Could Vitamins Help in the Fight Against COVID-19?
Abstract
:1. Introduction
2. Methods
Inclusion and Exclusion Criteria
3. Immunonutrition
3.1. Vitamin A
3.1.1. Source and Physiological Role
3.1.2. Mechanism of Action in Disease
3.1.3. Respiratory Infections
3.1.4. Relevance to COVID-19
3.2. Vitamins B
3.2.1. Sources and Physiological Role
3.2.2. Mechanism of Action in Disease
3.2.3. Respiratory Disease
3.2.4. Relevance to COVID-19
3.3. Vitamin C
3.3.1. Source and Physiological Role
3.3.2. Mechanism of Action in Disease
3.3.3. Respiratory Disease
3.3.4. Relevance to COVID-19
3.4. Vitamin D
3.4.1. Source and Physiological Role
3.4.2. Mechanism of Action in Disease
3.4.3. Respiratory Disease
3.4.4. Relevance to COVID-19
3.5. Vitamin E
3.5.1. Source and Physiological Role
3.5.2. Mechanism of Action in Disease
3.5.3. Respiratory Disease
3.5.4. Relevance to COVID-19
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Duffy, S. Why are RNA virus mutation rates so damn high? PLoS Biol. 2018, 16, e3000003. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- World Health Organisation. Coronavirus Disease (COVID-19) Pandemic. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 21 August 2020).
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Li, L. SARS-CoV-2: Virus dynamics and host response. Lancet Infect. Dis. 2020, 20, 515–516. [Google Scholar] [CrossRef] [Green Version]
- U.S. National Library of Medicine Safety and Immunogenicity. Study of 2019-nCoV Vaccine (mRNA-1273) to Prevent SARS-CoV-2 Infection (NCT04283461). Available online: https://clinicaltrials.gov/ct2/show/NCT04283461 (accessed on 21 August 2020).
- Calder, P.C. Immunonutrition. Br. Med. J. 2003, 327, 117–118. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.D.N.; Costa, K.A.; Wanner, S.P.; Santos, R.G.C.; Fernandes, S.O.A.; Martins, F.S.; Nicoli, J.R.; Coimbra, C.C.; Cardoso, V.N. Dietary glutamine prevents the loss of intestinal barrier function and attenuates the increase in core body temperature induced by acute heat exposure. Br. J. Nutr. 2014, 112, 1601–1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beale, R.J.; Bryg, D.J.; Bihari, D.J. Immunonutrition in the critically ill: A systematic review of clinical outcome. Crit. Care Med. 1999, 27, 2799–2805. [Google Scholar] [CrossRef] [PubMed]
- Heyland, D.K.; Dhaliwal, R.; Suchner, U.; Berger, M.M. Antioxidant nutrients: A systematic review of trace elements and vitamins in the critically ill patient. Intensive Care Med. 2005, 31, 327–337. [Google Scholar] [CrossRef]
- Mizock, B.A. Immunonutrition and critical illness: An update. Nutrition 2010, 26, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.L.; DeMichele, S.J.; Pacht, E.R.; Wennberg, A.K.; Gadek, J.; Drake, J.; Farmer, P.; Hart, J.; Karlstad, M.; Cruz, E.; et al. Effect of enteral feeding with eicosapentaenoic acid, γ-linolenic acid, and antioxidants on antioxidant status in patients with acute respiratory distress syndrome. J. Parenter. Enter. Nutr. 2003, 27, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Pontes-Arruda, A.; Aragão, A.M.A.; Albuquerque, J.D. Effects of enteral feeding with eicosapentaenoic acid, γ-linolenic acid, and antioxidants in mechanically ventilated patients with severe sepsis and septic shock. Crit. Care Med. 2006, 34, 2325–2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Bo, L.; Liu, W.; Lu, X.; Jin, F. Enteral immunomodulatory diet (omega-3 fatty acid, γ-linolenic acid and antioxidant supplementation) for acute lung injury and acute respiratory distress syndrome: An updated systematic review and meta-analysis. Nutrients 2015, 7, 5572–5585. [Google Scholar] [CrossRef]
- Dushianthan, A.; Cusack, R.; Burgess, V.A.; Grocott, M.P.W.; Calder, P.C. Immunonutrition for acute respiratory distress syndrome (ARDS) in adults. Cochrane Database Syst. Rev. 2019, 1, CD012041. [Google Scholar] [CrossRef]
- Caccialanza, R.; Laviano, A.; Lobascio, F.; Montagna, E.; Bruno, R.; Ludovisi, S.; Corsico, A.G.; Di Sabatino, A.; Belliato, M.; Calvi, M.; et al. Early nutritional supplementation in non-critically ill patients hospitalized for the 2019 novel coronavirus disease (COVID-19): Rationale and feasibility of a shared pragmatic protocol. Nutrition 2020, 74, 110835. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, Y.; Qi, G.; Brand, D.; Zheng, S. Role of vitamin A in the immune system. J. Clin. Med. 2018, 7, 258. [Google Scholar] [CrossRef] [Green Version]
- Retinol|C20H30O—PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Retinol (accessed on 17 August 2020).
- Lidén, M.; Eriksson, U. Understanding retinol metabolism: Structure and function of retinol dehydrogenases. J. Biol. Chem. 2006, 281, 13001–13004. [Google Scholar] [CrossRef] [Green Version]
- Sporn, M.B.; Dunlop, N.M.; Newton, D.L.; Smith, J.M. Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids). Fed. Proc. 1976, 35, 1332–1338. [Google Scholar]
- Lefebvre, P.; Martin, P.J.; Flajollet, S.; Dedieu, S.; Billaut, X.; Lefebvre, B. Transcriptional Activities of Retinoic Acid Receptors. Vitam. Horm. 2005, 70, 199–264. [Google Scholar] [CrossRef]
- Tanumihardjo, S.A. Vitamin A: Biomarkers of nutrition for development. Am. J. Clin. Nutr. 2011, 94, 658S–65S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, G. Bioconversion of dietary provitamin A carotenoids to vitamin A in humans. Am. J. Clin. Nutr. 2010, 91, 1468S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiser, P.D.; Palczewski, K. Retinoids and retinal diseases. Annu. Rev. Vis. Sci. 2016, 2, 197–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senoo, H.; Yoshikawa, K.; Morii, M.; Miura, M.; Imai, K.; Mezaki, Y. Hepatic stellate cell (vitamin A-storing cell) and its relative—Past, present and future. Cell Biol. Int. 2010, 34, 1247–1272. [Google Scholar] [CrossRef]
- Dao, D.Q.; Ngo, T.C.; Thong, N.M.; Nam, P.C. Is vitamin A an antioxidant or a pro-oxidant? J. Phys. Chem. B 2017, 121, 9348–9357. [Google Scholar] [CrossRef]
