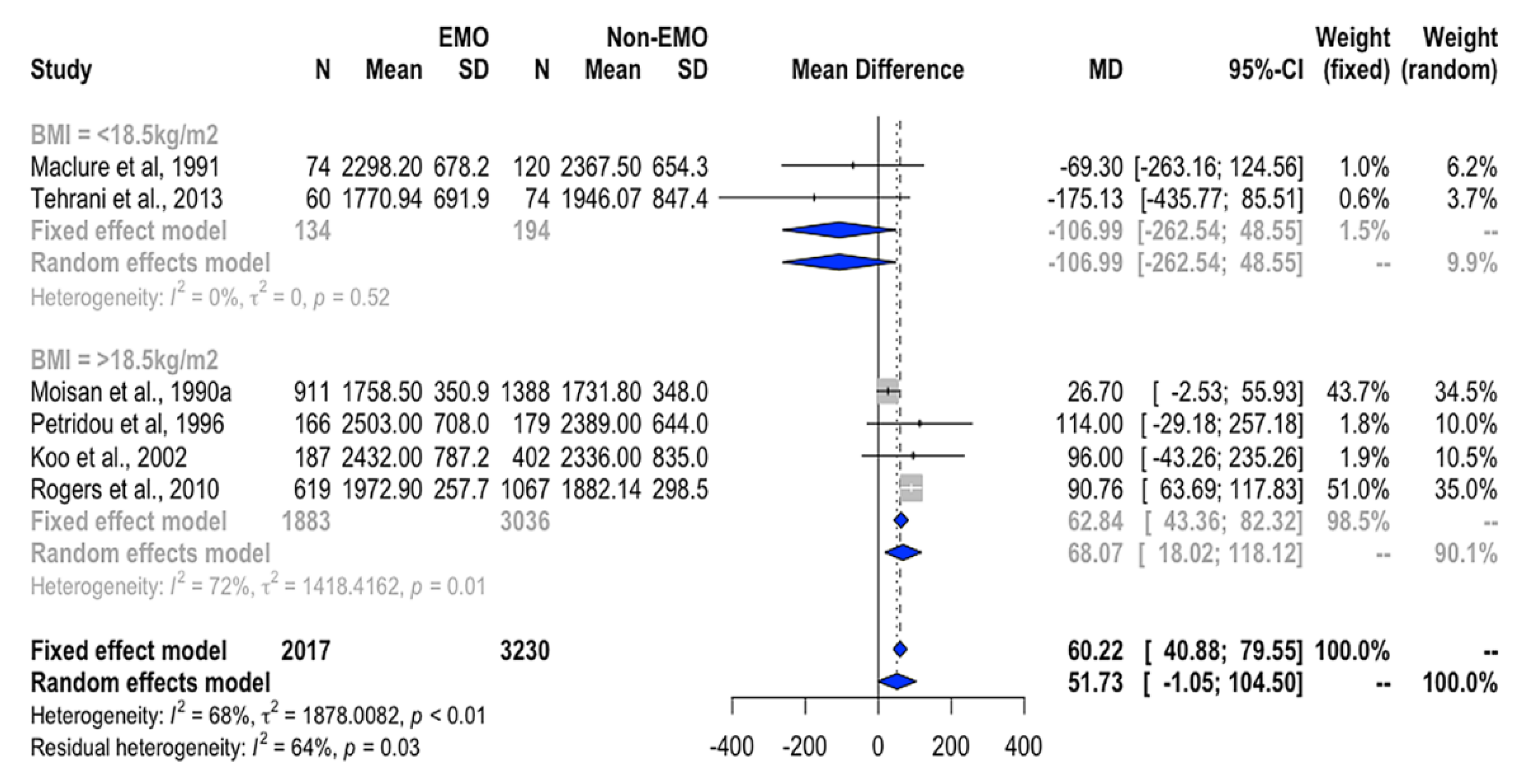
3.4.1. Association between Total EI and EMO
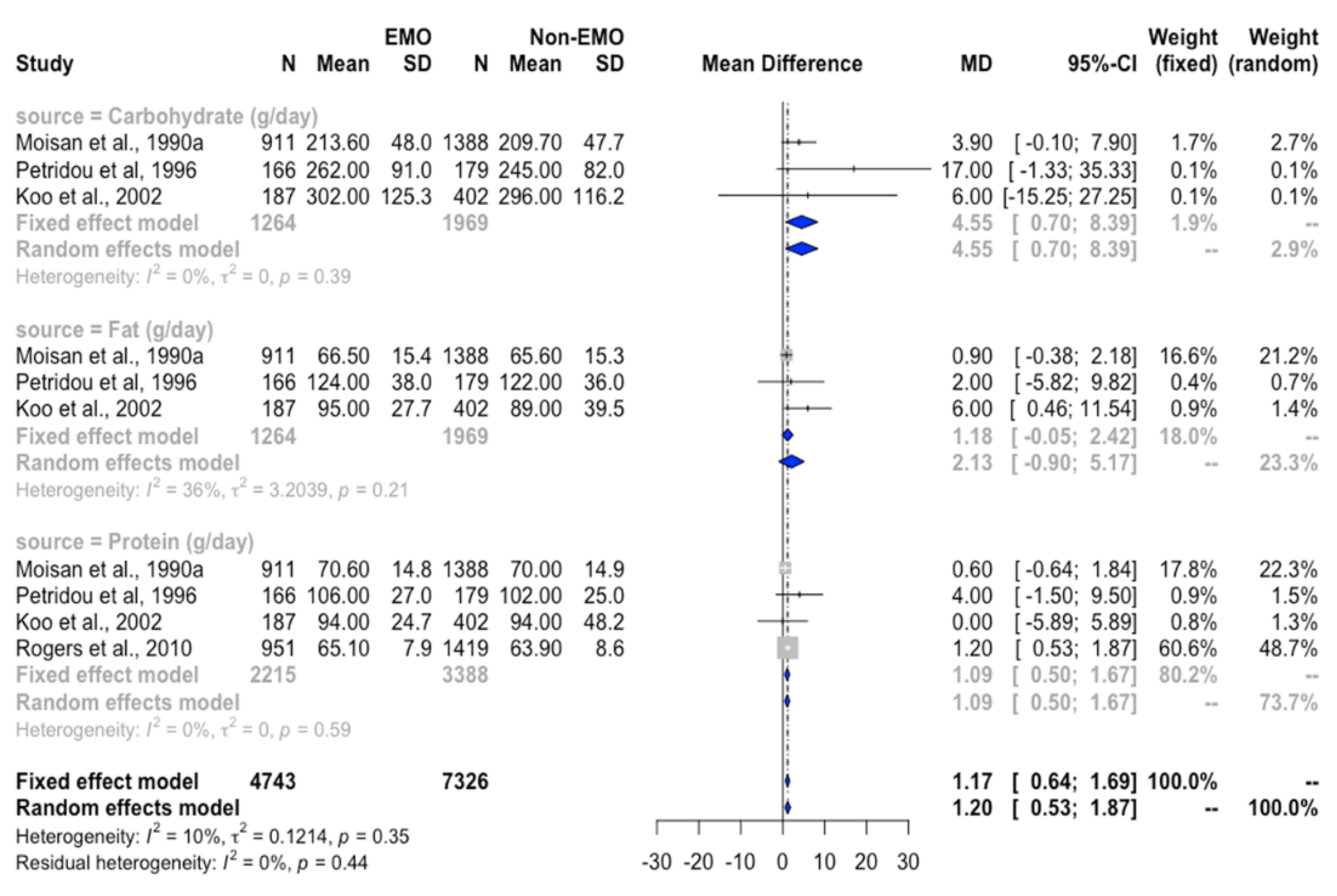
3.4.2. Association between Protein Intake and EMO
3.4.3. Association between Fat Intake and EMO
3.4.4. Association between Carbohydrate Intake and EMO
3.4.5. Association between Micronutrient Intake and EMO
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheng, G.; Buyken, A.E.; Shi, L.; Karaolis-Danckert, N.; Kroke, A.; Wudy, S.A.; Degen, G.H.; Remer, T. Beyond overweight: Nutrition as an important lifestyle factor influencing timing of puberty. Nutr. Rev. 2012, 70, 133–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Must, A.; Phillips, S.M.; Naumova, E.N.; Blum, M.; Harris, S.; Dawson-Hughes, B.; Rand, W.M. Recall of early menstrual history and menarcheal body size: After 30 years, how well do women remember? Am. J. Epidemiol. 2002, 155, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Calthorpe, L.; Brage, S.; Ong, K.K. Systematic review and meta-analysis of the association between childhood physical activity and age at menarche. Acta Paediatr. 2019, 108, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Dossus, L.; Allen, N.; Kaaks, R.; Bakken, K.; Lund, E.; Tjonneland, A.; Olsen, A.; Overvad, K.; Clavel-Chapelon, F.; Fournier, A.; et al. Reproductive risk factors and endometrial cancer: The European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer 2010, 127, 442–451. [Google Scholar] [CrossRef]
- Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: Individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012, 13, 1141–1151. [Google Scholar] [CrossRef]
- Charalampopoulos, D.; McLoughlin, A.; Elks, C.E.; Ong, K.K. Age at menarche and risks of all-cause and cardiovascular death: A systematic review and meta-analysis. Am. J. Epidemiol. 2014, 180, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, B.K.; Heuch, I.; Kvåle, G. Association of low age at menarche with increased all-cause mortality: A 37-year follow-up of 61,319 Norwegian women. Am. J. Epidemiol. 2007, 166, 1431–1437. [Google Scholar] [CrossRef] [Green Version]
- Kaplowitz, P.B. Link between body fat and the timing of puberty. Pediatrics 2008, 121, S208–S217. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-C.; Fan, H.-Y.; Yang, C.; Hsieh, R.-H.; Pan, W.-H.; Lee, Y.L. Assessing causality between childhood adiposity and early puberty: A bidirectional Mendelian randomization and longitudinal study. Metabolism 2019, 100, 153961. [Google Scholar] [CrossRef]
- Tapsell, L.C.; Neale, E.P.; Satija, A.; Hu, F.B. Foods, nutrients, and dietary patterns: Interconnections and implications for dietary guidelines. Adv. Nutr. 2016, 7, 445–454. [Google Scholar] [CrossRef]
