Dietary Complex and Slow Digestive Carbohydrates Prevent Fat Deposits During Catch-Up Growth in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Housing
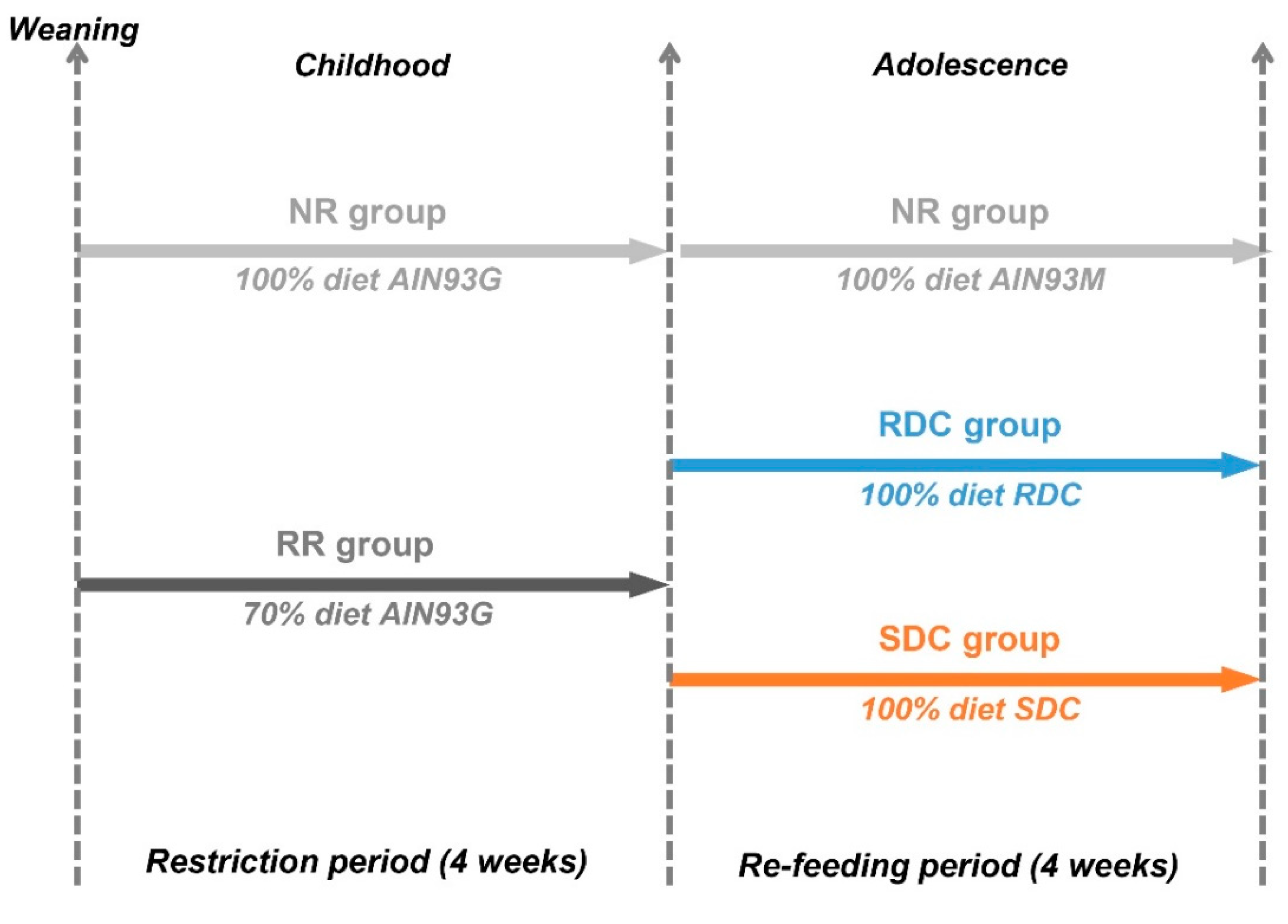
2.2. Experimental Design
2.3. Body Composition
2.4. Indirect Calorimetry
2.5. Biochemical Parameters
2.6. Western Blot Analysis
2.7. Quantitative Real-Time PCR
2.8. Glycogen Quantification
2.9. Quantitative Determination of Creatine Kinase (CK)
2.10. Statistical Analysis
3. Results
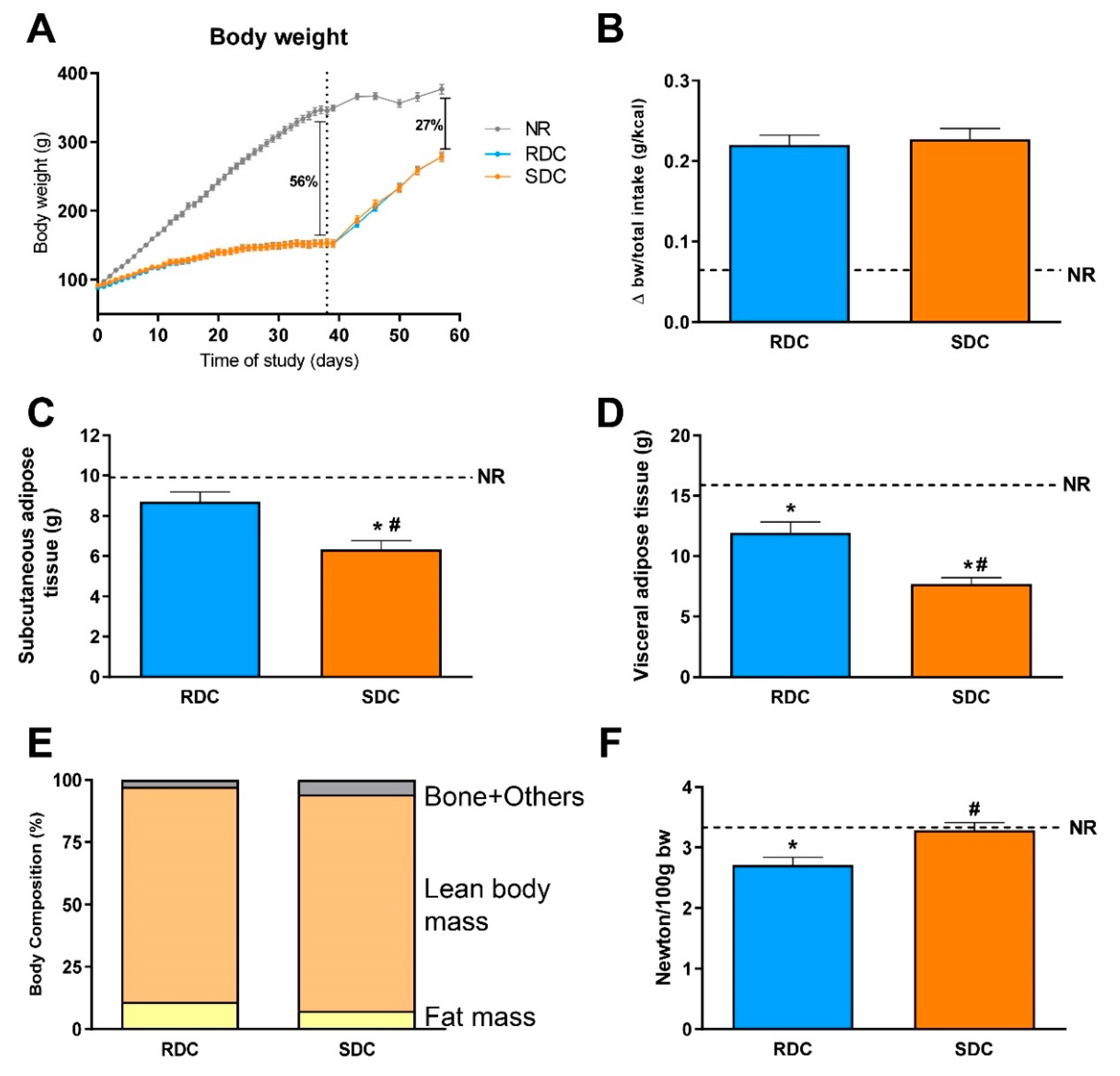
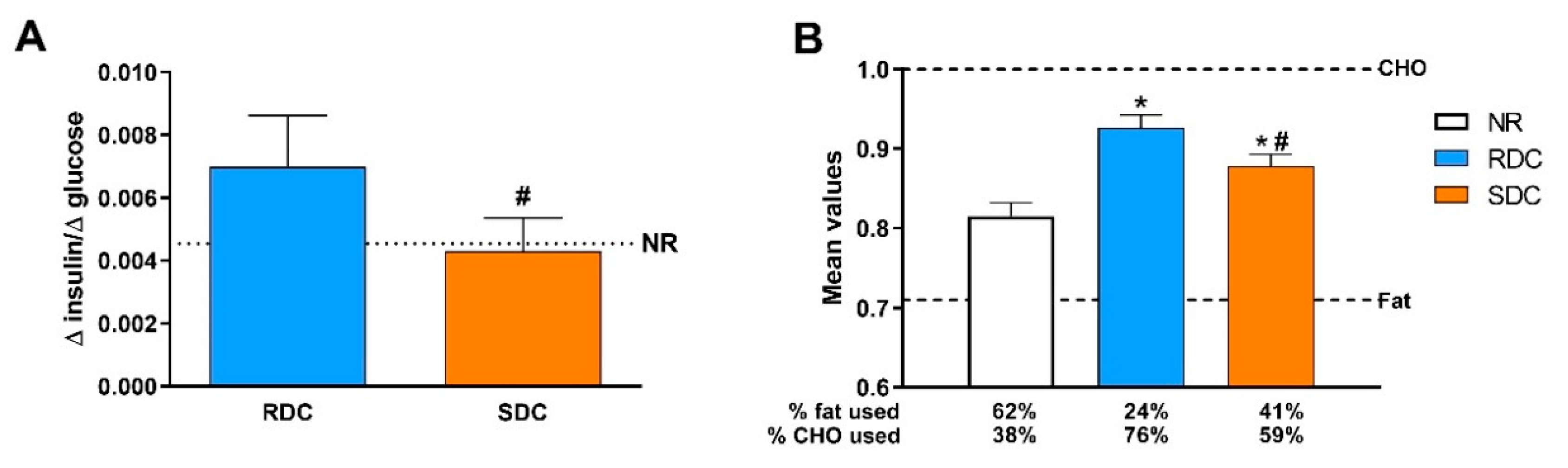
3.1. Experimental Catch-up Grow Model
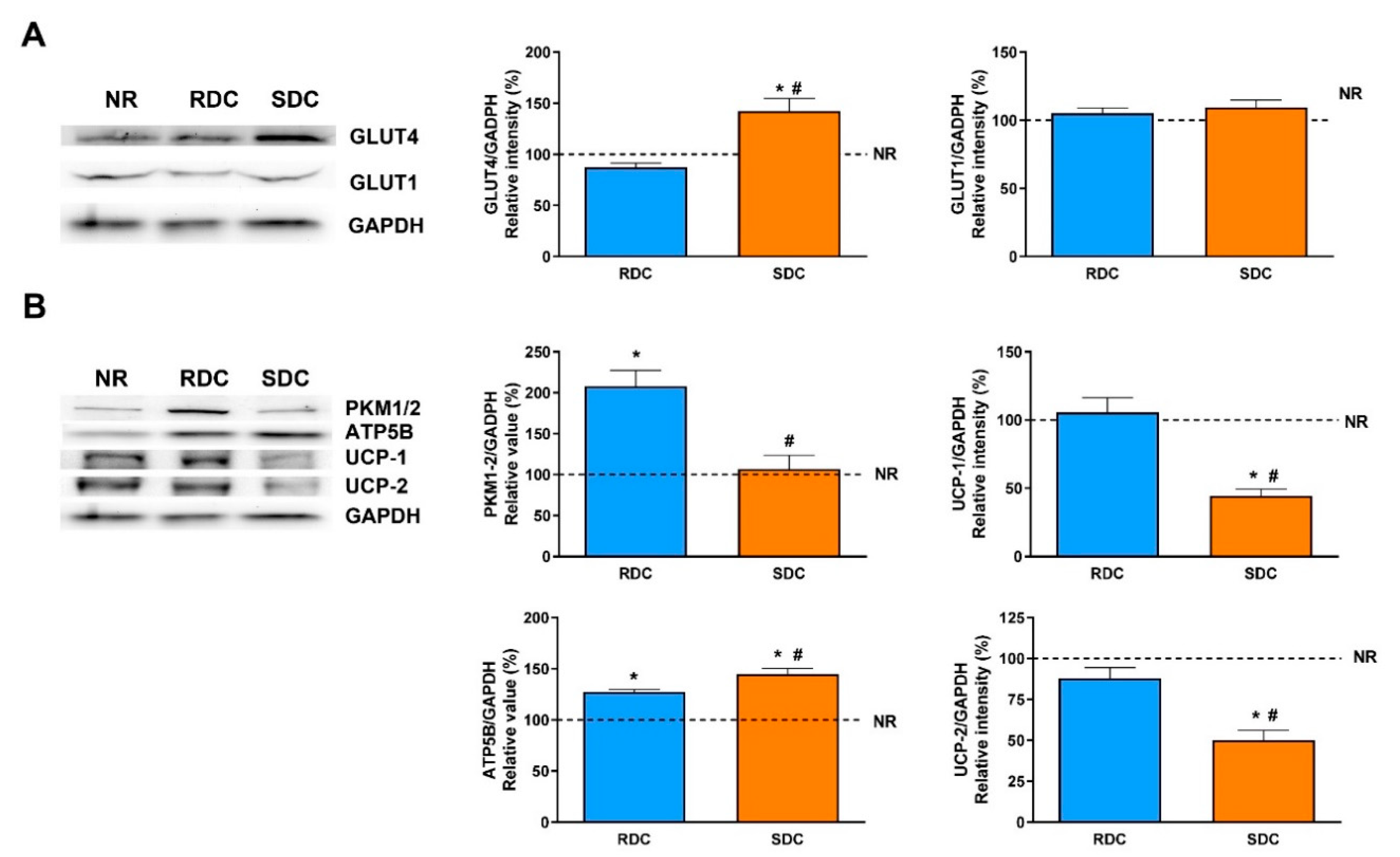
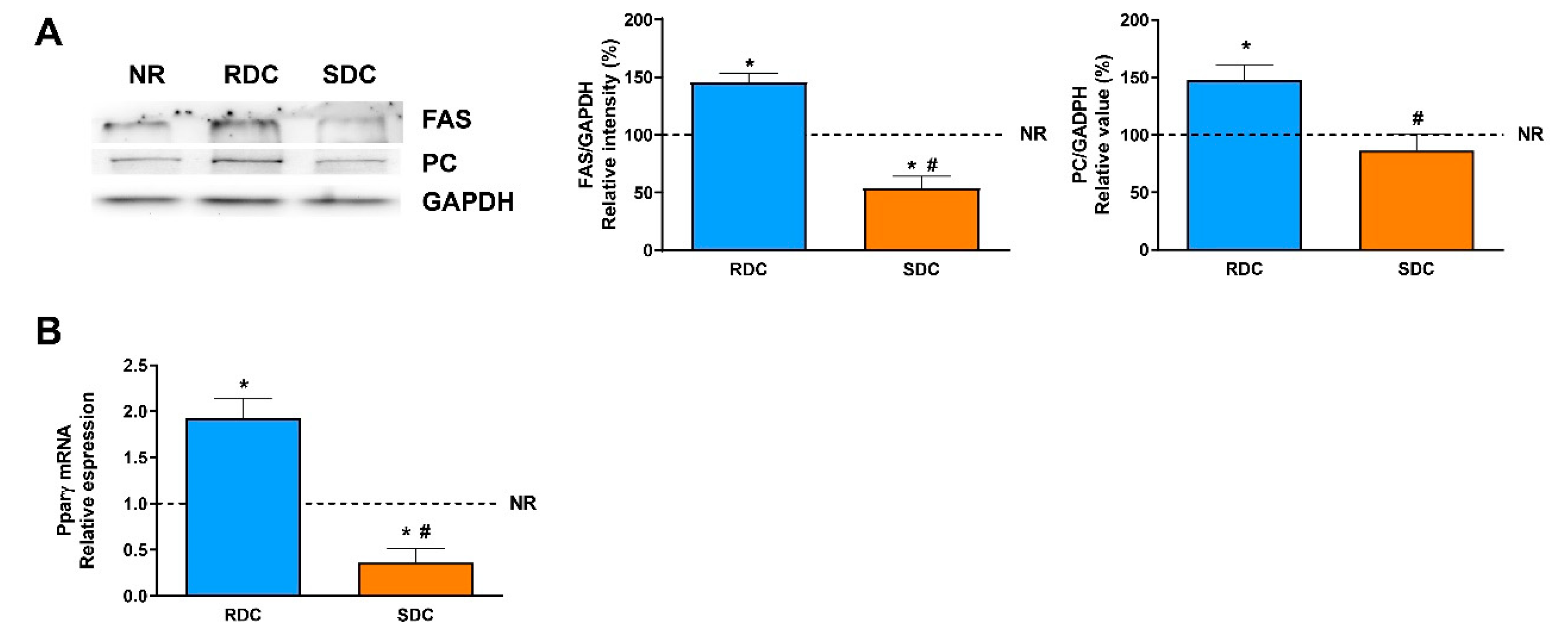
3.2. Dietary Effects on Gene Expression, Signaling and Metabolic Pathways in Adipose Tissue
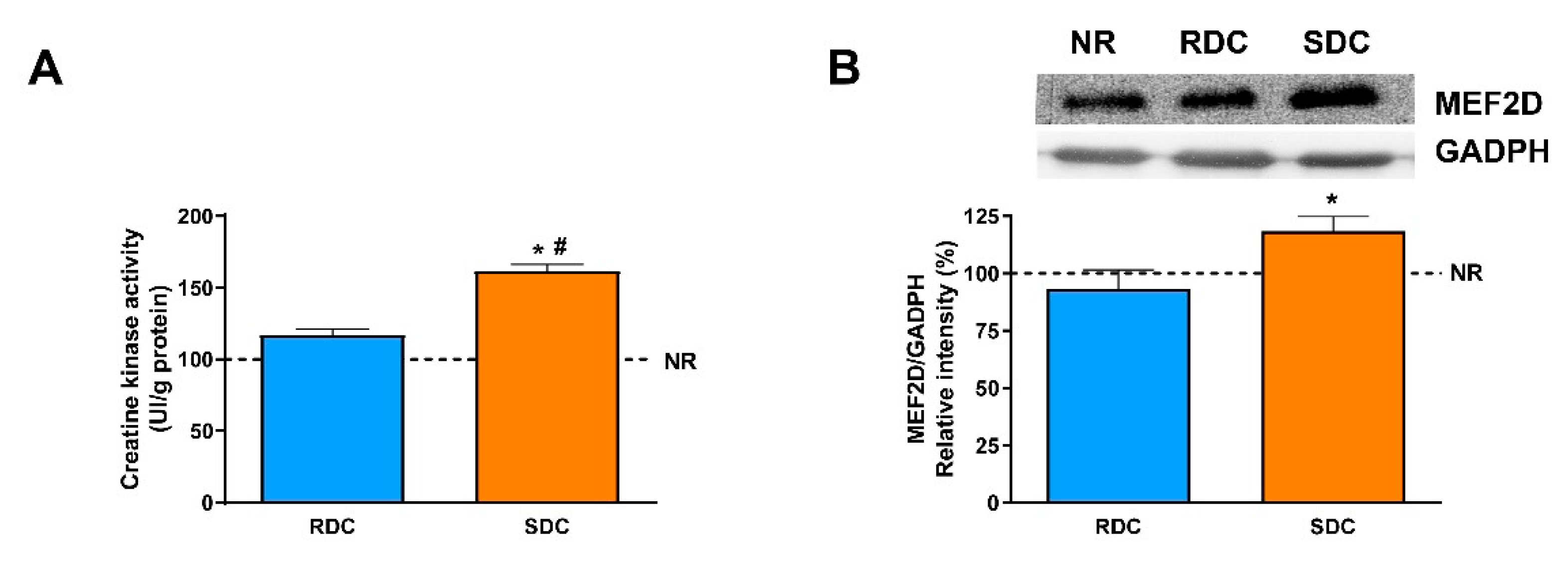
3.3. Dietary Effects on Gene Expression, Signaling and Metabolic Pathways in Skeletal Muscle
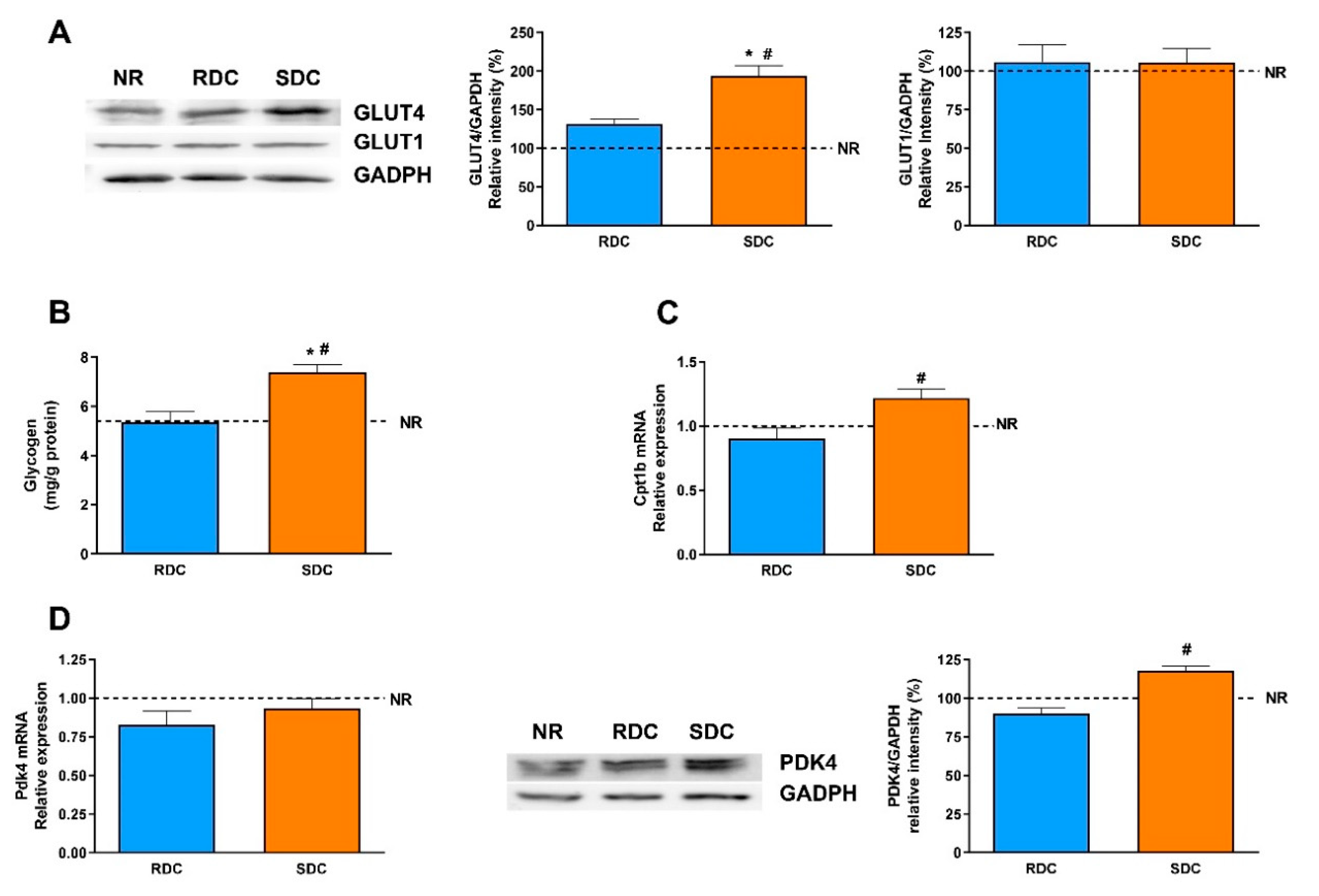
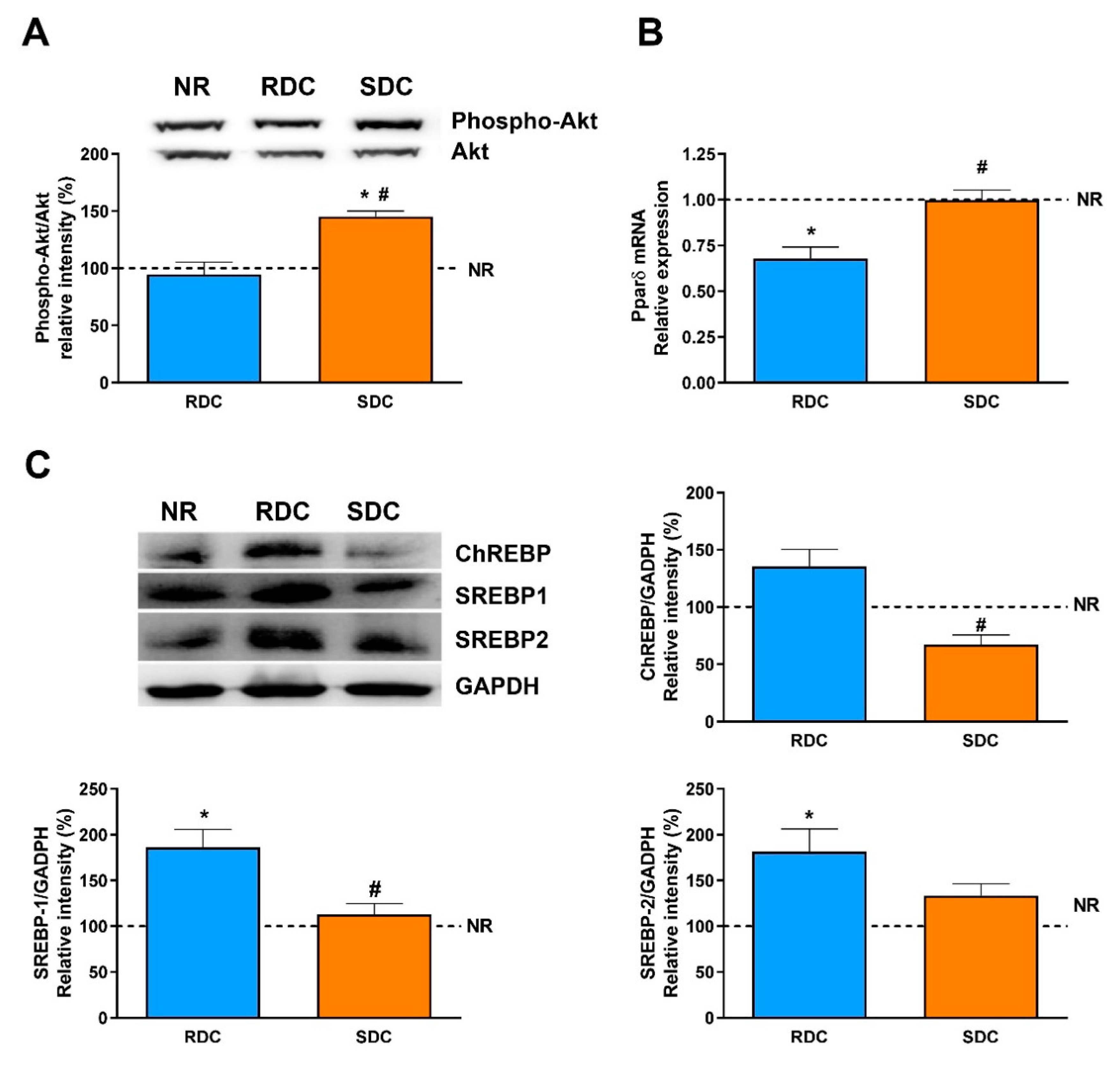
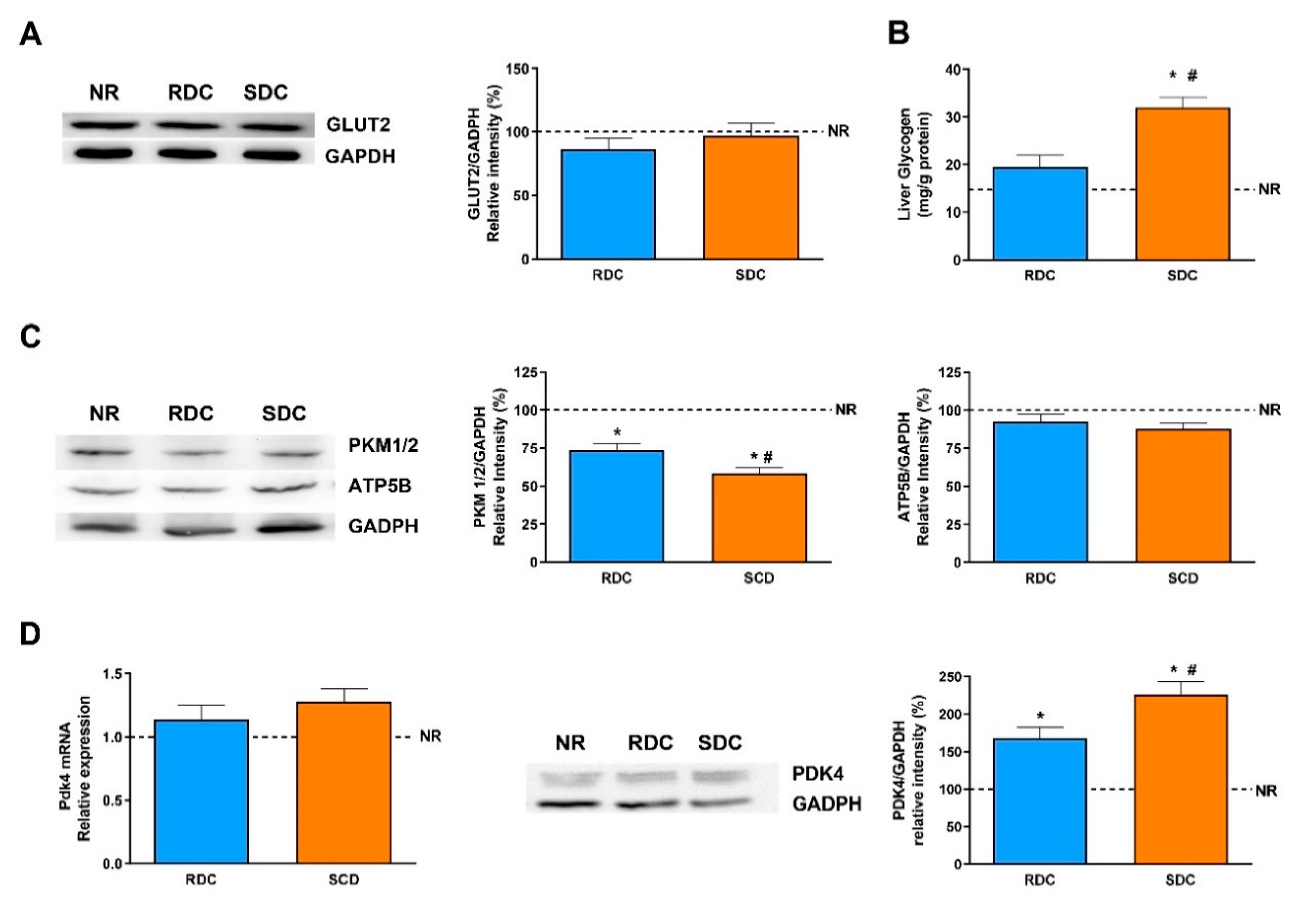
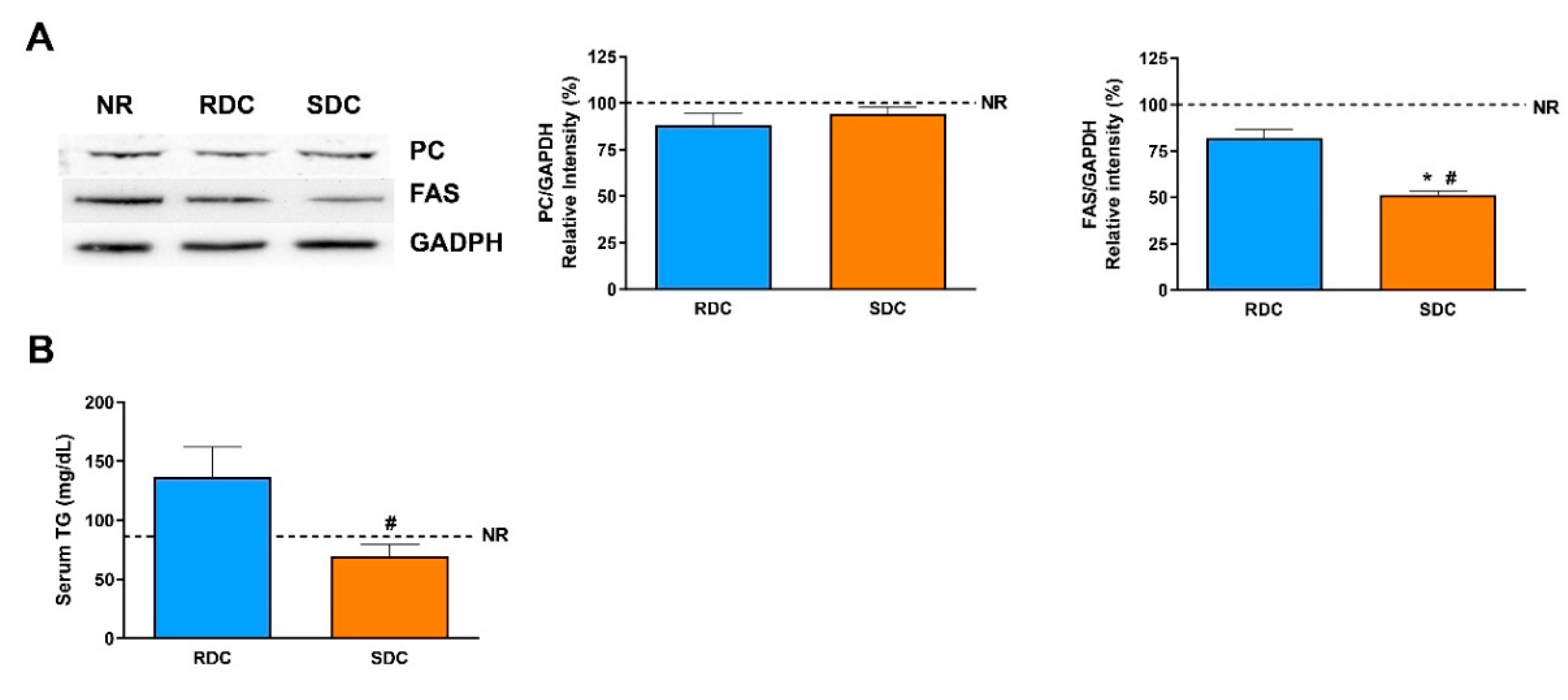
3.4. Dietary Effects on Gene Expression, Signaling and Metabolic Pathways in Liver
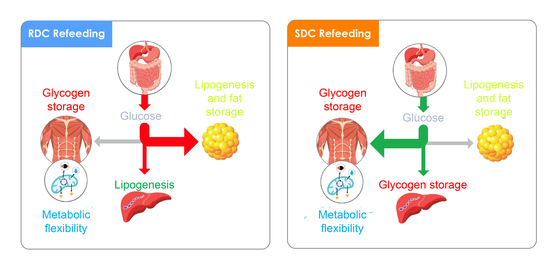
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dulloo, A.G.; Jacquet, J.; Seydoux, J.; Montani, J.P. The thrifty ‘catch-up fat’ phenotype: Its impact on insulin sensitivity during growth trajectories to obesity and metabolic syndrome. Int. J. Obes. 2006, 30 (Suppl. S4), S23–S35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Victora, C.G.; Adair, L.; Fall, C.; Hallal, P.C.; Martorell, R.; Richter, L.; Sachdev, H.S.; Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: Consequences for adult health and human capital. Lancet 2008, 371, 340–357. [Google Scholar] [CrossRef] [Green Version]
