The Effect of Resveratrol or Curcumin on Head and Neck Cancer Cells Sensitivity to the Cytotoxic Effects of Cisplatin
Abstract
:1. Introduction
2. Materials
3. Methods
3.1. Cell Lines and Treatment
3.1.1. Preparation of Stock Solution
3.1.2. Cell Cultures Treatment
3.2. Drug Sensitivity Assay (MTT)
3.3. Cell Proliferation Assay
3.4. Cell Lysates
3.5. ELISA Assay
3.6. Apoptosis Analysis
3.7. Cell Cycle Analysis by Flow Cytometry
3.8. RT-PCR [60,61]
3.8.1. RNA Isolation with TRIzol
3.8.2. Real-Time PCR
3.9. Statistical Analysis
4. Results
4.1. Effects of CisPt and/or Natural Compounds RSV, CRM on Cell Viability
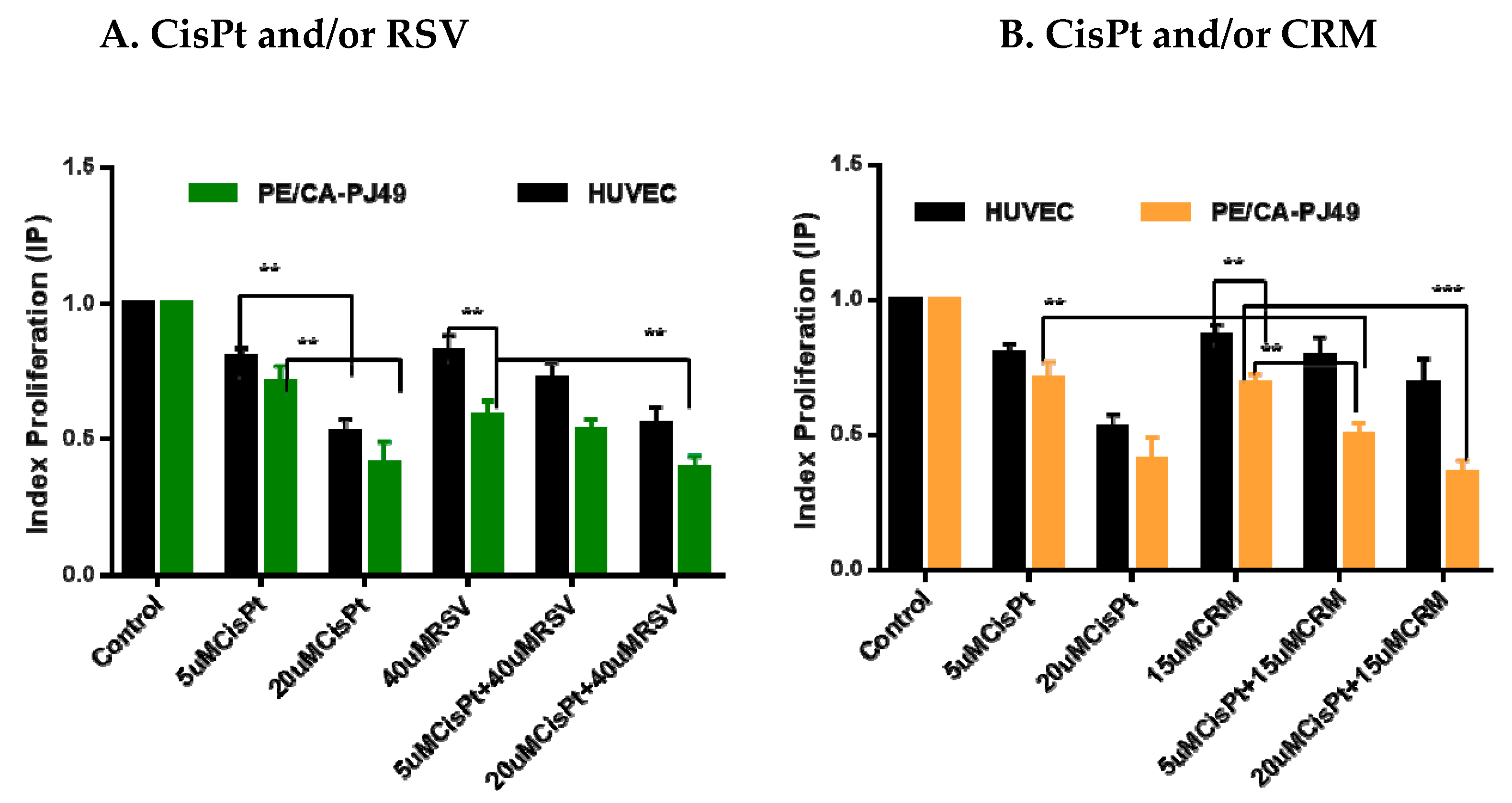
4.2. Effects of CisPt and/or Natural Compounds RSVor CRM on the Cell Proliferation Process
4.3. Modulation of p21 Protein Expression by Natural Compounds and/or Cisplatin Treatment
4.4. Modulation of P21 Gene Expression by Natural Compounds and/or Cisplatin Treatment
4.5. Effects of the Natural Compounds (RSV,CRM) on Cell Cycle Phases Distribution in Cisplatin Treated Cells
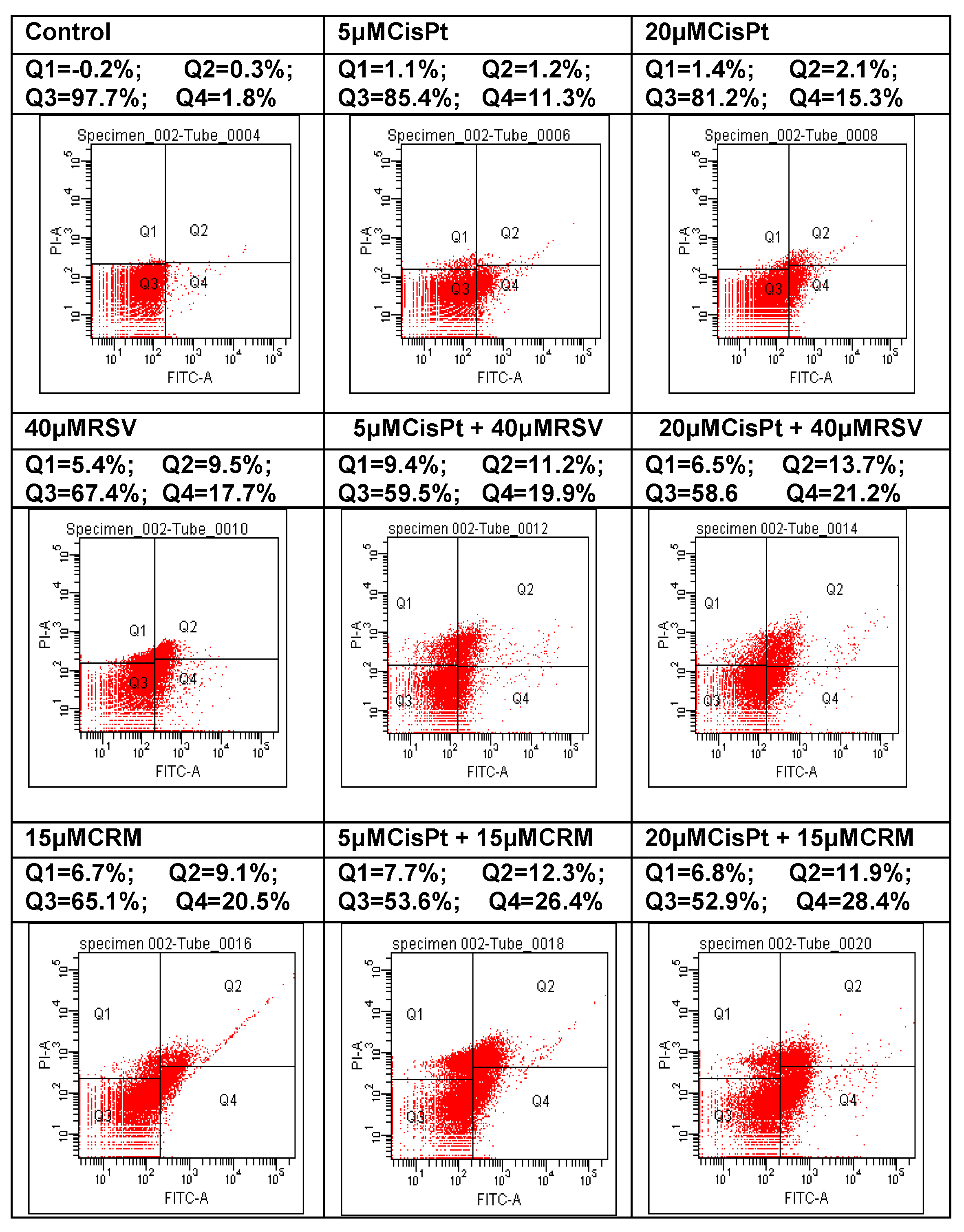
4.6. Effects of the Natural Compounds (RSV,CRM) on the Apoptotic Process in Cisplatin-Treated Cells
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lawrence, M.S. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Achkar, I.W.; Abdulrahman, N.; Al-Sulaiti, H.; Joseph, J.M.; Uddin, S.; Mraiche, F. Cisplatin based therapy: The role of the mitogen activated protein kinase signaling pathway. J. Transl. Med. 2018, 16, 96. [Google Scholar] [CrossRef] [PubMed]
- Thai, A.A.; Rischin, D. High-dose cisplatin for head and neck cancer lives on. J. Clin. Oncol. 2018, 36, 1055–1057. [Google Scholar] [CrossRef]
- Yang, Z.; Liao, J.; Carter-Cooper, B.A.; Lapidus, R.G.; Cullen, K.J.; Dan, H. Regulation of cisplatin-resistant head and neck squamous cell carcinoma by the SRC/ETS-1 signaling pathway. BMC Cancer 2019, 19, 485. [Google Scholar] [CrossRef] [Green Version]
- Gerhards, N.M.; Rottenberg, S. New tools for old drugs: Functional genetic screens to optimize current chemotherapy. Drug Resist. Updat. 2018, 36, 30–46. [Google Scholar] [CrossRef]