- Meyer, F.; Moisan, J.; Marcoux, D.; Bouchard, C. Dietary and physical determinants of menarche. Epidemiology 1990, 1, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Petridou, E.; Syrigou, E.; Toupadaki, N.; Zavitsanos, X.; Willett, W.; Trichopoulos, D. Determinants of age at menarche as early life predictors of breast cancer risk. Int. J. Cancer 1996, 68, 193–198. [Google Scholar] [CrossRef]
- Berkey, C.S.; Gardner, J.D.; Lindsay Frazier, A.; Colditz, G.A. Relation of childhood diet and body size to menarche and adolescent growth in girls. Am. J. Epidemiol. 2000, 152, 446–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunther, A.L.B.; Karaolis-Danckert, N.; Kroke, A.; Remer, T.; Buyken, A.E. Dietary protein intake throughout childhood is associated with the timing of puberty. J. Nutr. 2010, 140, 565–571. [Google Scholar] [CrossRef]
- Cheng, H.L.; Raubenheimer, D.; Steinbeck, K.; Baur, L.; Garnett, S. New insights into the association of mid-childhood macronutrient intake to pubertal development in adolescence using nutritional geometry. Br. J. Nutr. 2019, 122, 274–283. [Google Scholar] [CrossRef]
- Moisan, J.; Meyer, F.; Gingras, S. Diet and age at menarche. Cancer Causes Control. 1990, 1, 149–154. [Google Scholar] [CrossRef]
- De Ridder, C.M.; Thijssen, J.H.; Van’t Veer, P.; van Duuren, R.; Bruning, P.F.; Zonderland, M.L.; Erich, W.B. Dietary habits, sexual maturation, and plasma hormones in pubertal girls: A longitudinal study. Am. J. Clin. Nutr. 1991, 54, 805–813. [Google Scholar] [CrossRef]
- Koo, M.M.; Rohan, T.E.; Jain, M.; McLaughlin, J.R.; Corey, P.N. A cohort study of dietary fibre intake and menarche. Public Health Nutr. 2002, 5, 353–360. [Google Scholar] [CrossRef] [Green Version]
- Rogers, I.S.; Northstone, K.; Dunger, D.B.; Cooper, A.R.; Ness, A.R.; Emmett, P.M. Diet throughout childhood and age at menarche in a contemporary cohort of British girls. Public Health Nutr. 2010, 13, 2052–2063. [Google Scholar] [CrossRef] [Green Version]
- Merzenich, H.; Boeing, H.; Wahrendorf, J. Dietary fat and sports activity as determinants for age at menarche. Am. J. Epidemiol. 1993, 138, 217–224. [Google Scholar] [CrossRef]
- Koprowski, C.; Ross, R.K.; Mack, W.J.; Henderson, B.E.; Bernstein, L. Diet, body size and menarche in a multiethnic cohort. Br. J. Cancer 1999, 79, 1907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, G.; Remer, T.; Prinz-Langenohl, R.; Blaszkewicz, M.; Degen, G.H.; Buyken, A.E. Relation of isoflavones and fiber intake in childhood to the timing of puberty. Am. J. Clin. Nutr. 2010, 92, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Grace, P.B.; Taylor, J.I.; Low, Y.-L.; Luben, R.N.; Mulligan, A.A.; Botting, N.P.; Dowsett, M.; Welch, A.A.; Khaw, K.-T.; Wareham, N.J.; et al. Phytoestrogen concentrations in serum and spot urine as biomarkers for dietary phytoestrogen intake and their relation to breast cancer risk in European prospective investigation of cancer and nutrition-norfolk. Cancer Epidemiol. Biomarkers Prev. 2004, 13, 698–708. [Google Scholar] [PubMed]
