Clinical Trials for Use of Melatonin to Fight against COVID-19 Are Urgently Needed
Abstract
:1. Introduction
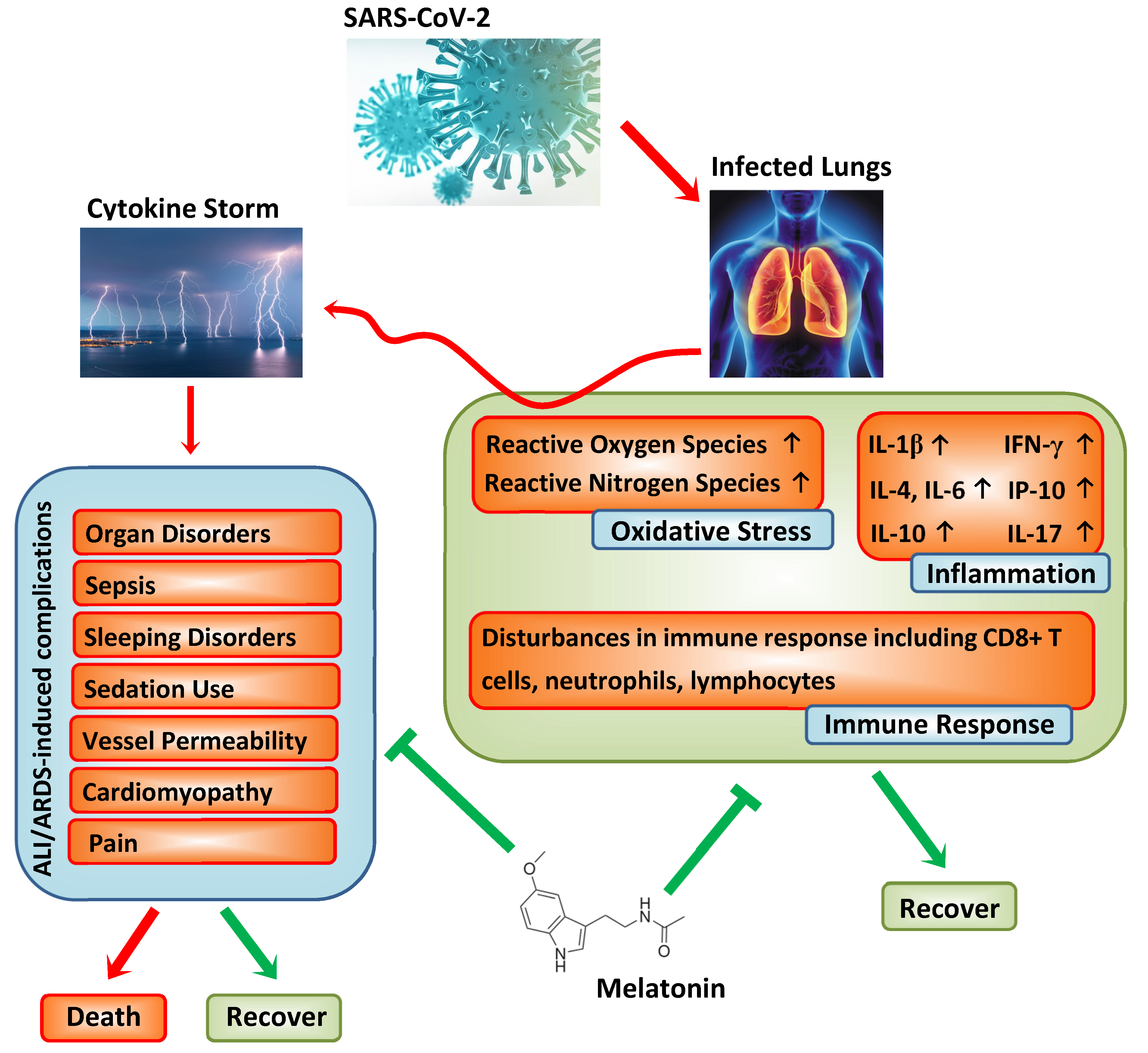
2. Pathogenesis of COVID-19
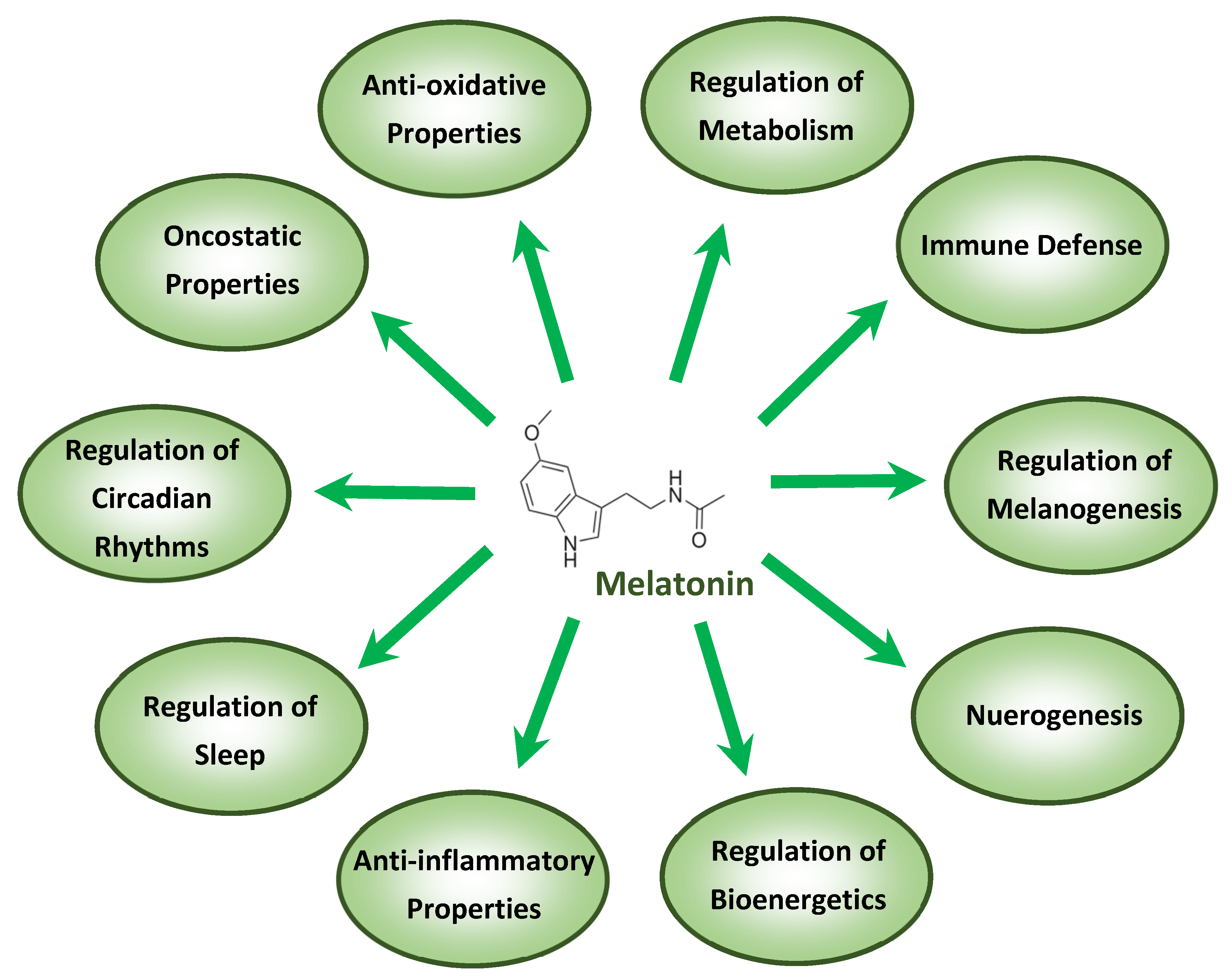
3. Melatonin and Its Anti-Inflammatory and Anti-Oxidative Properties
4. Melatonin and Immunomodulation
5. Melatonin and Its Adjuvant Effects
6. Melatonin and Its Safety
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Centre for Disease Prevention and Control. COVID-19 Situation Update Worldwide, as of 20 July 2020. Available online: https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases (accessed on 22 July 2020).