- Alderman, H.; Headey, D. The timing of growth faltering has important implications for observational analyses of the underlying determinants of nutrition outcomes. PLoS ONE 2018, 13, e0195904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dulloo, A.G. Adipose tissue plasticity in catch-up-growth trajectories to metabolic syndrome: Hyperplastic versus hypertrophic catch-up fat. Diabetes 2009, 58, 1037–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gellar, L.; Nansel, T.R. High and low glycemic index mixed meals and blood glucose in youth with type 2 diabetes or impaired glucose tolerance. J. Pediatr. 2009, 154, 455–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephen, A.; Alles, M.; de Graaf, C.; Fleith, M.; Hadjilucas, E.; Isaacs, E.; Maffeis, C.; Zeinstra, G.; Matthys, C.; Gil, A. The role and requirements of digestible dietary carbohydrates in infants and toddlers. Eur. J. Clin. Nutr. 2012, 66, 765–779. [Google Scholar] [CrossRef] [Green Version]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Even, P.C.; Nadkarni, N.A. Indirect calorimetry in laboratory mice and rats: Principles, practical considerations, interpretation and perspectives. Am. J. Physiol. Integr. Comp. Physiol. 2012, 303, R459–R476. [Google Scholar] [CrossRef]
- Ferrannini, E. The theoretical bases of indirect calorimetry: A review. Metab. Clin. Exp. 1988, 37, 287–301. [Google Scholar] [CrossRef]
- McClave, S.A.; Lowen, C.C.; Kleber, M.J.; McConnell, J.W.; Jung, L.Y.; Goldsmith, L.J. Clinical use of the respiratory quotient obtained from indirect calorimetry. JPEN J. Parenter. Enter. Nutr. 2003, 27, 21–26. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Chan, T.M.; Exton, J.H. A rapid method for the determination of glycogen content and radioactivity in small quantities of tissue or isolated hepatocytes. Anal. Biochem. 1976, 71, 96–105. [Google Scholar] [CrossRef]