- Vermorken, J.B.; Remenat, E.; Van Herpen, C.; Gorlia, T.; Mesia, R.; Degardin, M.; Stewart, J.S.; Jelić, S.; Betka, J.; Preiss, J.H.; et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N. Engl. J. Med. 2007, 357, 1695–1704. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.Y.; Wang, F.X.; Jia, K.K.; Kong, L.D. Natural product interventions for chemotherapy and radiotherapy-induced side effects. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Abotaleb, M.; Kubatka, P.; Caprnda, M.; Varghese, E.; Zolakova, B.; Zubor, P.; Opatřilová, R.; Kruzliak, P.; Stefanicka, P.; Büsselberg, D. Chemotherapeutic agents for the treatment of metastatic breast cancer: An update. Biomed. Pharmacother. 2018, 101, 458–477. [Google Scholar] [CrossRef]
- Ion, G.; Fazio, K.; Akinsete, J.A.; Hardman, W. Effects of canola and corn oil mimetic on Jurkat cells. Lipids Health Dis. 2011, 10, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotnog, D.; Mihaila., M.; Botezatu, A.; Matei, G.G.; Hotnog, C.; Anton, G.; Bostan, M.; Brasoveanu, L.I. Genistein potentiates the apoptotic effect of 5-fluorouracyl in colon cancer cell lines. Rom. Biotech. Lett. 2013, 18, 8751–8760. [Google Scholar]
- Radu, N.; Roman, V.; Bostan, M.; Radu, E.; Tanasescu, C. Influence of some spice food based bioproducts on human monocytic cells line type THP-1. Mol. Cryst. Liq. Cryst. 2017, 655, 114–123. [Google Scholar] [CrossRef]
- Chiou, C.T.; Wang, K.C.; Yang, Y.C.; Huang, C.L.; Yang, S.H.; Kuo, Y.H.; Huang, N.K. Liu Jun Zi Tang—A potential, multi-herbal complementary therapy for chemotherapy-induced neurotoxicity. Int. J. Mol. Sci. 2018, 19, 1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural polyphenols for prevention and treatment of cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef]
- Grosso, G.; Bella, F.; Godos, J.; Sciacca, S.; Del Rio, D.; Ray, S.; Galvano, F.; Giovannucci, E.L. Possible role of diet in cancer: Systematic review and multiple meta-analyses of dietary patterns, lifestyle factors, and cancer risk. Nutr. Rev. 2017, 75, 405–419. [Google Scholar] [CrossRef]
- Kumar, G.; Mittal, S.; Sak, K.; Tuli, H.S. Molecular mechanisms underlying chemopreventive potential of curcumin: Current challenges and future perspectives. Life Sci. 2016, 148, 313–328. [Google Scholar] [CrossRef]
- Teow, S.Y.; Liew, K.; Ali, S.A.; Khoo, A.S.B.; Peh, S.C. Antibacterial action of curcumin against staphylococcus aureus: A brief review. J. Trop. Med. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Farhood, B.; Mortezaee, K.; Goradel, N.H.; Khanlarkhani, N.; Salehi, E.; Nashtaei, M.S.; Najafi, M.; Sahebkar, A. Curcumin as an anti-inflammatory agent: Implications to radiotherapy and chemotherapy. J. Cell. Physiol. 2019, 234, 5728–5740. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, P.; Law, S.; Tian, H.; Leung, W.; Xu, C. Preventive effect of curcumin against chemotherapy-induced side-effects. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Shanmugam, M.K.; Rane, G.; Mathi, K.M.; Arfuso, F.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Tan, B.K.H.; Kumar, A.P.; Sethi, G. The multifaceted role of curcumin in cancer prevention and treatment. Molecules 2015, 20, 2728–2769. [Google Scholar] [CrossRef] [PubMed]
- Teiten, M.H.; Eifes, S.; Dicato, M.; Diederich, M. Curcumin―The paradigm of a multi-target natural compound with applications in cancer prevention and treatment. Toxins 2010, 2, 128–162. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.H.; Alsahli, M.A.; Aly, S.M.; Khan, M.A.; Aldebasi, Y.H. Role of curcumin in disease prevention and treatment. Adv. Biomed. Res. 2018, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Garg, T.; Yadav, V.K. Effects of resveratrol as an anticancer agent—A systematic review. Int. J. Pharm. Bio. Sci. 2014, 5, 534–542. [Google Scholar]
- Aluyen, J.K.; Ton, Q.N.; Tran, T.; Yang, A.E.; Gottlieb, H.B.; Bellanger, R.A. Resveratrol: Potential as anticancer agent. J. Diet. Suppl. 2012, 9, 45–56. [Google Scholar] [CrossRef]
- Crandall, J.P.; Barzilai, N. Exploring the promise of resveratrol: Where do we go from here? Diabetes 2013, 62, 1022–1023. [Google Scholar] [CrossRef] [Green Version]
- Santandreu, F.M.; Valle, A.; Oliver, J.; Roca, P. Resveratrol potentiates the cytotoxic oxidative stress induced by chemotherapy in human colon cancer cells. Cell. Physiol. Biochem. 2011, 28, 219–228. [Google Scholar] [CrossRef]
- Fouad, M.; Agha, A.; Al Merzabani, M.; Shouman, S.A. Resveratrol inhibits proliferation, angiogenesis and induces apoptosis in colon cancer cells. Hum. Exp. Toxicol. 2013, 32, 1067–1080. [Google Scholar] [CrossRef]
- Mihaila, M.; Bostan, M.; Hotnog, D.; Ferdes, M.; Brasoveanu, L.I. Real-time analysis of quercetin, resveratrol and /or doxorubicin effects in MCF-7 cells. Rom. Biotech. Lett. 2013, 18, 8106–8114. [Google Scholar]
- McCubrey, J.A.; Lertpiriyapong, K.; Steelman, L.S.; Abrams, S.L.; Yang, L.V.; Murata, R.M. Effects of resveratrol, curcumin, ber-berine and other nutraceuticals on aging, cancer development, cancer stem cells and microRNAs. Aging 2017, 9, 1477–1536. [Google Scholar] [CrossRef] [Green Version]