- Li, G.; Fan, Y.; Lai, Y.; Han, T.; Li, Z.; Zhou, P.; Pan, P.; Wang, W.; Hu, D.; Liu, X.; et al. Coronavirus infections and immune responses. J. Med. Virol. 2020, 92, 424–432. [Google Scholar] [CrossRef]
- Kleszczyński, K.; Tukaj, S.; Kruse, N.; Zillikens, D.; Fischer, T.W. Melatonin prevents ultraviolet radiation-induced alterations in plasma membrane potential and intracellular pH in human keratinocytes. J. Pineal Res. 2013, 54, 89. [Google Scholar] [CrossRef]
- Hardeland, R. Aging, melatonin, and the pro- and anti-inflammatory networks. Int. J. Mol. Sci. 2019, 20, 1223. [Google Scholar] [CrossRef] [Green Version]
- Hardeland, R. Melatonin and inflammation-story of a double-edged blade. J. Pineal Res. 2018, 65, e12525. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Zhang, Z.; Sun, H.; Song, J.; Chen, X.; Huang, J.; Lin, X.; Zhou, R. Effect of melatonin on T/B cell activation and immune regulation in pinealectomy mice. Life Sci. 2020, 242, 117191. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Castillo, R.R.; Quizon, G.R.A.; Juco, M.J.M.; Roman, A.D.E.; de Leon, D.G.; Punzalan, F.E.R.; Guingon, R.B.L.; Morales, D.D.; Tan, D.X.; Reiter, R.J. Melatonin as adjuvant treatment for coronavirus disease 2019 pneumonia patients requiring hospitalization (MAC-19 PRO): A case series. Melatonin Res. 2020, 3, 297–310. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Z.; Xu, L.; Xie, X.Y.; Yan, H.L.; Xie, B.J.; Xu, W.Z.; Liu, X.A.; Kang, G.J.; Jiang, W.L.; Yuan, J.P. Pulmonary pathology of early phase COVID-19 pneumonia in a patient with a benign lung lesion. Histopathology 2020. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, X.; Tong, Q.; Li, W.; Wang, B.; Sutter, K.; Trilling, M.; Lu, M.; Dittmer, U.; Yang, D. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV. J. Med. Virol. 2020, 92, 491–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Wang, X.; Ni, L.; Di, X.; Ma, B.; Niu, S.; Liu, C.; Reiter, R.J. COVID-19: Melatonin as a potential adjuvant treatment. Life Sci. 2020, 250, 117583. [Google Scholar] [CrossRef] [PubMed]
- Channappanavar, R.; Perlman, S. Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017, 39, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Channappanavar, R.; Fehr, A.R.; Vijay, R.; Mack, M.; Zhao, J.; Meyerholz, D.K.; Perlman, S. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe 2016, 19, 181–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smits, S.L.; de Lang, A.; van den Brand, J.M.A.; Leijten, L.M.; van IJcken, W.F.; Eijkemans, M.J.C.; van Amerongen, G.; Kuiken, T.; Andeweg, A.C.; Osterhaus, A.D.M.E.; et al. Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathog. 2010, 6, e1000756. [Google Scholar] [CrossRef] [Green Version]
- Anderson, G.; Reiter, R.J. Melatonin: Roles in influenza, Covid-19, and other viral infections. Rev. Med. Virol. 2020, 30, e2109. [Google Scholar] [CrossRef]
- Boga, J.A.; Coto-Montes, A.; Rosales-Corral, S.A.; Tan, D.X.; Reiter, R.J. Beneficial actions of melatonin in the management of viral infections: A new use for this “molecular handyman”? Rev. Med. Virol. 2012, 22, 323–338. [Google Scholar] [CrossRef]
- Kleszczyński, K.; Zillikens, D.; Fischer, T.W. Melatonin enhances mitochondrial ATP synthesis, reduces reactive oxygen species formation, and mediates translocation of the nuclear erythroid 2-related factor 2 resulting in activation of phase-2 antioxidant enzymes (γ-GCS, HO-1, NQO1) in ultraviolet radiation-treated normal human epidermal keratinocytes (NHEK). J. Pineal Res. 2016, 61, 187–197. [Google Scholar]