- Jitrapakdee, S.; Slawik, M.; Medina-Gomez, G.; Campbell, M.; Wallace, J.C.; Sethi, J.K.; O’Rahilly, S.; Vidal-Puig, A.J. The peroxisome proliferator-activated receptor-gamma regulates murine pyruvate carboxylase gene expression in vivo and in vitro. J. Boil. Chem. 2005, 280, 27466–27476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eissing, L.; Scherer, T.; Todter, K.; Knippschild, U.; Greve, J.W.; Buurman, W.A.; Pinnschmidt, H.O.; Rensen, S.S.; Wolf, A.M.; Bartelt, A.; et al. De novo lipogenesis in human fat and liver is linked to ChREBP-beta and metabolic health. Nat. Commun. 2013, 4, 1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertolio, R.; Napoletano, F.; Mano, M.; Maurer-Stroh, S.; Fantuz, M.; Zannini, A.; Bicciato, S.; Sorrentino, G.; Del Sal, G. Sterol regulatory element binding protein 1 couples mechanical cues and lipid metabolism. Nat. Commun. 2019, 10, 1326. [Google Scholar] [CrossRef] [Green Version]
- de Wit, C.C.; Sas, T.C.; Wit, J.M.; Cutfield, W.S. Patterns of catch-up growth. J. Pediatr. 2013, 162, 415–420. [Google Scholar] [CrossRef]
- Martin, A.; Connelly, A.; Bland, R.M.; Reilly, J.J. Health impact of catch-up growth in low-birth weight infants: Systematic review, evidence appraisal, and meta-analysis. Mater. Child Nutr. 2017, 13. [Google Scholar] [CrossRef] [Green Version]
- Singhal, A. Long-Term Adverse Effects of Early Growth Acceleration or Catch-Up Growth. Ann. Nutr. Metab. 2017, 70, 236–240. [Google Scholar] [CrossRef] [Green Version]
- Summermatter, S.; Mainieri, D.; Russell, A.P.; Seydoux, J.; Montani, J.P.; Buchala, A.; Solinas, G.; Dulloo, A.G. Thrifty metabolism that favors fat storage after caloric restriction: A role for skeletal muscle phosphatidylinositol-3-kinase activity and AMP-activated protein kinase. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2008, 22, 774–785. [Google Scholar] [CrossRef] [Green Version]