- McGrane, M.M. Vitamin A regulation of gene expression: Molecular mechanism of a prototype gene. J. Nutr. Biochem. 2007, 18, 497–508. [Google Scholar] [CrossRef]
- Zhong, M.; Kawaguchi, R.; Kassai, M.; Sun, H. Retina, retinol, retinal and the natural history of vitamin A as a light sensor. Nutrients 2012, 4, 2069–2096. [Google Scholar] [CrossRef] [Green Version]
- Herschel Conaway, H.; Henning, P.; Lerner, U.H. Vitamin a metabolism, action, and role in skeletal homeostasis. Endocr. Rev. 2013, 34, 766–797. [Google Scholar] [CrossRef] [Green Version]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [Green Version]
- Jørgensen, M.J.; Hein-Kristensen, L.; Hempel, C.; Ravn, H.; Wiese, L.; Kurtzhals, J.A.L.; Benn, C.S. The effect of vitamin A supplementation and diphtheria-tetanus-pertussis vaccination on parasitaemia in an experimental murine malaria model. Scand. J. Infect. Dis. 2011, 43, 296–303. [Google Scholar] [CrossRef]
- Benn, C.S. Combining vitamin A and vaccines: Convenience or conflict? Dan. Med. J. 2012, 59, B4378. [Google Scholar] [PubMed]
- Hollm-Delgado, M.G.; Piel, F.B.; Weiss, D.J.; Howes, R.E.; Stuart, E.A.; Hay, S.I.; Black, R.E. Vitamin A supplements, routine immunization, and the subsequent risk of plasmodium infection among children under 5 years in sub-Saharan Africa. Elife 2015, 4, e03925. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, R.; Sooriyaarachchi, P.; Chourdakis, M.; Jeewandara, C.; Ranasinghe, P. Enhancing immunity in viral infections, with special emphasis on COVID-19: A review. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Paiva, S.A.R.; Godoy, I.; Vannucchi, H.; Fávaro, R.M.D.; Geraldo, R.R.C.; Campana, A.O. Assessment of vitamin A status in chronic obstructive pulmonary disease patients and healthy smokers. Am. J. Clin. Nutr. 1996, 64, 928–934. [Google Scholar] [CrossRef] [Green Version]
- Arora, P.; Kumar, V.; Batra, S. Vitamin A status in children with asthma. Pediatr. Allergy Immunol. 2002, 13, 223–226. [Google Scholar] [CrossRef]
- Green, H.N.; Mellanby, E. Vitamin a as an anti-infective agent. Br. Med. J. 1928, 2, 691–696. [Google Scholar] [CrossRef] [Green Version]
- Riccioni, G.; Barbara, M.; Bucciarelli, T.; di Ilio, C.; D’Orazio, N. Antioxidant vitamin supplementation in asthma. Ann Clin Lab Sci 2007, 37, 96–101. [Google Scholar]
- Villamor, E.; Fawzi, W.W. Effects of vitamin A supplementation on immune responses and correlation with clinical outcomes. Clin. Microbiol. Rev. 2005, 18, 446–464. [Google Scholar] [CrossRef] [Green Version]
- Sommer, A.; Katz, J.; Tarwotjo, I. Increased risk of respiratory disease and diarrhea in children with preexisting mild vitamin A deficiency. Am. J. Clin. Nutr. 1984, 40, 1090–1095. [Google Scholar] [CrossRef]
- Brown, C.C.; Noelle, R.J. Seeing through the dark: New insights into the immune regulatory functions of vitamin A. Eur. J. Immunol. 2015, 45, 1287–1295. [Google Scholar] [CrossRef]
- Aibana, O.; Franke, M.F.; Huang, C.C.; Galea, J.T.; Calderon, R.; Zhang, Z.; Becerra, M.C.; Smith, E.R.; Ronnenberg, A.G.; Contreras, C.; et al. Impact of Vitamin A and carotenoids on the risk of tuberculosis progression. Clin. Infect. Dis. 2017, 65, 900–909. [Google Scholar] [CrossRef] [Green Version]
- Karim, T.; Muhit, M.; Khandaker, G. Interventions to prevent respiratory diseases—Nutrition and the developing world. Paediatr. Respir. Rev. 2017, 22, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, E.; Zelig, R.; Parker, A.; Johnson, S. Vitamin A supplementation for the prevention of bronchopulmonary dysplasia in preterm infants: An update. Nutr. Clin. Pract. 2017, 32, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Araki, S.; Kato, S.; Namba, F.; Ota, E. Vitamin A to prevent bronchopulmonary dysplasia in extremely low birth weight infants: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0207730. [Google Scholar] [CrossRef] [PubMed]
- Hustead, V.A.; Gutcher, G.R.; Anderson, S.A.; Zachman, R.D. Relationship of vitamin A (retinol) status to lung disease in the preterm infant. J. Pediatr. 1984, 116, 626–633. [Google Scholar] [CrossRef]
- Hashimoto, S.; Hayashi, S.; Yoshida, S.; Kujime, K.; Maruoka, S.; Matsumoto, K.; Gon, Y.; Koura, T.; Horie, T. Retinoic acid differentially regulates interleukin-1β and interleukin-1 receptor antagonist production by human alveolar macrophages. Leuk. Res. 1998, 22, 1057–1061. [Google Scholar] [CrossRef]
- Yang, C.; Yang, X.; Du, J.; Wang, H.; Li, H.; Zeng, L.; Gu, W.; Jiang, J. Retinoic acid promotes the endogenous repair of lung stem/progenitor cells in combined with simvastatin after acute lung injury: A stereological analysis. Respir. Res. 2015, 16, 140. [Google Scholar] [CrossRef] [Green Version]
- Trottier, C.; Colombo, M.; Mann, K.K.; Miller, W.H.; Ward, B.J. Retinoids inhibit measles virus through a type I IFN-dependent bystander effect. FASEB J. 2009, 23, 3203–3212. [Google Scholar] [CrossRef]
- Jee, J.; Hoet, A.E.; Azevedo, M.P.; Vlasova, A.N.; Loerch, S.C.; Pickworth, C.L.; Hanson, J.; Saif, L.J. Effects of dietary vitamin A content on antibody responses of feedlot calves inoculated intramuscularly with an inactivated bovine coronavirus vaccine. Am. J. Vet. Res. 2013, 74, 1353–1362. [Google Scholar] [CrossRef]
- West, C.E.; Sijtsma, S.R.; Kouwenhoven, B.; Rombout, J.H.W.M.; Van der Zijpp, A.J. Epithelia-damaging virus infections affect vitamin A status in chickens. J. Nutr. 1992, 122, 333–339. [Google Scholar] [CrossRef]
- Litonjua, A.A. Fat-soluble vitamins and atopic disease: What is the evidence? Proc. Nutr. Soc. 2012, 71, 67–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S. National Library of Medicine. Anti-Inflammatory/Antioxidant Oral Nutrition Supplementation in COVID-19 (ONSCOVID19). Available online: https://clinicaltrials.gov/ct2/show/NCT04323228 (accessed on 21 August 2020).