- Kleszczyński, K.; Zwicker, S.; Tukaj, S.; Kasperkiewicz, M.; Zillikens, D.; Wolf, R.; Fischer, T.W. Melatonin compensates silencing of heat shock protein 70 and suppresses ultraviolet radiation-induced inflammation in human skin ex vivo and cultured keratinocytes. J. Pineal Res. 2015, 58, 117–126. [Google Scholar] [CrossRef]
- Slominski, A.T.; Hardeland, R.; Zmijewski, M.A.; Slominski, R.M.; Reiter, R.J.; Paus, R. Melatonin: A cutaneous perspective on its production, metabolism, and functions. J. Invest. Dermatol. 2018, 138, 490–499. [Google Scholar] [CrossRef] [Green Version]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef]
- Huang, C.C.; Chiou, C.H.; Liu, S.C.; Hu, S.L.; Su, C.M.; Tsai, C.H.; Tang, C.H. Melatonin attenuates TNF-α and IL-1β expression in synovial fibroblasts and diminishes cartilage degradation: Implications for the treatment of rheumatoid arthritis. J. Pineal Res. 2019, 66, e12560. [Google Scholar] [CrossRef]
- Ling, Y.; Li, Z.Z.; Zhang, J.F.; Zheng, X.W.; Lei, Z.Q.; Chen, R.Y.; Feng, J.H. MicroRNA-494 inhibition alleviates acute lung injury through Nrf2 signaling pathway via NQO1 in sepsis-associated acute respiratory distress syndrome. Life Sci. 2018, 210, 1–8. [Google Scholar] [CrossRef]
- Pedrosa, A.M.C.; Weinlich, R.; Mognol, G.P.; Robbs, B.K.; Viola, J.P.B.; Campa, A.; Amarante-Mendes, G.P. Melatonin protects CD4+ T cells from activation-induced cell death by blocking NFAT-mediated CD95 ligand upregulation. J. Immunol. 2010, 184, 3487–3494. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.K.; Lee, F.Y.; Kao, Y.H.; Chiang, H.J.; Sung, P.H.; Tsai, T.H.; Lin, Y.C.; Leu, S.; Wu, Y.C.; Lu, H.I.; et al. Systemic combined melatonin-mitochondria treatment improves acute respiratory distress syndrome in the rat. J. Pineal Res. 2015, 58, 137–150. [Google Scholar] [CrossRef]
- Habtemariam, S.; Daglia, M.; Sureda, A.; Selamoglu, Z.; Gulhan, M.F.; Nabavi, S.M. Melatonin and respiratory diseases: A review. Curr. Top. Med. Chem. 2017, 17, 467–488. [Google Scholar] [CrossRef]
- Tan, D.X.; Chen, L.D.; Poeggeler, B.; Manchester, L.C.; Reiter, R.J. Melatonin: A potent, endogenous hydroxyl radical scavenger. Endocr. J. 1993, 1, 57–60. [Google Scholar]
- Reiter, R.J.; Poeggeler, B.; Tan, D.X.; Chen, L.D.; Manchester, L.C.; Guerrero, J.M. Antioxidant capacity of melatonin: A novel action not requiring a receptor. Neuroendocrinol. Lett. 1993, 15, 103–116. [Google Scholar]
- Tan, D.X.; Reiter, R.J.; Manchester, L.C.; Yan, M.T.; El-Sawi, M.; Sainz, R.M.; Mayo, J.C.; Kohen, R.; Allegra, M.; Hardeland, R. Chemical and physical properties and potential mechanisms: Melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2002, 2, 181–197. [Google Scholar] [CrossRef] [Green Version]
- Acuña-Castroviejo, D.; Rahim, I.; Acuña-Fernández, C.; Fernández-Ortiz, M.; Solera-Marín, J.; Sayed, R.K.A.; Díaz-Casado, M.E.; Rusanova, I.; López, L.C.; Escames, G. Melatonin, clock genes and mitochondria in sepsis. Cell. Mol. Life Sci. 2017, 74, 3965–3987. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and the electron transport chain. Cell. Mol. Life Sci. 2017, 74, 3883–3896. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Rosales-Corral, S.; Tan, D.X.; Jou, M.J.; Galano, A.; Xu, B. Melatonin as a mitochondria-targeted antioxidant: One of evolution’s best ideas. Cell. Mol. Life Sci. 2017, 74, 3863–3881. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Zhou, X.J.; Xu, B. Mitochondria: Central organelles for melatonin’s antioxidant and anti-aging actions. Molecules 2018, 23, 509. [Google Scholar] [CrossRef] [Green Version]