- Dulloo, A.G. Regulation of fat storage via suppressed thermogenesis: A thrifty phenotype that predisposes individuals with catch-up growth to insulin resistance and obesity. Horm. Res. 2006, 65 (Suppl. S3), 90–97. [Google Scholar] [CrossRef] [Green Version]
- Dulloo, A.G. Thrifty energy metabolism in catch-up growth trajectories to insulin and leptin resistance. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 155–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cettour-Rose, P.; Samec, S.; Russell, A.P.; Summermatter, S.; Mainieri, D.; Carrillo-Theander, C.; Montani, J.P.; Seydoux, J.; Rohner-Jeanrenaud, F.; Dulloo, A.G. Redistribution of glucose from skeletal muscle to adipose tissue during catch-up fat: A link between catch-up growth and later metabolic syndrome. Diabetes 2005, 54, 751–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cani, P.D.; Neyrinck, A.M.; Maton, N.; Delzenne, N.M. Oligofructose promotes satiety in rats fed a high-fat diet: Involvement of glucagon-like Peptide-1. Obes. Res. 2005, 13, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Joly, E.; Horsmans, Y.; Delzenne, N.M. Oligofructose promotes satiety in healthy human: A pilot study. Eur. J. Clin. Nutr. 2006, 60, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Hume, M.P.; Nicolucci, A.C.; Reimer, R.A. Prebiotic supplementation improves appetite control in children with overweight and obesity: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 105, 790–799. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, D.J.; Sawaya, A.L.; Verreschi, I.; Tucker, K.L.; Roberts, S.B. Why are nutritionally stunted children at increased risk of obesity? Studies of metabolic rate and fat oxidation in shantytown children from Sao Paulo, Brazil. Am. J. Clin. Nutr. 2000, 72, 702–707. [Google Scholar] [CrossRef]