- Fillies, T.; Woltering, M.; Brandt, B.; Van Diest, J.P.; Werkmeister, R.; Joos, U.; Buerger, H. Cell cycle regulating proteins p21 and p27 in prognosis of oral squamous cell carcinomas. Oncol. Rep. 2007, 17, 355–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Corbley, M.J.; Tang, Z.; Yang, L.; Peng, Y.; Zhang, Z.Y.; Tong, T.J. Down-regulation of p21WAF1 promotes apoptosis in senescent human fibroblasts: Involvement of retinoblastoma protein phosphorylation and delay of cellular aging. J. Cell. Physiol. 2004, 201, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Kreis, N.N.; Louwen, F.; Yuan, J. Less understood issues: P21Cip1 in mitosis and its therapeutic potential. Oncogene 2015, 34, 1758–1767. [Google Scholar] [CrossRef] [PubMed]
- Gartel, A.L. Is p21 an oncogene? Mol. Cancer Ther. 2006, 5, 1385–1386. [Google Scholar] [CrossRef] [Green Version]
- Efeyan, A.; Collado, M.; Velasco-Miguel, S.; Serrano, M. Genetic dissection of the role of p21Cip1/Waf1 inp53-mediated tumour suppression. Oncogene 2007, 26, 1645–1649. [Google Scholar] [CrossRef] [Green Version]
- Ishida, M.; Morita, M.; Saeki, H.; Ohga, T.; Sadanaga, N.; Watanabe, M.; Kakeji, Y.; Maehara, Y. Expression of p53 and p21 and the clinical response for hyperthermochemoradiotherapy in patients with squamous cell carcinoma of the esophagus. Anticancer. Res. 2007, 27, 3501–3506. [Google Scholar]
- Fisher, C.A.; Jung, M.; Zlobec, I.; Green, E.; Storck, C.; Tornillo, L.; Lugli, A.; Wolfensberger, M.; Terracciano, L.M. Co-overexpression of P2l and Ki67 in Head and Neck squamous cell carcinoma relative to a significant poor prognosis. Head Neck 2011, 33, 267–273. [Google Scholar] [CrossRef]
- Sadaf, S.; Loya, A.; Mushtaq, S.; Akhter, N.; Hussain, R.; Jamshed, A. Correlation of P21 expression in head and neck squamous cell carcinoma with clinicopathologic and prognostic parameters. J. Cancer Allied Spéc. 2015, 1, 5. [Google Scholar] [CrossRef] [Green Version]
- Shamloo, B.; Usluer, S. P21 in cancer research. Cancers 2019, 11, 1178. [Google Scholar] [CrossRef] [Green Version]
- Staalesen, V.; Leirvaag, B.; Lillehaug, J.R.; Lonning, P.E. Genetic and epigenetic changes in p21 and p21B do not correlate with resistance to doxorubicin or mitomycin and 5-fluorouracil in locally advanced breast cancer. Clin. Cancer Res. 2004, 10, 3438–3443. [Google Scholar] [CrossRef] [Green Version]