- Remer, T.; Shi, L.; Buyken, A.E.; Maser-Gluth, C.; Hartmann, M.F.; Wudy, S.A. Prepubertal adrenarchal androgens and animal protein intake independently and differentially influence pubertal timing. J. Clin. Endocrinol. Metab. 2010, 95, 3002–3009. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Is obesity associated with early sexual maturation? A comparison of the association in American boys versus girls. Pediatrics 2002, 110, 903–910. [Google Scholar] [CrossRef] [Green Version]
- Moisan, J.; Meyer, F.; Gingras, S. A nested case-control study of the correlates of early menarche. Am. J. Epidemiol. 1990, 132, 953–961. [Google Scholar] [CrossRef]
- Maclure, M.; Travis, L.B.; Willett, W.; MacMahon, B. A prospective cohort study of nutrient intake and age at menarche. Am. J. Clin. Nutr. 1991, 54, 649–656. [Google Scholar] [CrossRef]
- Tehrani, F.R.; Moslehi, N.; Asghari, G.; Gholami, R.; Mirmiran, P.; Azizi, F. Intake of dairy products, calcium, magnesium, and phosphorus in childhood and age at menarche in the Tehran Lipid and Glucose Study. PLoS ONE 2013, 8, e57696. [Google Scholar] [CrossRef]
- Wong, W.C.; Cheung, C.S.; Hart, G.J. Development of a quality assessment tool for systematic reviews of observational studies (QATSO) of HIV prevalence in men having sex with men and associated risk behaviours. Emerg. Themes Epidemiol. 2008, 5, 23. [Google Scholar] [CrossRef] [Green Version]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashino, Y.; Noguchi, Y.; Fukui, T. Systematic evaluation and comparison of statistical tests for publication bias. J. Epidemiol. 2005, 15, 235–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, F.; Khan, K.S.; Dinnes, J.; Sutton, A.J. Asymmetric funnel plots and publication bias in meta-analyses of diagnostic accuracy. Int. J. Epidemiol. 2002, 31, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Kissinger, D.G.; Sanchez, A. The association of dietary factors with the age of menarche. Nutr. Res. 1987, 7, 471–479. [Google Scholar] [CrossRef]
- Farahmand, M.; Tehrani, F.R.; Amiri, P.; Azizi, F. Barriers to healthy nutrition: Perceptions and experiences of Iranian women. BMC Public Health 2012, 12, 1064. [Google Scholar] [CrossRef] [Green Version]