- Olson, A.L.; Pessin, J.E. Transcriptional regulation of the human GLUT4 gene promoter in diabetic transgenic mice. J. Boil. Chem. 1995, 270, 23491–23495. [Google Scholar] [CrossRef] [Green Version]
- Cooke, D.W.; Lane, M.D. The transcription factor nuclear factor I mediates repression of the GLUT4 promoter by insulin. J. Boil. Chem. 1999, 274, 12917–12924. [Google Scholar] [CrossRef] [Green Version]
- Griesel, B.A.; Weems, J.; Russell, R.A.; Abel, E.D.; Humphries, K.; Olson, A.L. Acute inhibition of fatty acid import inhibits GLUT4 transcription in adipose tissue, but not skeletal or cardiac muscle tissue, partly through liver X receptor (LXR) signaling. Diabetes 2010, 59, 800–807. [Google Scholar] [CrossRef] [Green Version]
- Nurjhan, N.; Consoli, A.; Gerich, J. Increased lipolysis and its consequences on gluconeogenesis in non-insulin-dependent diabetes mellitus. J. Clin. Investig. 1992, 89, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Azzu, V.; Jastroch, M.; Divakaruni, A.S.; Brand, M.D. The regulation and turnover of mitochondrial uncoupling proteins. Biochim. Biophys. Acta 2010, 1797, 785–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Li, J.; Li, W.J.; Wang, C.M. The role of uncoupling proteins in diabetes mellitus. J. Diabetes Res. 2013, 2013, 585897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugden, M.C.; Zariwala, M.G.; Holness, M.J. PPARs and the orchestration of metabolic fuel selection. Pharmacol. Res. 2009, 60, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Ritze, Y.; Bardos, G.; D’Haese, J.G.; Ernst, B.; Thurnheer, M.; Schultes, B.; Bischoff, S.C. Effect of high sugar intake on glucose transporter