- Tan, D.X.; Manchester, L.C.; Qin, L.; Reiter, R.J. Melatonin: A mitochondrial targeting molecule involving mitochondrial protection and dynamics. Int. J. Mol. Sci. 2016, 17, 2124. [Google Scholar] [CrossRef]
- Wongprayoon, P.; Govitrapong, P. Melatonin as a mitochondrial protector in neurodegenerative diseases. Cell. Mol. Life Sci. 2017, 74, 3999–4014. [Google Scholar] [CrossRef]
- Pedreira, P.R.; García-Prieto, E.; Parra, D.; Astudillo, A.; Diaz, E.; Taboada, F.; Albaiceta, G.M. Effects of melatonin in an experimental model of ventilator-induced lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 295, L820–L827. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.C.; Peng, C.K.; Liao, W.I.; Pao, H.P.; Huang, K.L.; Chu, S.J. Melatonin receptor agonist protects against acute lung injury induced by ventilator through up-regulation of IL-10 production. Respir. Res. 2020, 21, 65. [Google Scholar] [CrossRef] [Green Version]
- Bouhafs, R.K.L.; Jarstrand, C. Effects of antioxidants on surfactant peroxidation by stimulated human polymorphonuclear leukocytes. Free Radic. Res. 2002, 36, 727–734. [Google Scholar] [CrossRef]
- Ceraulo, L.; Ferrugia, M.; Tesoriere, L.; Segreto, S.; Livrea, M.A.; Liveri, V.T. Interactions of melatonin with membrane models: Portioning of melatonin in AOT and lecithin reversed micelles. J. Pineal Res. 1999, 26, 108–112. [Google Scholar] [CrossRef]
- Tan, D.X.; Hardeland, R. Potential utility of melatonin in deadly infectious diseases related to the overreaction of innate immune response and destructive inflammation: Focus on COVID-19. Melatonin Res. 2020, 3, 120–143. [Google Scholar] [CrossRef]
- Reiter, R.J.; Ma, Q.; Sharma, R. Treatment of Ebola and other infectious diseases: Melatonin “goes viral”. Melatonin Res. 2020, 3, 43–57. [Google Scholar] [CrossRef]
- Chen, H.H.; Chang, C.L.; Lin, K.C.; Sung, P.H.; Chai, H.T.; Zhen, Y.Y.; Chen, Y.C.; Wu, Y.C.; Leu, S.; Tsai, T.H.; et al. Melatonin augments apoptotic adipose-derived mesenchymal stem cell treatment against sepsis-induced acute lung injury. Am. J. Transl. Res. 2014, 6, 439–458. [Google Scholar] [PubMed]
- Wang, M.L.; Wei, C.H.; Wang, W.D.; Wang, J.S.; Zhang, J.; Wang, J.J. Melatonin attenuates lung ischaemia-reperfusion injury via inhibition of oxidative stress and inflammation. Interact. Cardiov. Thorac. Surg. 2018, 26, 761–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarma, J.V.; Ward, P.A. Oxidants and redox signaling in acute lung injury. Compr. Physiol. 2011, 1, 1365–1381. [Google Scholar] [PubMed]
- Gitto, E.; Reiter, R.J.; Cordaro, S.P.; La, R.M.; Chiurazzi, P.; Trimarchi, G.; Gitto, P.; Calabrò, M.P.; Barberi, I. Oxidative and inflammatory parameters in respiratory distress syndrome of preterm newborns: Beneficial effects of melatonin. Am. J. Perinatol. 2004, 21, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Gitto, E.; Reiter, R.J.; Sabatino, G.; Buonocore, G.; Romeo, C.; Gitto, P.; Buggé, C.; Trimarchi, G.; Barberi, I. Correlation among cytokines, bronchopulmonary dysplasia and modality of ventilation in preterm newborns: Improvement with melatonin treatment. J. Pineal Res. 2005, 39, 287–293. [Google Scholar] [CrossRef]
- Rogers, M.C.; Williams, J.V. Quis Custodiet Ipsos Custodes? Regulation of cell mediated immune responses following viral lung infections. Annu. Rev. Virol. 2018, 5, 363–383. [Google Scholar] [CrossRef]
- Dubocovich, M.L.; Markowska, M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine 2005, 27, 101–110. [Google Scholar] [CrossRef]