- Thankamony, A.; Ong, K.K.; Ahmed, M.L.; Ness, A.R.; Holly, J.M.P.; Dunger, D.B. Higher levels of IGF-I and adrenal androgens at age 8 years are associated with earlier age at menarche in girls. J. Clin. Endocrinol. Metab. 2012, 97, E786–E790. [Google Scholar] [CrossRef] [Green Version]
- Villamor, E.; Marín, C.; Mora-Plazas, M.; Oliveros, H. Micronutrient status in middle childhood and age at menarche: Results from the Bogotá School Children Cohort. Br. J. Nutr. 2017, 118, 1097–1105. [Google Scholar] [CrossRef] [Green Version]
- Olson, L.K.; Tan, Y.; Zhao, Y.; Aupperlee, M.D.; Haslam, S.Z. Pubertal exposure to high fat diet causes mouse strain-dependent alterations in mammary gland development and estrogen responsiveness. Int. J. Obes. 2010, 34, 1415–1426. [Google Scholar] [CrossRef] [Green Version]
- Maher, J.Y.; Zacur, H.A. Menarche/menopause. In Encyclopedia of Reproduction; Elsevier: Amsterdam, The Netherlands, 2018; pp. 245–249. [Google Scholar]
- Valeggia, C.R.; Núñez-de la Mora, A. Human reproductive ecology. In Basics in Human Evolution; Elsevier: Amsterdam, The Netherlands, 2015; pp. 295–308. [Google Scholar]
- Anderson, B.M.; MacLennan, M.B.; Hillyer, L.M.; Ma, D.W.L. Lifelong exposure to n-3 PUFA affects pubertal mammary gland development. Appl. Physiol. Nutr. Metab. 2014, 39, 699–706. [Google Scholar] [CrossRef] [Green Version]
- Sampath, H.; Ntambi, J.M. Polyunsaturated fatty acid regulation of genes of lipid metabolism. Annu. Rev. Nutr. 2005, 25, 317–340. [Google Scholar] [CrossRef]
- Hilakivi-Clarke, L.; Clarke, R.; Onojafe, I.; Raygada, M.; Cho, E.; Lippman, M. A maternal diet high in n−6 polyunsaturated fats alters mammary gland development, puberty onset, and breast cancer risk among female rat offspring. Proc. Natl. Acad. Sci. USA 1997, 94, 9372–9377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowler, L.A.; Dennis-Cornelius, L.N.; Dawson, J.A.; Barry, R.J.; Davis, J.L.; Powell, M.L.; Yuan, Y.; Williams, M.B.; Makowsky, R.; D’Abramo, L.R.; et al. Both dietary ratio of n–6 to n–3 fatty acids and total dietary lipid are positively associated with adiposity and reproductive health in zebrafish. Curr. Dev. Nutr. 2020, 4, nzaa034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Y.; Zhang, J.; Yuan, C.; Zhang, F.; Fu, Q.; Su, H.; Zhu, X.; Wang, L.; Gao, P.; Shu, G.; et al. Oleic acid stimulates HC11 mammary epithelial cells proliferation and mammary gland development in peripubertal mice through activation of CD36-Ca2+ and PI3K/Akt signaling pathway. Oncotarget 2018, 9, 12982. [Google Scholar] [CrossRef] [PubMed]
- C.C.O. Canada Canola Oil: Nutritional Properties. Available online: https://www.canolacouncil.org/media/515159/canola_oil_nutritional_properties.pdf (accessed on 7 November 2019).