and weight regulating hormones in mice and humans. PLoS ONE 2014, 9, e101702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Hulver, M.W.; McMillan, R.P.; Cline, M.A.; Gilbert, E.R. The pivotal role of pyruvate dehydrogenase kinases in metabolic flexibility. Nutr. Metab. 2014, 11, 10. [Google Scholar] [CrossRef] [Green Version]
- Esteves, J.V.; Enguita, F.J.; Machado, U.F. MicroRNAs-Mediated Regulation of Skeletal Muscle GLUT4 Expression and Translocation in Insulin Resistance. J. Diabetes Res. 2017, 2017, 7267910. [Google Scholar] [CrossRef]
- Kelley, D.E.; Mandarino, L.J. Fuel selection in human skeletal muscle in insulin resistance: A reexamination. Diabetes 2000, 49, 677–683. [Google Scholar] [CrossRef] [Green Version]
- Nordfors, L.; Hoffstedt, J.; Nyberg, B.; Thorne, A.; Arner, P.; Schalling, M.; Lonnqvist, F. Reduced gene expression of UCP2 but not UCP3 in skeletal muscle of human obese subjects. Diabetologia 1998, 41, 935–939. [Google Scholar] [CrossRef]
- Aziz, A.; Liu, Q.-C.; Dilworth, F.J. Regulating a master regulator: Establishing tissue-specific gene expression in skeletal muscle. Epigenetics 2010, 5, 691–695. [Google Scholar] [CrossRef] [Green Version]
- Fan, W.; Waizenegger, W.; Lin, C.S.; Sorrentino, V.; He, M.X.; Wall, C.E.; Li, H.; Liddle, C.; Yu, R.T.; Atkins, A.R.; et al. PPARdelta Promotes Running Endurance by Preserving Glucose. Cell Metab. 2017, 25, 1186–1193.e4. [Google Scholar] [CrossRef]
- Reilly, S.M.; Lee, C.H. PPAR delta as a therapeutic target in metabolic disease. FEBS Lett. 2008, 582, 26–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, M.T.; Yudell, B.E.; Loor, J.J. Regulation of energy metabolism by long-chain fatty acids. Prog. Lipid Res. 2014, 53, 124–144. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.; Soundarapandian, M.M.; Sessions, H.; Peddibhotla, S.; Roth, G.P.; Li, J.L.; Sugarman, E.; Koo, A.; Malany, S.; Wang, M.; et al. MondoA coordinately regulates skeletal myocyte lipid homeostasis and insulin signaling. J. Clin. Investig. 2016, 126, 3567–3579. [Google Scholar] [CrossRef] [PubMed]