- Gottifredi, V.; McKinney, K.; Poyurovsky, M.V.; Prives, C. Decreased p21 levels are required for efficient restart of DNA synthesis after S phase block. J. Biol. Chem. 2004, 279, 5802–5810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsahafi, E.; Begg, K.; Amelio, I.; Raulf, N.; Lucarelli, P.; Sauter, T.; Tavassoli, M. Clinical update on head and neck cancer: Molecular biology and ongoing challenges. Cell Death Dis. 2019, 10, 540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.A.; Amin, A.R.M.R.; Shin, D.M. Chemopreventive potential of natural compounds in head and neck cancer. Nutr. Cancer 2010, 62, 973–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saba, N.F.; Haigentz, M.; Vermorken, J.B.; Strojan, P.; Bossi, P.; Rinaldo, A.; Takes, R.P.; Ferlito, A. Prevention of head and neck squamous cell carcinoma: Removing the “chemo” from “chemoprevention”. Oral Oncol. 2015, 51, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Crooker, K.; Aliani, R.; Ananth, M.; Arnold, L.; Anant, S.; Thomas, S.M. A review of promising natural chemopreventive agents for head and neck cancer. Cancer Prev. Res. 2018, 11, 441–450. [Google Scholar] [CrossRef] [Green Version]
- Petrica-Matei, G.G.; Roman, V.; Mihaila, M.; Hotnog, C.M.; Brasoveanu, L.I.; Bostan, M. Role of p38-mitogen-activated protein kinase in modulation of response to therapy in FaDu human pharyngeal carcinoma cell line. Rom. Biotech. Lett 2019, 24, 118–128. [Google Scholar] [CrossRef]
- Bostan, M.; Petrica-Matei, G.G.; Ion, G.; Radu, N.; Mihaila, M.; Hainarosie, R.; Brasoveanu, L.I.; Roman, V.; Constantin, C.; Neagu, M.T. Cisplatin effect on head and neck squamous cell carcinoma cells is modulated by ERK1/2 protein kinases. Exp. Ther. Med. 2019, 18, 5041–5051. [Google Scholar] [CrossRef] [Green Version]
- Sylvester, P.W. Optimization of the tetrazolium dye (MTT) colorimetric assay for cellular growth and viability. Methods Mol. Biol. 2011, 716, 157–168. [Google Scholar] [CrossRef]
- Bostan, M.; Radu, N.; Babeanu, N.; Zaharie, M.G.; Horatiu Tanasescu, C.A. Biological properties of a biomaterialobtained from Syzygiumaromaticum. Mol. Cryst. Liq. Cryst. 2019, 695, 45–52. [Google Scholar] [CrossRef]
- Machana, S.; Weerapreeyakul, N.; Barusrux, S.; Nonpunya, A.; Sripanidkulchai, B.; Thitimetharoch, T. Cytotoxic and apoptotic effects of six herbal plants against the human hepatocarcinoma (HepG2) cell line. Chin. Med. 2011, 6, 39. [Google Scholar] [CrossRef] [Green Version]