- Li, W.; Liu, Q.; Deng, X.; Chen, Y.; Liu, S.; Story, M. Association between obesity and puberty timing: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2017, 14, 1266. [Google Scholar] [CrossRef]
- Anderson, K.E.; Kappas, A.; Conney, A.H.; Bradlow, H.L.; Fishman, J. The influence of dietary protein and carbohydrate on the principal oxidative biotransformations of estradiol in normal subjects. J. Clin. Endocrinol. Metab. 1984, 59, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Leenstra, T.; Petersen, L.T.; Kariuki, S.K.; Oloo, A.J.; Kager, P.A.; Ter Kuile, F.O. Prevalence and severity of malnutrition and age at menarche; cross-sectional studies in adolescent schoolgirls in western Kenya. Eur. J. Clin. Nutr. 2005, 59, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Hughes, R.E.; Jones, E. Intake of dietary fibre and the age of menarche. Ann. Hum. Biol. 1985, 12, 325–332. [Google Scholar] [CrossRef]
- Williams, C.L. Importance of dietary fiber in childhood. J. Am. Diet. Assoc. 1995, 95, 1140–1149. [Google Scholar] [CrossRef]
- Hayes, D.P. Adverse effects of nutritional inadequacy and excess: A hormetic model. Am. J. Clin. Nutr. 2008, 88, 578S–581S. [Google Scholar] [CrossRef] [Green Version]
- Sinai, T.; Ben-Avraham, S.; Guelmann-Mizrahi, I.; Goldberg, M.R.; Naugolni, L.; Askapa, G.; Katz, Y.; Rachmiel, M. Consumption of soy-based infant formula is not associated with early onset of puberty. Eur. J. Nutr. 2019, 58, 681–687. [Google Scholar] [CrossRef]
- Eliassen, A.H.; Liao, X.; Rosner, B.; Tamimi, R.M.; Tworoger, S.S.; Hankinson, S.E. Plasma carotenoids and risk of breast cancer over 20 y of follow-up. Am. J. Clin. Nutr. 2015, 101, 1197–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folman, Y.; Ascarelli, I.; Kraus, D.; Barash, H. Adverse effect of β-carotene in diet on fertility of dairy cows. J. Dairy Sci. 1987, 70, 357–366. [Google Scholar] [CrossRef]
- Boeing, H. Nutritional epidemiology: New perspectives for understanding the diet-disease relationship? Eur. J. Clin. Nutr. 2013, 67, 424–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Authors, Year, Country | Subjects(Mean ± SD/Age Range) (Yrs) | Dietary Method | Predictors (Nutrient Intake) | Follow-Up (Yrs) | Outcomes: AAM (Mean ± SD/Median, Min, Max) (Yrs) | Sample Size Menarche: Non-Menarche | Confounders | Main Findings | |
|---|---|---|---|---|---|---|---|---|---|
| Kissinger and Sanchez, 1987, United States [34] | Premenarcheal girls aged 9–15 | Multiple 24-h recalls Child reported | EI, intakes of carbohydrate, protein, fat, vitamins Food groups | >1 | AAM Mother reported | 230:770 | NA NA | (1) No mean difference data between EMO and NEMO groups (2) Upper quartile intake of carbohydrate, thiamine, and iron was linked to 7–8-month later menarche compared with the lowest quartile (p < 0.05) | |
| Moisan et al., 1990a, Canada [16] | 2299 Caucasian girls aged 11.1 ± 0.6 | 3-Day dietary record Child reported | EI, intakes of macronutrients, SFAs, MUFAs, PUFAs, cholesterol, calcium, phosphorus, iron, crude fiber, and vitamins | 1.4 | 12.1 (10.1–14.7) Mother reported | 911:1388 | Adjusted for age and mother’s AAM Adjusted for age and mother’s AAM | (1) No differences in mean intake of energy and macronutrients between menarche and premenarche groups (p > 0.05). Higher intake of vitamin C was linked to earlier AAM (2) Girls with the highest quartile of MUFA intake were likely to have early menarche | |