- Spinas, E.; Saggini, A.; Kritas, S.K.; Cerulli, G.; Caraffa, A.; Antinolfi, P.; Pantalone, A.; Frydas, A.; Tei, M.; Speziali, A.; et al. Crosstalk Between Vitamin B and Immunity. J. Biol. Regul. Homeost. Agents 2015, 29, 283–288. [Google Scholar] [PubMed]
- Neri, M.; Cantatore, S.; Pomara, C.; Riezzo, I.; Bello, S.; Turillazzi, E.; Fineschi, V. Immunohistochemical expression of proinflammatory cytokines IL-1β, IL-6, TNF-α and involvement of COX-2, quantitatively confirmed by Western blot analysis, in Wernicke’s encephalopathy. Pathol. Res. Pract. 2011, 207, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.; Li, Y.; Yao, M.; Wei, Z.; Fu, Y.; Yang, Z. Niacin attenuates the production of pro-inflammatory cytokines in LPS-induced mouse alveolar macrophages by HCA2 dependent mechanisms. Int. Immunopharmacol. 2014, 23, 121–126. [Google Scholar] [CrossRef]
- Rodriguez-Melendez, R.; Zempleni, J. Regulation of gene expression by biotin (review). J. Nutr. Biochem. 2003, 14, 680–690. [Google Scholar] [CrossRef]
- Marik, P.E.; Khangoora, V.; Rivera, R.; Hooper, M.H.; Catravas, J. Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: A retrospective before-after study. Chest 2017, 151, 1229–1238. [Google Scholar] [CrossRef]
- Keil, S.D.; Bowen, R.; Marschner, S. Inactivation of Middle East respiratory syndrome coronavirus (MERS-CoV) in plasma products using a riboflavin-based and ultraviolet light-based photochemical treatment. Transfusion 2016, 56, 12. [Google Scholar] [CrossRef] [Green Version]
- Kandeel, M.; Al-Nazawi, M. Virtual screening and repurposing of FDA approved drugs against COVID-19 main protease. Life Sci. 2020, 251, 15. [Google Scholar] [CrossRef]
- Serseg, T.; Benarous, K.; Yousfi, M. Hispidin and lepidine E: Two natural compounds and folic acid as potential inhibitors of 2019-novel coronavirus Main Protease (2019-nCoVMpro), molecular docking and SAR study. Curr. Comput. Aided. Drug Des. 2020. [Google Scholar] [CrossRef]
- European Union. EU Register on Nutrition and Health Claims. Available online: https://ec.europa.eu/food/safety/labelling_nutrition/claims/register/public (accessed on 21 August 2020).
- Calder, P.C.; Carr, A.C.; Gombart, A.F.; Eggersdorfer, M. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef] [Green Version]
- Kishimoto, K.; Kobayashi, R.; Sano, H.; Suzuki, D.; Maruoka, H.; Yasuda, K.; Chida, N.; Yamada, M.; Kobayashi, K. Impact of folate therapy on combined immunodeficiency secondary to hereditary folate malabsorption. Clin. Immunol. 2014, 153, 17–22. [Google Scholar] [CrossRef]
- Vogiatzoglou, A.; Refsum, H.; Johnston, C.; Smith, S.M.; Bradley, K.M.; De Jager, C.; Budge, M.M.; Smith, A.D. Vitamin B12 status and rate of brain volume loss in community-dwelling elderly. Neurology 2008, 71, 826–832. [Google Scholar] [CrossRef]
- Rocco, A.; Compare, D.; Coccoli, P.; Esposito, C.; Spirito, A.D.; Barbato, A.; Strazzullo, P.; Nardone, G. Vitamin B12 supplementation improves rates of sustained viral response in patients chronically infected with hepatitis C virus. Gut 2013, 62, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, W.; Hardy, G. Thiamine supplementation in the critically ill. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Mallat, J.; Lemyze, M.; Thevenin, D. Do not forget to give thiamine to your septic shock patient! J. Thorac. Dis. 2016, 8, 1062–1066. [Google Scholar] [CrossRef]
- Donnino, M.W.; Andersen, L.W.; Chase, M.; Berg, K.M.; Tidswell, M.; Giberson, T.; Wolfe, R.; Moskowitz, A.; Smithline, H.; Ngo, L.; et al. Randomized, double-blind, placebo-controlled trial of thiamine as a metabolic resuscitator in septic shock: A pilot study. Crit. Care Med. 2016, 44, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.Y.; Jo, E.J.; Eom, J.S.; Mok, J.; Kim, M.H.; Kim, K.U.; Park, H.K.; Lee, M.K.; Lee, K. Combined vitamin C, hydrocortisone, and thiamine therapy for patients with severe pneumonia who were admitted to the intensive care unit: Propensity score-based analysis of a before-after cohort study. J. Crit. Care 2018, 47, 211–218. [Google Scholar] [CrossRef]
- Fimognari, F.L.; Loffredo, L.; Di Simone, S.; Sampietro, F.; Pastorelli, R.; Monaldo, M.; Violi, F.; D’Angelo, A. Hyperhomocysteinaemia and poor vitamin B status in chronic obstructive pulmonary disease. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 654–659. [Google Scholar] [CrossRef]
- Tsiligianni, I.G.; van der Molen, T. A systematic review of the role of vitamin insufficiencies and supplementation in COPD. Respir. Res. 2010, 11, 171. [Google Scholar] [CrossRef] [Green Version]
- Hilgenfeld, R. From SARS to MERS: Crystallographic studies on coronaviral proteases enable antiviral drug design. FEBS J. 2014, 281, 4085–4096. [Google Scholar] [CrossRef] [Green Version]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of Mpro from COVID-19 virus and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padayatty, S.J.; Levine, M. Vitamin C: The known and the unknown and Goldilocks. Oral Dis. 2016, 22, 463–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Russo, T.A.; Kwon, O.; Chanock, S.; Rumsey, S.C.; Levine, M. Ascorbate recycling in human neutrophils: Induction by bacteria. Proc. Natl. Acad. Sci. USA 1997, 94, 13816–13819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nualart, F.J.; Rivas, C.I.; Montecinos, V.P.; Godoy, A.S.; Guaiquil, V.H.; Golde, D.W.; Vera, J.C. Recycling of vitamin C by a bystander effect. J. Biol. Chem. 2003, 278, 10128–10133. [Google Scholar] [CrossRef] [Green Version]
- Marik, P.E. Vitamin C: An essential “stress hormone” during sepsis. J. Thorac. Dis. 2020, 2, S84–S88. [Google Scholar] [CrossRef]
- Haworth, W.; Hirst, E. Synthesis of ascorbic acid. J. Soc. Chem. Ind. 1933, 52, 645–646. [Google Scholar] [CrossRef]
- Pauling, L. Vitamin C and Common Cold. JAMA J. Am. Med. Assoc. 1971, 216, 332. [Google Scholar] [CrossRef] [Green Version]
- Pauling, L. Evolution and the need for ascorbic acid. Proc. Natl. Acad. Sci. USA 1970, 67, 1643–1648. [Google Scholar] [CrossRef] [Green Version]
- Thomas, W.R.; Holt, P.G. Vitamin C and immunity: An assessment of the evidence. Clin. Exp. Immunol. 1978, 32, 370–379. [Google Scholar]
- Webb, A.L.; Villamor, E. Update: Effects of antioxidant and non-antioxidant vitamin supplementation on immune function. Nutr. Rev. 2007, 65, 181–217. [Google Scholar] [CrossRef]
- Hemilä, H. Vitamin C and infectious diseases. In Vitamin C; Springer: Milano, Italy, 1998; pp. 73–85. [Google Scholar]
- Goode, H.F.; Webster, N.R. Free radicals and antioxidants in sepsis. Crit. Care Med. 1993, 21, 1770–1776. [Google Scholar] [CrossRef] [PubMed]
- Akaike, T.; Suga, M.; Maeda, H. Free radicals in viral pathogenesis: Molecular mechanisms involving superoxide and NO. Proc. Soc. Exp. Biol. Med. 1998, 217. [Google Scholar] [CrossRef] [PubMed]
- Peterhans, E. Oxidants and antioxidants in viral diseases: Disease mechanisms and metabolic regulation. J. Nutr. 1997, 127, 962S–965S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemilä, H. Do Vitamins C and E Affect Respiratory Infections? Doctoral Thesis, University of Helsinki, Helsinki, Iceland, 2006. [Google Scholar]