- Oberbach, A.; Bossenz, Y.; Lehmann, S.; Niebauer, J.; Adams, V.; Paschke, R.; Schon, M.R.; Bluher, M.; Punkt, K. Altered fiber distribution and fiber-specific glycolytic and oxidative enzyme activity in skeletal muscle of patients with type 2 diabetes. Diabetes Care 2006, 29, 895–900. [Google Scholar] [CrossRef] [Green Version]
- Dessalle, K.; Euthine, V.; Chanon, S.; Delarichaudy, J.; Fujii, I.; Rome, S.; Vidal, H.; Nemoz, G.; Simon, C.; Lefai, E. SREBP-1 transcription factors regulate skeletal muscle cell size by controlling protein synthesis through myogenic regulatory factors. PLoS ONE 2012, 7, e50878. [Google Scholar] [CrossRef] [Green Version]
- Oosterveer, M.H.; Schoonjans, K. Hepatic glucose sensing and integrative pathways in the liver. Cell. Mol. Life Sci. CMLS 2014, 71, 1453–1467. [Google Scholar] [CrossRef] [Green Version]
- Rui, L. Energy metabolism in the liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [CrossRef] [Green Version]
- Bayley, J.P.; Devilee, P. The Warburg effect in 2012. Curr. Opin. Oncol. 2012, 24, 62–67. [Google Scholar] [CrossRef]
- Parillo, M.; Licenziati, M.R.; Vacca, M.; De Marco, D.; Iannuzzi, A. Metabolic changes after a hypocaloric, low-glycemic-index diet in obese children. J. Endocrinol. Investig. 2012, 35, 629–633. [Google Scholar] [CrossRef]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef] [Green Version]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Macronutrients | AIN93M | RDC | SDC |
|---|---|---|---|
| NR | RDC | SDC | |
| CHO (g/100 g diet) | 78.5 | 67.0 | 67.0 |