| Moisan et al., 1990b, Canada [26] | 666 Caucasia-n girls aged 9.5–12.5 years | 3-Day dietary record Child reported | EI, intakes of macronutrients, saturated FAs, MUFAs, PUFAs, cholesterol, calcium, phosphorus, iron, crude fiber, and vitamins | 1 | 11.4 (10.1–13.3) Mother reported | 333:333 | Adjusted for age and EI Adjusted for age and EI | (1) EMO girls consumed more energy, carbohydrate, lipids, MUFAs, iron, niacin, and vitamin A than NEMO girls (p < 0.05) (2) EI was positively associated with EMO in girls (OR = 1.5, 95% CI = 0.9–2.3) Girls with the highest MUFA and vitamin A intakes experienced menarche later (OR = 0.6, 95% CI = 0.4–0.9; OR = 0.5, 95% CI = 0.3–0.8, respectively) | |
| Maclure et al., 1991, United States [27] | 194 girls, aged 11.0±0.7 | Semiquantitative FFQ Parent and child reported | Intakes of carbohydrate, sucrose, fiber, protein, total fat, FAs, cholesterol, and vitamins | 4 | 12.5 Mother reported | 74:120 | NA Adjusted for baseline EI, height, and QI | (1) Early menarche was associated with higher intake of PUFAs, vitamins A, C, and B12, carotene, and retinol (p < 0.05) (2) The highest intake of saturated FAs, n-3 FAs, was associated with later menarche than the reference level (RR = 0.7, 95% CI = 0.4–1.2; RR = 2.7, 95% CI = 1.6–4.6, respectively). The highest intake of vitamin A was linked to AAM compared with the reference (RR = 1.3, 95% CI = 0.7–2.3) | |
| de Ridder et al., 1991, Netherla-nds [17] | 63 girls aged 9.6±0.04 | 7-Day food record Child reported | EI, intakes of fiber, grain fiber, vegetable fiber, protein fat, carbohydrate | 3 | 14.3 Child reported | 31:29 | NA Adjusted for EI, height, dietary methods, timing, poly-saccharides | (1) No mean difference data between EMO and NEMO groups (2) Vegetable protein and fiber intakes were inversely associated with earlier AAM (β = −3.3 (1.5); β = −2.6 (1.2), respectively) | |
| Merzenich et al., 1993, Germany [20] | 261 girls aged 8–15 | Semiquantitative FFQ; Parent and child reported | Intakes of carbohydrate, protein, and fat | 2 | 9.7–15.6 Parent and child reported | 94:167 | NA Adjusted by age and total EI | (1) No mean difference data between EMO and NEMO groups (2) The highest quartile accelerated menarche onset (RR = 2.1; 95% CI = 1.1–4.0) | |
| Petridou et al., 1996, Greece [12] | 345 girls, aged 9–16 | FFQ Child reported | EI, intakes of carbohydrate, protein, saturated FAs, MUFAs, and PUFAs | 1 | 13 (9.9–16.0) Mother reported | 166:179 | NA Adjusted for total EI | (1) No mean difference data between EMO and NEMO groups (2) Girls with higher PUFA intake reached menarche later than those with the reference intake (RR= 0.79, 95% CI = 0.65–0.95) | |
| Koprowski et al. 1999, United States [21] | 1378 girls aged 8–13 | 7-Day FFQ Child reported | Total EI and intakes of carbohydrate, protein, and fat | 4 | 9.7–14.8 Parent reported | 464:215 | NA Adjusted for age at dietary assessment, height, QI, EI, and ethnicity | (1) No mean difference data between EMO and NEMO groups (2) Higher EI was inversely associated with AAM (p trend < 0.05). Carbohydrate, protein, and fat intakes were unrelated to AAM (p < 0.05) | |
| Berkey et al., 2000, United States [13] | 67 Caucasian girls aged 3–5 or 6–8 | Dietary history interview regarding the preceding 6 months Mother reported | EI, intakes of animal protein, vegetable protein, and total fat | 4.83 and 8.83 | 12.8 ± 1.1 Medical records | NA | NA Adjusted for age and EI | (1) No mean difference data between EMO and NEMO groups (2) Vegetable protein intake at age 3–5 years was linked to delayed AAM (β = 2.19 (0.91)). Animal protein intake at age 6–8 years was linked to earlier AAM (β = −1.69 (0.76)) | |
| Koo et al., 2002, Canada [18] | 637 girls aged 9.7±1.2 | Semiqu-antitative FFQ Mother reported | Intakes of fiber, fat, and FAs | 3 | 13.6 (8.5–15.6) Mother reported | 187:402 | NA Adjusted for EI, age at entry, time-dependent age at entry, body weight, birth weight, the logarithm of EI, mother’s AAM, and father’s career | (1) No mean difference data between EMO and NEMO groups (2) The fiber intake was negatively associated with EMO (HR = 0.54, 95% CI = 0.31–0.94). Increased cellulose and animal fat intakes were negatively associated with menarche onset (p trend = 0.009 and 0.03, respectively). | |