- Atherton, J.G.; Kratzing, C.C.; Fisher, A. The effect of ascorbic acid on infection of chick-embryo ciliated tracheal organ cultures by coronavirus. Arch. Virol. 1978, 56, 195–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davelaar, F.G.; Van Den Bos, J. Ascorbic acid and infectious bronchitis infections in broilers. Avian Pathol. 1992, 21, 581–589. [Google Scholar] [CrossRef] [Green Version]
- Ziegler-Heitbrock, H.W.L.; Sternsdorf, T.; Liese, J.; Belohradsky, B.; Weber, C.; Wedel, A.; Schreck, R.; Bauerle, P.; Strobel, M. Pyrrolidine dithiocarbamate inhibits NF-κB mobilization and TNF production in human monocytes. J. Immunol. 1993, 151, 6986–6993. [Google Scholar]
- Lo, S.K.; Janakidevi, K.; Lai, L.; Malik, A.B. Hydrogen peroxide-induced increase in endothelial adhesiveness is dependent on ICAM-1 activation. Am. J. Physiol. Lung Cell. Mol. Physiol. 1993, 264, L406–L412. [Google Scholar] [CrossRef]
- DeForge, L.E.; Preston, A.M.; Takeuchi, E.; Kenney, J.; Boxer, L.A.; Remick, D.G. Regulation of interleukin 8 gene expression by oxidant stress. J. Biol. Chem. 1993, 268, 25568–25576. [Google Scholar]
- Chen, Y.; Luo, G.; Yuan, J.; Wang, Y.; Yang, X.; Wang, X.; Li, G.; Liu, Z.; Zhong, N. Vitamin C mitigates oxidative stress and tumor necrosis factor-alpha in severe community-acquired pneumonia and LPS-induced macrophages. Mediat. Inflamm. 2014, 2014, 426740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auer, B.L.; Auer, D.; Rodgers, A.L. Relative hyperoxaluria, crystalluria and haematuria after megadose ingestion of vitamin C. Eur. J. Clin. Investig. 1998, 28, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.N.; Stampfer, M.J.; Curhan, G.C. Dietary factors and the risk of incident kidney stones in men: New insights after 14 years of follow-up. J. Am. Soc. Nephrol. 2004, 15, 3225–3232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemilä, H.; Chalker, E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef] [Green Version]
- Hemilä, H. Vitamin C and infections. Nutrients 2017, 9, 339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, E.M.; Goetzsche, J.M.; Grobbelaar, B.; Noakes, T.D. Vitamin C supplementation reduces the incidence of postrace symptoms of upper-respiratory-tract infection in ultramarathon runners. Am. J. Clin. Nutr. 1993, 57, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Peters, T.D. Vitamin C as effective as combinations of anti-oxidant nutrients in reducing symptoms of upper respiratory tract infection in ultramarathon runners. South Afr. J. Sport. Med. 1996, 3, 23–27. [Google Scholar]
- Moolla, M.E. The Effect of Supplemental Anti-Oxidants on the Incidence and Severity of Upper Respiratory Infections in Ultra Marathon Runners. Master’s Thesis, University of Capetown, Cape Town, South Africa, 1996. [Google Scholar]
- Sabiston, B.H.; Radomski, M.W. Health Problems and Vitamin C in Canadian Northern Military Operations; Defence and Civil Institute of Environmental Medicine Report No. 74-R-M2; University of Helsinki: Helsinki, Finland, 1974. [Google Scholar]
- Bosmann, M.; Ward, P.A. The inflammatory response in sepsis. Trends Immunol. 2013, 34, 129–136. [Google Scholar] [CrossRef]
- Grommes, J.; Soehnlein, O. Contribution of neutrophils to acute lung injury. Mol. Med. 2011, 17, 293–307. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Mohammed, B.M.; Fisher, B.J.; Kraskauskas, D.; Farkas, D.; Brophy, D.F.; Fowler, A.A.; Natarajan, R. Vitamin C: A novel regulator of neutrophil extracellular trap formation. Nutrients 2013, 5, 3131–3151. [Google Scholar] [CrossRef] [Green Version]
- Erol, N.; Saglam, L.; Saglam, Y.S.; Erol, H.S.; Altun, S.; Aktas, M.S.; Halici, M.B. The protection potential of antioxidant vitamins against acute respiratory distress syndrome: A rat trial. Inflammation 2019, 42, 1585–1594. [Google Scholar] [CrossRef]
- Fisher, B.J.; Kraskauskas, D.; Martin, E.J.; Farkas, D.; Wegelin, J.A.; Brophy, D.; Ward, K.R.; Voelkel, N.F.; Fowler, A.A.; Natarajan, R. Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, L20–L32. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maulén, N.P.; Henríquez, E.A.; Kempe, S.; Cárcamo, J.G.; Schmid-Kotsas, A.; Bachem, M.; Grünert, A.; Bustamante, M.E.; Nualart, F.; Vera, J.C. Up-regulation and polarized expression of the sodium-ascorbic acid transporter SVCT1 in post-confluent differentiated CaCo-2 cells. J. Biol. Chem. 2003, 278, 9035–9041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashiouris, M.G.; L’heureux, M.; Cable, C.A.; Fisher, B.J.; Leichtle, S.W.; Fowler, A.A. The emerging role of vitamin C as a treatment for sepsis. Nutrients 2020, 12, 292. [Google Scholar] [CrossRef] [Green Version]
- Carr, A.C.; Rosengrave, P.C.; Bayer, S.; Chambers, S.; Mehrtens, J.; Shaw, G.M. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit. Care 2017, 21, 300. [Google Scholar] [CrossRef] [Green Version]
- Fowler, A.A.; Truwit, J.D.; Hite, R.D.; Morris, P.E.; Dewilde, C.; Priday, A.; Fisher, B.; Thacker, L.R.; Natarajan, R.; Brophy, D.F.; et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: The CITRIS-ALI randomized clinical trial. JAMA J. Am. Med Assoc. 2019, 322, 1261–1270. [Google Scholar] [CrossRef]
- Marik, P.E.; Payen, D. CITRIS-ALI: How statistics were used to obfuscate the true findings. Anaesth. Crit. Care Pain Med. 2019, 38, 575–577. [Google Scholar] [CrossRef]
- Hemilä, H.; Chalker, E. Vitamin C can shorten the length of stay in the ICU: A meta-analysis. Nutrients 2019, 11, 708. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Jativa, D.F. Vitamin C supplementation in the critically ill: A systematic review and meta-analysis. SAGE Open Med. 2018, 6, 2050312118807615. [Google Scholar] [CrossRef] [Green Version]
- Marik, P.E. Patterns of death in patients with sepsis and the use of hydrocortisone, ascorbic acid, and thiamine to prevent these deaths. Surg. Infect. (Larchmt) 2018, 19, 8. [Google Scholar] [CrossRef]
- Rice, T.W.; Wheeler, A.P.; Thompson, B.T.; DeBoisblanc, B.P.; Steingrub, J.; Rock, P. Enteral omega-3 fatty acid, γ-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA J. Am. Med. Assoc. 2011, 306, 1574–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Hu, C.; Hood, M.; Zhang, X.; Zhang, L.; Kan, J.; Du, J. A novel combination of vitamin C, curcumin and glycyrrhizic acid potentially regulates immune and inflammatory response associated with coronavirus infections: A perspective from system biology analysis. Nutrients 2020, 12, 1193. [Google Scholar] [CrossRef] [PubMed]
- Hager, D.N.; Hooper, M.H.; Bernard, G.R.; Busse, L.W.; Ely, E.W.; Fowler, A.A.; Gaieski, D.F.; Hall, A.; Hinson, J.S.; Jackson, J.C.; et al. The Vitamin C, Thiamine and Steroids in Sepsis (VICTAS) Protocol: A prospective, multi-center, double-blind, adaptive sample size, randomized, placebo-controlled, clinical trial. Trials 2019, 20, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senses, H. Vitamin C Effective Against COVID-19: Expert; Anadolu Agency: Ankara, Turkey, 2020. [Google Scholar]
- Australian Government. No Evidence to Support Intravenous High-Dose Vitamin C in the Management of COVID-19; Therapeutic Goods Administration: Symonston, Australia, 2020. [Google Scholar]
- M2 PressWIRE. Single vitamins see 166% growth as consumers look to boost immunity during COVID-19 lockdown. Eastern Daylight Time, 28 April 2020.