- Maestroni, G.J.; Conti, A.; Pierpaoli, W. Role of the pineal gland in immunity. Circadian synthesis and release of melatonin modulates the antibody response and antagonizes the immunosuppressive effect of corticosterone. J. Neuroimmunol. 1986, 13, 19–30. [Google Scholar] [CrossRef]
- Guerrero, J.M.; Reiter, R.J. Melatonin-Immune system relationships. Curr. Top. Med. Chem. 2002, 2, 167–179. [Google Scholar] [CrossRef]
- Maestroni, G.J. The immunotherapeutic potential of melatonin. Expert Opin. Investig. Drugs 2001, 10, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Y.; Zhang, C.; Huang, F.; Wang, F.; Yuan, J.; Wang, Z.; Li, J.; Feng, C.; Zhang, Z.; et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020, 63, 364–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, S.C.; Pandi-Perumal, S.R.; Esquifino, A.I.; Cardinali, D.P.; Maestroni, G.J.M. The role of melatonin in immuno-enhancement: Potential application in cancer. Int. J. Exp. Pathol. 2006, 87, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Zhang, Z.; Xie, T.; Ji, J.; Xu, J.; Lin, L.; Yan, J.; Kang, A.; Dai, Q.; Dong, Y.; et al. Rhein suppresses lung inflammatory injury induced by human respiratory syncytial virus through inhibiting NLRP3 inflammasome activation via NF-κB pathway in mice. Front. Pharmacol. 2019, 10, 1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tate, M.D.; Ong, J.D.H.; Dowling, J.K.; McAuley, J.L.; Robertson, A.B.; Latz, E.; Drummond, G.R.; Cooper, M.A.; Hertzog, P.J.; Mansell, A. Reassessing the role of the NLRP3 inflammasome during pathogenic influenza a virus infection via temporal inhibition. Sci. Rep. 2016, 6, 27912. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Ji, H.; Wang, Y.; Gu, C.; Gu, W.; Hu, L.; Zhu, L. Melatonin alleviates radiation-induced lung injury via regulation of miR-30e/NLRP3 axis. Oxidat. Med. Cell. Longev. 2019, 2019, 4087298. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.M.; Xie, Q.M.; Zhao, C.C.; Xu, J.; Fan, X.Y.; Fei, G.H. Melatonin biosynthesis restored by CpG oligodeoxynucleotides attenuates allergic airway inflammation via regulating NLRP3 inflammasome. Life Sci. 2019, 239, 117067. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.X.; Grailer, J.J.; Wang, N.; Wang, M.; Yao, J.; Zhong, R.; Gao, G.F.; Ward, P.A.; Tan, D.X.; et al. Melatonin alleviates acute lung injury through inhibiting the NLRP3 inflammasome. J. Pineal Res. 2016, 60, 405–414. [Google Scholar] [CrossRef]
- Cheng, J.; Yang, H.L.; Gu, C.J.; Liu, Y.K.; Shao, J.; Zhu, R.; He, Y.Y.; Zhu, X.Y.; Li, M.Q. Melatonin restricts the viability and angiogenesis of vascular endothelial cells by suppressing HIF-1alpha/ROS/VEGF. Int. J. Mol. Med. 2019, 43, 945–955. [Google Scholar]
- Chen, J.; Xia, H.; Zhang, L.; Zhang, H.; Wang, D.; Tao, X. Protective effects of melatonin on sepsis-induced liver injury and dysregulation of gluconeogenesis in rats through activating SIRT1/STAT3 pathway. Biomed. Pharmacother. 2019, 117, 109150. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Xie, W.; Hu, S.; Zhou, H.; Zhu, P.; Zhu, H. Melatonin attenuates ER stress and mitochondrial damage in septic cardiomyopathy: A new mechanism involving BAP31 upregulation and MAPK-ERK pathway. J. Cell. Physiol. 2020, 235, 2847–2856. [Google Scholar] [CrossRef] [PubMed]