- Prayong, P.; Barusrux, S.; Weerapreeyakul, N. Cytotoxic activity screening of some indigenous Thai plants. Fitoterapia 2008, 79, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Hotnog, C.M.; Mihaila, M.; Puiu, L.; Botezatu, A.; Roman, V.; Popescu, I.D.; Bostan, M.; Brasoveanu, L.I. Modulation of the interplay between p53, ICAM-1 and VEGF in drug-treated LoVo colon cancer cells. Rom. Biotechnol. Lett. 2019, 24, 261–270. [Google Scholar] [CrossRef]
- Ciulu-Costinescu, F. Antimicrobial assay of a capsaicin—α-cyclodextrin inclusion complex against planktonic and adherent cells. Farmacia 2019, 67, 496–503. [Google Scholar] [CrossRef]
- Zhao, G.; Bae, J.Y.; Zheng, Z.; Park, H.S.; Chung, K.Y.; Roh, M.R.; Jin, Z. Overexpression and implications of melanoma-associated antigen A12 in pathogenesis of human cutaneous squamous cell carcinoma. Anticancer. Res. 2019, 39, 1849–1857. [Google Scholar] [CrossRef] [PubMed]
- Tak, J.; Sabarwal, A.; Shyanti, R.K.; Singh, R.P. Berberine enhances posttranslational protein stability of p21/cip1 in breast cancer cells via down-regulation of Akt. Mol. Cell. Biochem. 2019, 458, 49–59. [Google Scholar] [CrossRef]
- Bundscherer, A.C.; Malsy, M.; Lange, R.; Hofmann, P.; Metterlein, T.; Graf, B.M.; Gruber, M. Cell harvesting method influences results of apoptosis analysis by annexin V staining. Anticancer. Res. 2013, 33, 3201–3204. [Google Scholar]
- Roman, V.; Radu, N.; Bostan, M.; Popa, O.; Tanasescu, C.A.H. Effect of Melaleuca alternifolia bioproduct on acute monocytic leukemia cells line. Mol. Cryst. Liq. Cryst. 2019, 695, 29–36. [Google Scholar] [CrossRef]
- Iancu, I.V.; Botezatu, A.; Plesa, A.; Huica, I.; Fudulu, A.; Albulescu, A.; Bostan, M.; Mihaila, M.; Grancea, C.; Manda, D.A.; et al. Alterations of regulatory factors and DNA methylation pattern in thyroid cancer. Cancer Biomarkers 2020, 28, 255–268. [Google Scholar] [CrossRef]
- Petrică-Matei, G.G.; Iordache, F.; Hainăroşie, R.; Bostan, M. Characterization of the tumor cells from human head and neck cancer. Rom. J. Morphol. Embryol. 2016, 57, 791–799. [Google Scholar]
- Botezatu, A.; Iancu, I.V.; Plesa, A.; Manda, D.; Popa, O.; Bostan, M.; Mihaila, M.; Albulescu, A.; Fudulu, A.; Vladoiu, S.V.; et al. Methylation of tumour suppressor genes associated with thyroid cancer. Cancer Biomarkers 2019, 25, 53–65. [Google Scholar] [CrossRef]