- U.S. National Library of Medicine. Use of Ascorbic Acid in Patients with COVID 19. Available online: https://clinicaltrials.gov/ct2/show/NCT04323514 (accessed on 21 August 2020).
- U.S. National Library of Medicine. Vitamin C Infusion for the Treatment of Severe 2019-nCoV Infected Pneumonia (NCT04264533). Available online: https://clinicaltrials.gov/ct2/show/NCT04264533 (accessed on 21 August 2020).
- Mahmoudi, M.; Rezaei, N. Nutrition and Immunity; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Lips, P.; Van Schoor, N.M. The effect of vitamin D on bone and osteoporosis. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Chemistry & biology review vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.; Eisman, J.A.; Center, J.R. Vitamin D Deficiency in Critically Ill Patients. N. Engl. J. Med. 2011, 364, 1882. [Google Scholar] [CrossRef]
- Quraishi, S.A.; Litonjua, A.A.; Moromizato, T.; Gibbons, F.K.; Camargo, C.A.; Giovannucci, E.; Christopher, K.B. Association between prehospital vitamin D status and hospitalacquired bloodstream infections. Am. J. Clin. Nutr. 2013, 98, 952–959. [Google Scholar] [CrossRef] [Green Version]
- Braun, A.B.; Litonjua, A.A.; Moromizato, T.; Gibbons, F.K.; Giovannucci, E.; Christopher, K.B. Association of low serum 25-hydroxyvitamin D levels and acute kidney injury in the critically ill. Crit. Care Med. 2012, 40, 3170–3179. [Google Scholar] [CrossRef]
- Thickett, D.R.; Moromizato, T.; Litonjua, A.A.; Amrein, K.; Quraishi, S.A.; Lee-Sarwar, K.A.; Mogensen, K.M.; Purtle, S.W.; Gibbons, F.K.; Camargo, C.A.; et al. Association between prehospital vitamin d status and incident acute respiratory failure in critically ill patients: A retrospective cohort study. BMJ Open Respir. Res. 2015, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Moromizato, T.; Litonjua, A.A.; Braun, A.B.; Gibbons, F.K.; Giovannucci, E.; Christopher, K.B. Association of low serum 25-hydroxyvitamin D levels and sepsis in the critically ill. Crit. Care Med. 2014, 42, 97–107. [Google Scholar] [CrossRef]
- Braun, A.B.; Gibbons, F.K.; Litonjua, A.A.; Giovannucci, E.; Christopher, K.B. Low serum 25-hydroxyvitamin D at critical care initiation is associated with increased mortality. Crit. Care Med. 2012, 40, 63–72. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, L.; Clarke, L.; Khalilidehkordi, E.; Butzkueven, H.; Taylor, B.; Broadley, S.A. Vitamin D for the treatment of multiple sclerosis: A meta-analysis. J. Neurol. 2018, 265, 2893–2905. [Google Scholar] [CrossRef] [PubMed]
- Law, A.D.; Dutta, U.; Kochhar, R.; Vaishnavi, C.; Kumar, S.; Noor, T.; Bhadada, S.; Singh, K. Vitamin D deficiency in adult patients with ulcerative colitis: Prevalence and relationship with disease severity, extent, and duration. Indian J. Gastroenterol. 2019, 38, 6–14. [Google Scholar] [CrossRef] [PubMed]
- White, J.H. Vitamin D deficiency and the pathogenesis of Crohn’s disease. J. Steroid Biochem. Mol. Biol. 2018, 175, 23–28. [Google Scholar] [CrossRef]
- Pfeffer, P.E.; Hawrylowicz, C.M. Vitamin D in asthma: Mechanisms of action and considerations for clinical trials. Chest 2018, 153, 1229–1239. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F. Medical progress: Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Gröber, U.; Kisters, K. Influence of drugs on vitamin D and calcium metabolism. Dermatoendocrinol. 2012, 4, 158–166. [Google Scholar] [CrossRef] [Green Version]
- Medicine, I. Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Scientific Advisory Committee on Nutrition (SACN). Vitamin D and Health 2016 ii. 2016. Available online: https://www.gov.uk/government/publications/sacn-vitamin-d-and-health-report (accessed on 21 August 2020).