| Cheng et al., 2010, Germany [22] | 119 girls, aged 7.2 ± 1.0 | 3-Day dietary record | Intakes of isoflavones and dietary fiber at 1 and 2 years before age at take-off | 1–2 | 12.6 ± 1.0 to 13.1 ± 1.2 Parent and child reported | 108:11 | NA Adjusted for smoking, baseline isoflavone intake, and baseline EI z-score | (1) No mean difference data between EMO and NEMO groups (2) Fiber and isoflavone intakes were not associated with AAM in the unadjusted model or adjusted model (p_trend = 0.4) | |
| Gunther et al., 2010, Germany [14] | 112 children (57 girls, 50.9%) | 3-Day weighed dietary record for girls aged 3–4 or 5–6 years Parent reported | Intakes of protein, animal protein, and vegetable protein | Average = 9.3 and 7.3 | 12.8 ± 1.2 | 47:10 | NA Adjusted for sex, EI, breastfeeding, birth year, and father’s university degree | (1) No mean difference data between EMO and NEMO groups (2) Higher animal protein intake at 5–6 years was related to earlier menarche (p trend = 0.02). Children with higher vegetable protein intake experienced later menarche (p trend = 0.02–0.03) | |
| Remer et al., 2010, Germany [24] | 109 German children (55 girls) | 3-Day weighed dietary record Child reported | Intake of animal protein | 1 and 2 before ATO | 13.1 ± 0.8 Parent and child reported | NA | NA Adjusted for sex, EI, fat mass index, gestational age, birth weight, breastfeeding, and maternal overweight | (1) No mean difference data between EMO and NEMO groups (2) Animal protein intake was negatively associated with AAM (p = 0.07) | |
| Roger et al., 2010, England [19] | 3298 girls aged 12.9±0.2 | FFQ at ages 3 and 7 years. 3-Day weight dietary record at age 10–11 years, Child reported | EI, intakes of total fat, saturated FAs, MUFAs, PUFAs, starch, sugar, total protein, animal protein, vegetable protein, fiber, vitamins | 3.5 | 12.8 ± 0.2 Clinic reported | 951:1419 | NA Adjusted for BMI and height at the time of diet measurement | (1) At 3 years, protein, animal protein, and carotene intakes were higher in girls with EMO (p < 0.05). At 7 years, PUFA, protein, animal protein, and Zn intakes were higher in girls with EMO (p = 0.019). At 10 years, EI was higher in girls with EMO (p = 0.002) (2) At aged 3 and 7 years, protein, animal protein PUFA intakes were positively associated with EMO, but not at 10 years of age | |
| Tehrani et al., 2013, Iran [28] | 134 prepubertal girls aged 8.9 ± 2.4 | 24-h Dietary recalls Child reported | EI, intakes of carbohydrate, fat, protein, magnesium, phosphorus, milk, yogurt, and cheese | Medi-an follow-up = 6.5 | 12.7 ± 1.3 Child reported | 60:74 | NA Adjusted for EI, protein intake, the interval between the age at study initiation and the AAM, and mother’s AAM | (1) No significant differences in EI or carbohydrate, protein, or total fat intake between EMO and NEMO groups (p < 0.05) (2) Girls with EMO had higher intakes of calcium (OR = 3.20, 95% CI = 1.39–7.42), magnesium (OR = 2.43, 95% CI = 1.12–5.27), and phosphorus (OR = 3.37, 95% CI = 1.44–7.87) than girls with NEMO. | |
| Cheng et al., 2019, Australia [15] | 142 prepuber-tal children aged 8 | 3-Day food record Parent and child reported | EI, intakes of carbohydrate, fat, total protein, and animal protein | 5–8 | 12.6 ± 1.0 Child reported | 92:50 | NA Adjusted for total energy, birth weight, height, zBMI, and mother’s occupation | (1) No mean difference data between EMO and NEMO groups (2) Lower absolute (p = 0.04) and energy-adjusted (p = 0.03) protein intake was linked to EMO. Lower dietary protein (relative to carbohydrate and fat) intake consistently predicted EMO. Animal protein intake was not associated with menarche onset | |