- Kim, D.K.; Lee, J.H.; Lee, O.J.; Park, C.H. Expression and mutational analysis of Cip/Kip family in early glottic cancer. J. Laryngol. Otol. 2015, 129, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Badisa, R.B.; Darling-Reed, S.F.; Joseph, P.; Cooperwood, J.S.; Latinwo, L.M.; Goodman, C.B. Selective cytotoxic activities of two novel synthetic drugs on human breast carcinoma MCF-7 cells. Anticancer. Res. 2009, 29, 2993–2996. [Google Scholar] [PubMed]
- Georgakilas, A.G.; Martin, O.A.; Bonner, W.M. P21: A two-faced genome guardian. Trends Mol. Med. 2017, 23, 310–319. [Google Scholar] [CrossRef]
- Parveen, A.; Akash, M.S.H.; Rehman, K.; Kyunn, W.W. Dual role of p21 in the progression of cancer and its treatment. Crit. Rev. Eukaryot. Gene Expr. 2016, 26, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Xia, R.; Lu, K.; Xie, M.; Yang, F.; Sun, M.; De, W.; Wang, C.; Ji, G. LincRNAFEZF1-AS1 represses p21 expression to promote gastric cancer proliferation through LSD1-Mediated H3K4me2 demethylation. Mol. Cancer 2017, 16, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, D.; Lu, X.; Su, J.; He, X.; De, W.; Yang, J.; Li, W.; Han, L.; Zhang, E. Long noncoding RNA AFAP1-AS1 predicts a poor prognosis and regulates non-small cell lung cancer cell proliferation by epigenetically repressing p21 expression. Mol. Cancer 2018, 17, 92. [Google Scholar] [CrossRef]
- Li, G.; Liu, Z.; Sturgis, E.M.; Shi, Q.; Chamberlain, R.M.; Spitz, M.R.; Wei, Q. Genetic polymorphisms of p21 are associated with risk of squamous cell carcinoma of the head and neck. Carcinogenesis 2005, 26, 1596–1602. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.R.; Park, K.H.; Yang, J.O.; Lee, C.W.; Oh, S.J.; Yun, J.; Lee, M.Y.; Han, S.B.; Kang, J.S. MiR-6734 up-regulates p21 gene expression and induces cell cycle arrest and apoptosis in colon cancer cells. PLoS ONE 2016, 11, e0160961. [Google Scholar] [CrossRef]
- Szturz, P.; Cristina, V.; Gómez, R.G.; Bourhis, J.; Simon, C.; Vermorken, J.B. Cisplatin eligibility issues and alternative regimens in locoregionally advanced head and neck cancer: Recommendations for clinical practice. Front. Oncol. 2019, 9, 464. [Google Scholar] [CrossRef] [Green Version]
- Mehanna, H.; Robinson, M.; Hartley, A.; Kong, A.; Foran, B.; Fulton-Lieuw, T.; Dalby, M.; Mistry, P.; Sen, M.; O’Toole, L.; et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): An open-label randomised controlled phase 3 trial. Lancet 2019, 393, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Kunnumakkara, A.B.; Anand, P.; Aggarwal, B.B. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008, 269, 199–225. [Google Scholar] [CrossRef] [PubMed]