- Nduhirabandi, F.; Lamont, K.; Albertyn, Z.; Opie, L.H.; Lecour, S. Role of toll-like receptor 4 in melatonin-induced cardioprotection. J. Pineal Res. 2016, 60, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, functions and therapeutic benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.R.; Pritchard, M.W.; Schofield-Robinson, O.J.; Alderson, P.; Smith, A.F. Melatonin for the promotion of sleep in adults in the intensive care unit. Cochrane Database Syst. Rev. 2018, 5, CD012455. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, K.; Malkiewicz, M.A.; Sieminski, M.; Cubala, W.J.; Winklewski, P.J.; Medrzycka-Dabrowska, W.A. The role of melatonin and melatonin receptor agonist in the prevention of sleep disturbances and delirium in intensive care unit—A clinical review. Sleep Med. 2020, 69, 127–134. [Google Scholar] [CrossRef]
- Mistraletti, G.; Umbrello, M.; Sabbatini, G.; Miori, S.; Taverna, M.; Cerri, B.; Mantovani, E.S.; Formenti, P.; Spanu, P.; D’Agostino, A.; et al. Melatonin reduces the need for sedation in ICU patients: A randomized controlled trial. Minerva Anestesiol. 2015, 81, 1298–1310. [Google Scholar]
- Bi, X.; Su, Z.; Yan, H.; Du, J.; Wang, J.; Chen, L.; Peng, M.; Chen, S.; Shen, B.; Li, J. Prediction of severe illness due to COVID-19 based on an analysis of initial fibrinogen to albumin ratio and platelet count. Platelets 2020, 31, 674–679. [Google Scholar] [CrossRef]
- Tan, D.X.; Korkmaz, A.; Reiter, R.J.; Manchester, L.C. Ebola virus disease: Potential use of melatonin as a treatment. J. Pineal Res. 2014, 57, 381–384. [Google Scholar] [CrossRef]
- Galley, H.F.; Lowes, D.A.; Allen, L.; Cameron, G.; Aucott, L.S.; Webster, N.R. Melatonin as a potential therapy for sepsis: A phase I dose escalation study and an ex vivo whole blood model under conditions of sepsis. J. Pineal Res. 2014, 56, 427–438. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, V.; Spence, D.W.; Moscovitch, A.; Pandi-Perumal, S.R.; Trakht, I.; Brown, G.M.; Cardinali, D.P. Malaria: Therapeutic implications of melatonin. J. Pineal Res. 2010, 48, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Michaelides, M.; Stover, N.B.; Francis, P.J.; Weleber, R.G.M. Retinal toxicity associated with hydroxychloroquine and chloroquine. Arch. Ophthalmol. 2011, 129, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Kaptanoglu, E.; Tuncel, M.; Palaoglu, S.; Konan, A.; Demirpençe, E.; Kilinç, K. Comparison of the effects of melatonin and methylprednisolone in experimental spinal cord injury. J. Neurosur. 2000, 93, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Cayli, S.R.; Kocak, A.; Yilmaz, U.; Tekiner, A.; Erbil, M.; Ozturk, C.; Batcioglu, K.; Yologlu, S. Effect of combined treatment with melatonin and methylprednisolone on neurological recovery after experimental spinal cord injury. Eur. Spine J. 2004, 13, 724–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.H.; Lia, C.L.; Chen, S.J.; Shia, L.G.; Lin, L.; Chen, Y.W.; Cheng, C.P.; Sytwu, H.K.; Shang, S.T.; Lin, G.J. Melatonin possesses an anti-influenza potential through its immune modulatory effect. J. Funct. Foods 2019, 58, 189–198. [Google Scholar] [CrossRef]
- Hardeland, R.; Cardinali, D.P.; Srinivasan, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin —A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011, 93, 350–384. [Google Scholar] [CrossRef] [Green Version]
- Lanoix, D.; Lacasse, A.A.; Reiter, R.J.; Vaillancourt, C. Melatonin: The smart killer: The human trophoblast as a model. Mol. Cell. Endocrinol. 2012, 348, 1–11. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Zhou, Z.; Cruz, M.H.; Fuentes-Broto, L.; Galano, A. Phytomelatonin: Assisting plants to survive and thrive. Molecules 2015, 20, 7396–7437. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.; Tobin, D.J.; Zmijewski, M.A.; Wortsman, J.; Paus, R. Melatonin in the skin: Synthesis, metabolism and functions. Trends Endocrinol. Metab. 2008, 19, 17–24. [Google Scholar] [CrossRef]