| Energy and Nutrient Intake | EMO (N) | NEMO (N) | Weighted mean Difference (WMD, 95% CI) | I2 | |
|---|---|---|---|---|---|
| Fixed Effect | Random Effect | ||||
| Energy (kcal/day) | 2017 | 3230 | 60.2 (40.9, 79.6) * | 51.7 (-1.1, 104.5) | 68% * |
| Carbohydrate (g/day) | 1398 | 2163 | −0.1 (−3.7, 3.5) | −2.8 (−22.2, 16.7) | 92% |
| Carbohydrate (% energy) | 1398 | 2163 | −0.1 (−3.8, 0.8) | −0.1 (−3.8, 0.8) | 0% |
| Total fiber (g/day) | 1172 | 1910 | 0.0 (−0.1, 0.1) | 0.0 (−0.1, 0.1) | 0% |
| Protein (g/day) | 2349 | 3582 | 0.9 (0.3, 1.5) * | 0.2 (−1.4, 1.8) | 62% * |
| Protein (% energy) | 2349 | 3582 | 0.2 (0.1, 0.3) * | 0.04 (−0.3, 0.3) | 56% |
| Fat (g/day) | 1398 | 2163 | 1.2 (0.02, 2.4) * | 1.8 (−0.7, 4.2) | 34% |
| Fat (% energy) | 1398 | 2163 | -0.8 (-1.1, -0.5) * | -0.03 (−1.0, 0.9) | 73% * |
| Saturated FAs (g/day) | 1338 | 2089 | 0.2 (−0.4, 0.8) | 0.3 (−1.4, 2.1) | 60% |
| MUFAs (g/day) | 1338 | 2089 | 0.5 (0.04, 1.0) * | 0.5 (−0.3, 1.4) | 18% |
| PUFAs (g/day) | 2289 | 3508 | 0.4 (0.2, 0.6) * | 0.5 (0.2, 0.8) * | 21% |
| Cholesterol (g/day) | 1172 | 1910 | 1.9 (−4.3, 8.1) | 1.9 (4.3, 8.1) | 0% |
| Calcium (mg/day) | 1404 | 1649 | −2.4 (−31.5, 26.7) | −2.4 (−31.5, 26.7) | 0% |
| Phosphate (mg/day) | 971 | 1462 | 8.9 (−17.5, 35.3) | 8.9 (−17.5, 35.3) | 0% |
| Magnesium (mg/day) | 679 | 1141 | 3.1 (0.3, 5.9) * | 3.1 (0.3, 5.9) * | 0% |
| Iron (mg/day) | 911 | 1388 | 0.2 (−0.1; 0.4) | NA | NA |
| Vitamin B1 (mg/day) | 985 | 1508 | 0.0 (−0.03, 0.03) | 0.0 (−0.03, 0.03) | 0%* |
| Riboflavine (µg/day) | 985 | 1508 | −1.0 (−1.0, −0.9) * | −0.4 (−1.6, 0.7) | 96% * |
| Vitamin B3 (µg/day) | 985 | 1508 | 0.2 (−0.3, 0.6) | 0.2 (−0.3, 0.6) | 0% |
| Vitamin B6 (mg/day) | 985 | 1508 | 0.0 (−0.03, 0.03) | 0.0 (−0.03, 0.03) | 0% |
| Vitamin B9 (µg/day) | 985 | 1508 | −0.7 (−7.8, 6.4) | −0.7 (−7.8, 6.4) | 0% |
| Vitamin B12 (µg/day) | 985 | 1508 | 0.01 (−0.1, 0.1) | 0.2 (−0.4, 0.8) | 59% |
| Vitamin C (mg/day) | 1418 | 1695 | 5.4 (1.1, 9.7) * | 8.6 (−9.7, 26.9) | 23% |
| Vitamin A (IU/day) | 1418 | 1695 | −83.8 (−218.3,50.7) | 1476.9 (−2262.5, 5261.4) | 81% * |
| Carotene (µg/day) | 1068 | 1600 | 78.1(13.9, 142.4) * | 744.7 (−992.9, 2482.2) | 72% |
| Vitamin D (IU/day) | 985 | 1508 | −0.5 (−10.4, 9.4) | −0.5 (−10.4, 9.4) | 0% |
| Vitamin E (mg/day) | 985 | 1508 | 0.1 (−0.1, 0.3) | 0.1 (−0.1, 0.3) | 0% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, N.T.K.; Fan, H.-Y.; Tsai, M.-C.; Tung, T.-H.; Huynh, Q.T.V.; Huang, S.-Y.; Chen, Y.C. Nutrient Intake through Childhood and Early Menarche Onset in Girls: Systematic Review and Meta-Analysis. Nutrients 2020, 12, 2544. https://doi.org/10.3390/nu12092544
Nguyen NTK, Fan H-Y, Tsai M-C, Tung T-H, Huynh QTV, Huang S-Y, Chen YC. Nutrient Intake through Childhood and Early Menarche Onset in Girls: Systematic Review and Meta-Analysis. Nutrients. 2020; 12(9):2544. https://doi.org/10.3390/nu12092544
Chicago/Turabian StyleNguyen, Ngan Thi Kim, Hsien-Yu Fan, Meng-Che Tsai, Te-Hsuan Tung, Quynh Thi Vu Huynh, Shih-Yi Huang, and Yang Ching Chen. 2020. "Nutrient Intake through Childhood and Early Menarche Onset in Girls: Systematic Review and Meta-Analysis" Nutrients 12, no. 9: 2544. https://doi.org/10.3390/nu12092544
APA StyleNguyen, N. T. K., Fan, H.-Y., Tsai, M.-C., Tung, T.-H., Huynh, Q. T. V., Huang, S.-Y., & Chen, Y. C. (2020). Nutrient Intake through Childhood and Early Menarche Onset in Girls: Systematic Review and Meta-Analysis. Nutrients, 12(9), 2544. https://doi.org/10.3390/nu12092544