- Beard, J.A.; Bearden, A.; Striker, R. Vitamin D and the anti-viral state. J. Clin. Virol. 2011, 50, 194–200. [Google Scholar] [CrossRef]
- Spector, S.A. Vitamin D and HIV: Letting the sun shine in. Top. Antivir. Med. 2011, 19, 6. [Google Scholar]
- Campbell, G.R.; Spector, S.A. Autophagy induction by vitamin D inhibits both Mycobacterium tuberculosis and human immunodeficiency virus type 1. Autophagy 2012, 8, 1523–1525. [Google Scholar] [CrossRef] [Green Version]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that vitamin D supplementation could reduce risk of influenza and covid-19 infections and deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rondanelli, M.; Miccono, A.; Lamburghini, S.; Avanzato, I.; Riva, A.; Allegrini, P.; Faliva, M.A.; Peroni, G.; Nichetti, M.; Perna, S. Self-care for common colds: The pivotal role of vitamin D, vitamin C, zinc, and echinacea in three main immune interactive clusters (physical barriers, innate and adaptive immunity) involved during an episode of common colds—Practical advice on dosages and on the time to take these nutrients/botanicals in order to prevent or treat common colds. Evid. Based Complement. Altern. Med. 2018, 2018, 5813095. [Google Scholar] [CrossRef] [Green Version]
- Schwalfenberg, G.K. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol. Nutr. Food Res. 2011, 55, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Kast, J.I.; McFarlane, A.J.; Głobińska, A.; Sokolowska, M.; Wawrzyniak, P.; Sanak, M.; Schwarze, J.; Akdis, C.A.; Wanke, K. Respiratory syncytial virus infection influences tight junction integrity. Clin. Exp. Immunol. 2017, 190, 351–359. [Google Scholar] [CrossRef] [Green Version]
- McCartney, D.M.; Byrne, D.G. Optimisation of vitamin D status for enhanced immuno-protection against COVID-19. Ir. Med. J. 2020, 113, 58. [Google Scholar]
- Vankadari, N.; Wilce, J.A. Emerging WuHan (COVID-19) coronavirus: Glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg. Microbes Infect. 2020, 9, 601–604. [Google Scholar] [CrossRef]
- Skariyachan, S.; Challapilli, S.B.; Packirisamy, S.; Kumargowda, S.T.; Sridhar, V.S. Recent aspects on the pathogenesis mechanism, animal models and novel therapeutic interventions for middle east respiratory syndrome coronavirus infections. Front. Microbiol. 2019, 10, 569. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef]
- Adams, J.S.; Ren, S.; Liu, P.T.; Chun, R.F.; Lagishetty, V.; Gombart, A.F.; Borregaard, N.; Modlin, R.L.; Hewison, M. Vitamin d-directed rheostatic regulation of monocyte antibacterial responses. J. Immunol. 2009, 182, 4289–4295. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Moreno, J.; Hernandez, J.C.; Urcuqui-Inchima, S. Effect of high doses of vitamin D supplementation on dengue virus replication, toll-like receptor expression, and cytokine profiles on dendritic cells. Mol. Cell. Biochem. 2020, 464, 169–180. [Google Scholar] [CrossRef]
- Dancer, R.C.A.; Parekh, D.; Lax, S.; D’Souza, V.; Zheng, S.; Bassford, C.R.; Park, D.; Bartis, D.G.; Mahida, R.; Turner, A.M.; et al. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). Thorax 2015, 70, 617–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnett, N.; Zhao, Z.; Koyama, T.; Janz, D.R.; Wang, C.Y.; May, A.K.; Bernard, G.R.; Ware, L.B. Vitamin D deficiency and risk of acute lung injury in severe sepsis and severe trauma: A case-control study. Ann. Intensive Care 2014, 4, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, A.; Chang, D.; Mahadevappa, K.; Gibbons, F.K.; Liu, Y.; Giovannucci, E.; Christopher, K.B. Association of low serum 25-hydroxyvitamin D levels and mortality in the critically ill. Crit. Care Med. 2011, 39, 671–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantorna, M.T.; Snyder, L.; Lin, Y.D.; Yang, L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients 2015, 7, 3011–3021. [Google Scholar] [CrossRef] [Green Version]
- Jeffery, L.E.; Burke, F.; Mura, M.; Zheng, Y.; Qureshi, O.S.; Hewison, M.; Walker, L.S.K.; Lammas, D.A.; Raza, K.; Sansom, D.M. 1,25-Dihydroxyvitamin D 3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and foxp3. J. Immunol. 2009, 183, 5458–5467. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Wu, H.; Wang, C.; Xiao, Z.; Xu, F. Regulatory T cells and acute lung injury: Cytokines, uncontrolled inflammation, and therapeutic implications. Front. Immunol. 2018, 9, 1545. [Google Scholar] [CrossRef] [Green Version]
- Lei, G.S.; Zhang, C.; Cheng, B.H.; Lee, C.H. Mechanisms of action of vitamin D as supplemental therapy for Pneumocystis pneumonia. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Yang, J.; Chen, J.; Luo, Q.; Zhang, Q.; Zhang, H. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol. Med. Rep. 2017, 16, 7432–7438. [Google Scholar] [CrossRef] [Green Version]
- Hansdottir, S.; Monick, M.M.; Hinde, S.L.; Lovan, N.; Look, D.C.; Hunninghake, G.W. Respiratory epithelial cells convert inactive vitamin D to its active form: Potential effects on host defense. J. Immunol. 2008, 181, 7090–7099. [Google Scholar] [CrossRef] [Green Version]
- Imai, Y.; Kuba, K.; Rao, S.; Huan, Y.; Guo, F.; Guan, B.; Yang, P.; Sarao, R.; Wada, T.; Leong-Poi, H.; et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005, 436, 112–116. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, Y.; Kuba, K.; Penninger, J.M. The discovery of angiotensin-converting enzyme 2 and its role in acute lung injury in mice. Exp. Physiol. 2008, 93, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Jia, H. Pulmonary Angiotensin-Converting Enzyme 2 (ACE2) and inflammatory lung disease. Shock 2016, 46, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, L.; Feng, Z.; Wan, S.; Huang, P.; Sun, X.; Wen, F.; Huang, X.; Ning, G.; Wang, W. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020, 6, 1–4. [Google Scholar] [CrossRef] [Green Version]
- La Vignera, S.; Cannarella, R.; Condorelli, R.A.; Torre, F.; Aversa, A.; Calogero, A.E. Sex-Specific SARS-CoV-2 mortality: Among hormone-modulated ACE2 expression, risk of venous thromboembolism and hypovitaminosis, D. Int. J. Mol. Sci. 2020, 21, 2948. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, M.; Shyamsundar, M.; McNamee, J.; Thickett, D.; O’Kane, C.; McAuley, D. S67 Vitamin D deficiency drives pulmonary inflammation in a human model of the acute respiratory distress syndrome induced by inhaled lipopolysaccharide in healthy volunteers. Thorax 2015, 70, A40–A41. [Google Scholar] [CrossRef] [Green Version]
- Panarese, A.; Shahini, E. Letter: Covid-19, and vitamin D. Aliment. Pharmacol. Ther. 2020, 51, 993–995. [Google Scholar] [CrossRef]
- Jakovac, H. COVID-19 and vitamin D—Is there a link and an opportunity for intervention? Am. J. Physiol. Endocrinol. Metab. 2020, 318, E589. [Google Scholar] [CrossRef]
- Knibbs, J. Vitamin D deficiency: How lacking in the essential vitamin increases risk from Covid-19. Express Online 2020, 8, 570. [Google Scholar]
- Prema, S. Health expert explains why getting just 10 min of sunshine every day could make you less vulnerable to COVID-19—With low vitamin D levels increasing the risk of lung infections. Daily Mail, 28 April 2020. [Google Scholar]
- Bieber, N. Coronavirus: Vitamin D could “treat” Covid-19 as scientists launch investigation. Daily Star, 20 April 2020. [Google Scholar]
- Pinkstone, J. People with low levels of Vitamin D may be more likely to catch coronavirus and die from COVID-19 infection, study suggests. Daily. Mail, 1 May 2020. [Google Scholar]
- Pinkstone, J. More evidence vitamin D can help against coronavirus: Study finds patients with a severe deficiency are TWICE as likely to die from COVID-19. Daily Mail Online, 8 May 2020. [Google Scholar]
- Ridley, M. It is time to take seriously the link between Vitamin D deficiency and more serious Covid-19 symptoms. Telegr, 3 May 2020. [Google Scholar]
- Chapman, A. Coronavirus warning: Low levels of this vitamin is linked to worse treatment outcomes. Express Online, 30 April 2020. [Google Scholar]
- NPA Pharmacy Services Team. Updated Advice on Vitamin D Intake during COVID-19 Pandemic; National Pharmacy Association: St Albans, UK, 2020. [Google Scholar]
- Knibbs, J. Vitamin D and coronavirus: How the sunshine vitamin could help with COVID-19 infection. Express Online, 2 May 2020. [Google Scholar]
- Sunderland, C. Trinity College study says Vitamin D could help fight against Covid-19 infections. Irish Exam, 3 April 2020. [Google Scholar]
- Chapman, A. Best supplements for the immune system: The vitamin proven to prevent infection. Express Online, 4 April 2020. [Google Scholar]
- Allister Vitamin D could help in fight against Covid-19. Irish Medical Times, 6 April 2020.