- Sánchez-López, A.L.; Ortiz, G.G.; Pacheco-Moises, F.P.; Mireles-Ramírez, M.A.; Bitzer-Quintero, O.K.; Delgado-Lara, D.L.C.; Ramírez-Jirano, R.J.; Velázquez-Brizuela, I.E. Efficacy of melatonin on serum pro-inflammatory cytokines and oxidative stress markers in relapsing remitting multiple sclerosis. Arch. Med. Res. 2018, 49, 391–398. [Google Scholar] [CrossRef]
- Zhao, Z.; Lu, C.; Li, T.; Wang, W.; Ye, W.; Zeng, R.; Ni, L.; Lai, Z.; Wang, X.; Liu, C. The protective effect of melatonin on brain ischemia and reperfusion in rats and humans: In Vivo assessment and a randomized controlled trial. J. Pineal Res. 2018, 65, e12521. [Google Scholar] [CrossRef]
- Shafiei, E.; Bahtoei, M.; Raj, P.; Ostovar, A.; Iranpour, D.; Akbarzadeh, S.; Shahryari, H.; Anvaripour, A.; Tahmasebi, R.; Netticadan, T.; et al. Effects of N-acetyl cysteine and melatonin on early reperfusion injury in patients undergoing coronary artery bypass grafting: A randomized, open-labeled, placebo-controlled trial. Medicine 2018, 97, e11383. [Google Scholar] [CrossRef] [PubMed]
- Nordlund, J.J.; Lerner, A.B. The effects of oral melatonin on skin color and on the release of pituitary hormones. J. Clin. Endocrinol. Metab. 1977, 45, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Weishaupt, J.H.; Bartels, C.; Pölking, E.; Dietrich, J.; Rohde, G.; Poeggeler, B.; Mertens, N.; Sperling, S.; Bohn, M.; Hüther, G.; et al. Reduced oxidative damage in ALS by high-dose enteral melatonin treatment. J. Pineal Res. 2006, 41, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Nickkholgh, A.; Schneider, H.; Sobirey, M.; Venetz, W.P.; Hinz, U.; Pelzl, L.H.; Gotthardt, D.N.; Cekauskas, A.; Manikas, M.; Mikalauskas, S.; et al. The use of high-dose melatonin in liver resection is safe: First clinical experience. J. Pineal Res. 2011, 50, 381–388. [Google Scholar] [CrossRef]
- Henderson, R.; Kim, S.; Lee, E. Use of melatonin as adjunctive therapy in neonatal sepsis: A systematic review and meta-analysis. Complement. Ther. Med. 2018, 39, 131–136. [Google Scholar] [CrossRef]
- El-Gendy, F.M.; El-Hawy, M.A.; Hassan, M.G. Beneficial effect of melatonin in the treatment of neonatal sepsis. J. Matern. Fetal. Neonatal. Med. 2018, 31, 2299–2303. [Google Scholar] [CrossRef]
- Slominski, A.T.; Semak, I.; Fischer, T.W.; Kim, T.K.; Kleszczyński, K.; Hardeland, R.; Reiter, R.J. Metabolism of melatonin in the skin: Why is it important? Exp. Dermatol. 2017, 26, 563–568. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kleszczyński, K.; Slominski, A.T.; Steinbrink, K.; Reiter, R.J. Clinical Trials for Use of Melatonin to Fight against COVID-19 Are Urgently Needed. Nutrients 2020, 12, 2561. https://doi.org/10.3390/nu12092561
Kleszczyński K, Slominski AT, Steinbrink K, Reiter RJ. Clinical Trials for Use of Melatonin to Fight against COVID-19 Are Urgently Needed. Nutrients. 2020; 12(9):2561. https://doi.org/10.3390/nu12092561
Chicago/Turabian StyleKleszczyński, Konrad, Andrzej T. Slominski, Kerstin Steinbrink, and Russel J. Reiter. 2020. "Clinical Trials for Use of Melatonin to Fight against COVID-19 Are Urgently Needed" Nutrients 12, no. 9: 2561. https://doi.org/10.3390/nu12092561
APA StyleKleszczyński, K., Slominski, A. T., Steinbrink, K., & Reiter, R. J. (2020). Clinical Trials for Use of Melatonin to Fight against COVID-19 Are Urgently Needed. Nutrients, 12(9), 2561. https://doi.org/10.3390/nu12092561