| Sucrose (g/100 g CHO) | 13.0 | 32.0 | |
| Isomaltulose (g/100 g CHO) | 26.4 | ||
| Sucromalt® (g/100 g CHO) | 22.1 | ||
| Cornstarch (g/100 g CHO) | 61.0 | ||
| MDs (g/100 g CHO) | 20.0 | 64.0 | 23.0 |
| IMOs (g/100 g CHO) | 11.5 | ||
| Resistant MD (g/100 g CHO) | 10.0 | ||
| Soluble fiber (g/100 g CHO) | 4.0 | 7.0 | |
| Insoluble fiber (g/100 g CHO) | 5.0 | ||
| Glycemic load (index) | 1269 | 851 | 468 |
| Protein (g/100 g diet) | 14.0 | 15.0 | 15.0 |
| Fat (g/100 g diet) | 4.0 | 9.8 | 9.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salto, R.; Girón, M.D.; Ortiz-Moral, C.; Manzano, M.; Vílchez, J.D.; Reche-Perez, F.J.; Bueno-Vargas, P.; Rueda, R.; Lopez-Pedrosa, J.M. Dietary Complex and Slow Digestive Carbohydrates Prevent Fat Deposits During Catch-Up Growth in Rats. Nutrients 2020, 12, 2568. https://doi.org/10.3390/nu12092568
Salto R, Girón MD, Ortiz-Moral C, Manzano M, Vílchez JD, Reche-Perez FJ, Bueno-Vargas P, Rueda R, Lopez-Pedrosa JM. Dietary Complex and Slow Digestive Carbohydrates Prevent Fat Deposits During Catch-Up Growth in Rats. Nutrients. 2020; 12(9):2568. https://doi.org/10.3390/nu12092568
Chicago/Turabian StyleSalto, Rafael, María D Girón, Carolina Ortiz-Moral, Manuel Manzano, Jose D Vílchez, Francisco J Reche-Perez, Pilar Bueno-Vargas, Ricardo Rueda, and Jose M Lopez-Pedrosa. 2020. "Dietary Complex and Slow Digestive Carbohydrates Prevent Fat Deposits During Catch-Up Growth in Rats" Nutrients 12, no. 9: 2568. https://doi.org/10.3390/nu12092568
APA StyleSalto, R., Girón, M. D., Ortiz-Moral, C., Manzano, M., Vílchez, J. D., Reche-Perez, F. J., Bueno-Vargas, P., Rueda, R., & Lopez-Pedrosa, J. M. (2020). Dietary Complex and Slow Digestive Carbohydrates Prevent Fat Deposits During Catch-Up Growth in Rats. Nutrients, 12(9), 2568. https://doi.org/10.3390/nu12092568