- Ring, E. Irish studies find Vitamin D can build Covid-19 resistance. Irish Exam, 3 April 2020. [Google Scholar]
- Atherton, M. Coronavirus—the cheap supplements that could ‘fight off’’ COVID-19 infection. Express Online, 24 April 2020. [Google Scholar]
- Rhodes, J.; Subramanian, S.; Laird, E.; Kenny, R.A. COVID-19, vitamin D and latitude. University of Liverpool News, 21 April 2020. [Google Scholar]
- Ilie, P.C.; Stefanescu, S.; Smith, L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 2020, 1. [Google Scholar] [CrossRef]
- Rhodes, J.M.; Subramanian, S.; Laird, E.; Anne Kenny, R. Editorial: Low population mortality from COVID-19 in countries south of latitude 35 degrees North—supports vitamin D as a factor determining severity. Aliment. Pharmacol. Ther. 2020, 51, 1434–1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- COvid-19 and Vitamin D Supplementation: A Multicenter Randomized Controlled Trial of High Dose Versus Standard Dose Vitamin D3 in High-Risk COVID-19 Patients (CoVitTrial)—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04344041 (accessed on 4 May 2020).
- Vitamin D on Prevention and Treatment of COVID-19—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04334005 (accessed on 4 May 2020).
- Traber, M.G. Vitamin E Regulatory Mechanisms. Annu. Rev. Nutr. 2007, 27, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Toyoshima, K.; Yamashita, K. Dietary sesame seeds elevate α- and γ-tocotrienol concentrations in skin and adipose tissue of rats fed the tocotrienol-rich fraction extracted from palm oil. J. Nutr. 2001, 131, 2892–2897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peh, H.Y.; Tan, W.S.D.; Liao, W.; Wong, W.S.F. Vitamin E therapy beyond cancer: Tocopherol versus tocotrienol. Pharmacol. Ther. 2016, 162, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Serbinova, E.; Kagan, V.; Han, D.; Packer, L. Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Free Radic. Biol. Med. 1991, 10, 263–275. [Google Scholar] [CrossRef]
- Suzuki, Y.J.; Tsuchiya, M.; Wassall, S.R.; Choo, Y.M.; Govil, G.; Kagan, V.E.; Packer, L. Structural and dynamic membrane properties of α-tocopherol and α- tocotrienol: Implication to the molecular mechanism of their antioxidant potency. Biochemistry 1993, 32, 10692–10699. [Google Scholar] [CrossRef]
- Bieri, J.G.; Corash, L.; Van Hubbard, S. Medical Uses of Vitamin, E. N. Engl. J. Med. 1983, 308, 1063–1071. [Google Scholar] [CrossRef]
- Tappel, A.L.; Dillard, C.J. In vivo lipid peroxidation: Measurement via exhaled pentane and protection by vitamin E. Fed. Proc. 1981, 40, 174–178. [Google Scholar]
- Hafeman, D.G.; Hoekstra, W.G. Lipid Peroxidation in Vivo during Vitamin E and Selenium Deficiency in the Rat as Monitored by Ethane Evolution. J. Nutr. 1977, 107, 666–672. [Google Scholar] [CrossRef]
- Richard, C.; Lemonnier, F.; Thibault, M.; Couturier, M.; Auzepy, P. Vitamin E deficiency and lipoperoxidation during adult respiratory distress syndrome. Crit. Care Med. 1990, 18, 4–9. [Google Scholar] [CrossRef]
- Lee, G.Y.; Han, S.N. The role of vitamin E in immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meydani, S.N.; Leka, L.S.; Fine, B.C.; Dallal, G.E.; Keusch, G.T.; Singh, M.F.; Hamer, D.H. Vitamin E and respiratory tract infections in elderly nursing home residents: A randomized controlled trial. J. Am. Med. Assoc. 2004, 292, 828–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, B.T.; Chambers, R.C.; Liu, K.D. Acute respiratory distress syndrome. N. Engl. J. Med. 2017, 377, 562–572. [Google Scholar] [CrossRef]
- Wong, C.; Flynn, J.; Demling, R.H. Role of oxygen radicals in endotoxin-induced lung injury. Arch. Surg. 1984, 119, 77–82. [Google Scholar] [CrossRef]
- Takeda, K.; Shimada, Y.; Amano, M.; Sakai, T.; Okada, T.; Yoshiya, I. Plasma lipid peroxides and alpha-tocopherol in critically ill patients. Crit. Care Med. 1984, 12, 957–959. [Google Scholar] [CrossRef]
- Bertrand, Y.; Pincemail, J.; Hanique, G.; Denis, B.; Leenaerts, L.; Vankeerberghen, L.; Deby, C. Differences in tocopherol-lipid ratios in ARDS and non-ARDS patients. Intensive Care Med. 1989, 15, 87–93. [Google Scholar] [CrossRef]
- Hybertson, B.M.; Kitlowski, R.P.; Jepson, E.K.; Repine, J.E. Supercritical fluid-aerosolized vitamin E pretreatment decreases leak in isolated oxidant-perfused rat lungs. J. Appl. Physiol. 1998, 84, 263–268. [Google Scholar] [CrossRef]
- Hybertson, B.M.; Leff, J.A.; Beehler, C.J.; Barry, P.C.; Repine, J.E. Effect of vitamin E deficiency and supercritical fluid aerosolized vitamin E supplementation on interleukin-1-induced oxidative lung injury in rats. Free Radic. Biol. Med. 1995, 18, 537–542. [Google Scholar] [CrossRef]
- Wolf, H.R.D.; Seeger, H.W. Experimental and clinical results in shock lung treatment with vitamin E. Ann. N. Y. Acad. Sci. 1982, 93, 392–410. [Google Scholar] [CrossRef]
- Meydani, S.N. Vitamin E supplementation and in vivo immune response in healthy elderly subjects. JAMA 1997, 277, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Meydani, S. Age-associated changes in immune function: Impact of vitamin E intervention and the underlying mechanisms. Endocrine Metab. Immune Disord. Targets 2014, 14, 283–289. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M.; Hernanz, A.; Guayerbas, N.; Victor, V.M.; Arnalich, F. Vitamin E ingestion improves several immune functions in elderly men and women. Free Radic. Res. 2008, 42, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Hu, W.; Niu, L.; Liu, H.; Xu, H.; Xiao, S.Y. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J. Thorac. Oncol. 2020, 15, 700–704. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Beck, M.A.; Handy, J.; Levander, O.A. Host nutritional status: The neglected virulence factor. Trends Microbiol. 2004, 12, 417–423. [Google Scholar] [CrossRef]
- Front Line COVID-19 Critical Care Working Group Treatment Protocol. Available online: https://covid19criticalcare.com/math-hospital-treatment/ (accessed on 21 August 2020).
- Butt, J.; Okoli, G. BAME Covid-19 deaths demand a broader inquiry. The Guardian, 20 April 2020. [Google Scholar]
- Atherton, M. Coronavirus: Why you should be adding a supplement to your diet after government advice. Express Online, 23 April 2020. [Google Scholar]
- Welsh Government. Vitamin D Advice for Everyone: Coronavirus. Available online: https://gov.wales/vitamin-d-advice-everyone-coronavirus (accessed on 8 May 2020).
- Public Health Agency PHA Recommends Daily Vitamin D Supplement During Lockdown. Available online: https://www.publichealth.hscni.net/news/pha-recommends-daily-vitamin-d-supplement-during-lockdown (accessed on 15 May 2020).
- Scottish Government Vitamin D: Advice for All Age Groups. Available online: https://www.gov.scot/publications/vitamin-d-advice-for-all-age-groups/ (accessed on 15 May 2020).
- NHS England Vitamin, D. Available online: https://www.nhs.uk/conditions/vitamins-and-minerals/vitamin-d/ (accessed on 15 May 2020).
- Hastie, C.E.; Mackay, D.F.; Ho, F.; Celis-Morales, C.A.; Katikireddi, S.V.; Niedzwiedz, C.L.; Jani, B.D.; Welsh, P.; Mair, F.S.; Gray, S.R.; et al. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 561–565. [Google Scholar] [CrossRef]





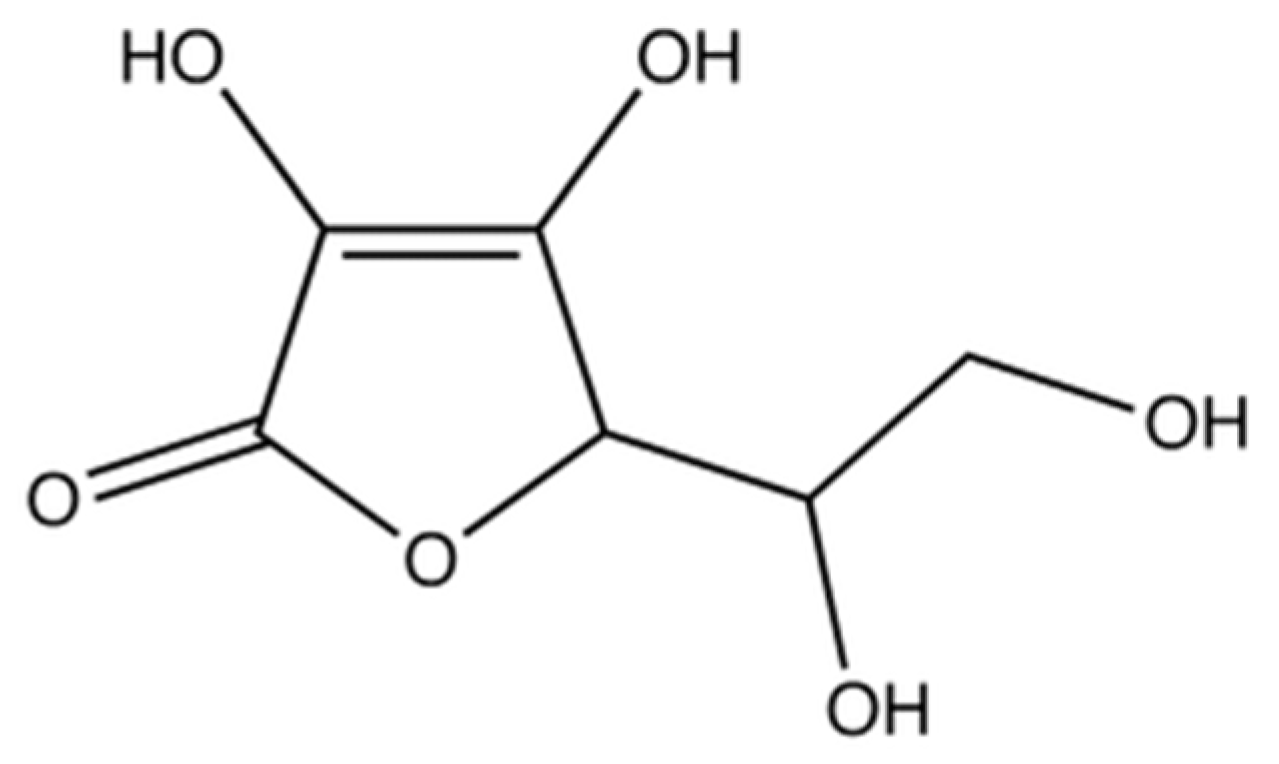




| B Vitamin | Chemical Name | Chemical Structure | Physiological Role | Evidence Related to SARS-CoV-2 Pandemic |
|---|---|---|---|---|
| B1 | Thiamine |  | Precursor of coenzymes in sugar and amino acid catabolism | IV thiamine (together with high dose vitamin C and corticosteroids) shown to prevent deaths in people with sepsis [60] |
| B2 | Riboflavin |  | Precursor of coenzymes needed for flavoprotein enzyme reactions | Riboflavin (B2) and UV light effectively reduced the titer of MERS-CoV in human plasma [61] |
| B3 | Niacin (nicotinic acid), nicotinamide, nicotinamide riboside |  | Precursor of coenzymes needed in many metabolic processes | Nicotinamide identified to have potential binding affinity for the SARS-CoV-2 protease [62] |
| B5 | Pantothenic acid |  | Precursor of coenzyme A | None to date |
| B6 | Pyridoxine, pyridoxal, pyridoxamine |  | Precursor of coenzyme in metabolic reactions | None to date |
| B7 | Biotin | Coenzyme for carboxylase enzymes needed for gluconeogenesis and fatty acid synthesis | None to date | |
| B9 | Folate |  | Precursor needed for DNA synthesis and repair especially during rapid cell division | Folate identified to have potential binding affinity to the SARS-CoV-2 protease [63] |
| B12 | Cobalamins e.g., cyanocobalamin, methylcobalamin |  | Coenzyme in metabolic reactions affecting DNA, fatty acid and amino acid metabolism | Vitamin B12 identified to have potential binding affinity to the SARS-CoV-2 protease [62] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jovic, T.H.; Ali, S.R.; Ibrahim, N.; Jessop, Z.M.; Tarassoli, S.P.; Dobbs, T.D.; Holford, P.; Thornton, C.A.; Whitaker, I.S. Could Vitamins Help in the Fight Against COVID-19? Nutrients 2020, 12, 2550. https://doi.org/10.3390/nu12092550
Jovic TH, Ali SR, Ibrahim N, Jessop ZM, Tarassoli SP, Dobbs TD, Holford P, Thornton CA, Whitaker IS. Could Vitamins Help in the Fight Against COVID-19? Nutrients. 2020; 12(9):2550. https://doi.org/10.3390/nu12092550
Chicago/Turabian StyleJovic, Thomas H, Stephen R Ali, Nader Ibrahim, Zita M Jessop, Sam P Tarassoli, Thomas D Dobbs, Patrick Holford, Catherine A Thornton, and Iain S Whitaker. 2020. "Could Vitamins Help in the Fight Against COVID-19?" Nutrients 12, no. 9: 2550. https://doi.org/10.3390/nu12092550
APA StyleJovic, T. H., Ali, S. R., Ibrahim, N., Jessop, Z. M., Tarassoli, S. P., Dobbs, T. D., Holford, P., Thornton, C. A., & Whitaker, I. S. (2020). Could Vitamins Help in the Fight Against COVID-19? Nutrients, 12(9), 2550. https://doi.org/10.3390/nu12092550