- Leischner, C.; Burkard, M.; Pfeiffer, M.M.; Lauer, U.M.; Busch, C.; Venturelli, S. Nutritional immunology: Function of natural killer cells and their modulation by resveratrol for cancer prevention and treatment. Nutr. J. 2016, 15, 47. [Google Scholar] [CrossRef] [Green Version]
- Baharuddin, P.; Satar, N.; Fakiruddin, K.S.; Zakaria, N.; Lim, M.N.; Yusoff, N.M.; Zakaria, Z.; Yahaya, B.H. Curcumin improves the efficacy of cisplatin by targeting cancer stem-like cells through p21 and cyclin D1-mediated tumour cell inhibition in non-small cell lung cancer cell lines. Oncol. Rep. 2015, 35, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.M.M.; Al-Malki, H.S.; Al-Harthi, S.E.; El-Hanafy, A.A.; Elashmaoui, H.M.; ElShal, M.F. Modulatory role of resveratrol on cytotoxic activity of cisplatin, sensitization and modification of cisplatin resistance in colorectal cancer cells. Mol. Med. Rep. 2012, 12, 1368–1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, Z.; Guan, H.; You, Z.; Wang, C.; Hu, L.; Zhang, L.; Wang, Y.; Chen, S.; Xu, B.; Chen, M. Aloperine executes antitumor effects through the induction of apoptosis and cell cycle arrest in prostate cancer in vitro and in vivo. OncoTargets Ther. 2018, 11, 2735–2743. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Reaction Components | Volume |
|---|---|
| RT-Buffer 10× | 2.0 μL |
| Mix dNTP 25× (100 mM) | 0.8 μL |
| Randomics RT-primeri 10× | 2.0 μL |
| MultiScribe™ Reverse Transcriptase | 1.0 μL |
| Ultrapure water | 4.2 μL |
| Total Volume/reaction | 10 μL |
| Reaction Components | Volume |
|---|---|
| QPCR master mix | 10 μL |
| Reference dye | 0.3 μL |
| cDNA | 5 μL |
| Ultrapure water | 4.7 μL |
| Total Volume/reaction | 20 μL |
| (a) | ||||||
|---|---|---|---|---|---|---|
| Treatment (24 h) | HUVEC | PE/CA-PJ49 | ||||
| IC 25 | IC 50 | IC 25 | IC 50 | |||
| CisPt (μM) | 5.82 ± 1.1 | 20.93 ± 2.1 | 3.95 ± 1.2 | 9.72 ± 1.7 | ||
| RSV (μM) | 40.6 ± 3.3 | 110.4 ± 8.6 | 17.6 ± 1.5 | 46.8 ± 2.6 | ||
| CRM (μM) | 19.2 ± 2.1 | 59.3 ± 6.1 | 7.9 ± 1.8 | 16.3 ± 3.4 | ||
| (b) | ||||||
| CisPt (μM) | RSV (μM) | CRM (μM) | ||||
| HUVEC (IC 50) | 20.93 ± 2.1 | 110.4 ± 8.6 | 59.3 ± 6.1 | |||
| PE/CA-PJ49 (IC 50) | 9.72 ± 1.7 | 46.8 ± 2.6 | 16.3 ± 3.4 | |||
| Selectivity Index (SI) | 2.15 | 2.36 | 3.66 | |||
| Treatment | p21 pg/mL HUVEC | n-fold p21 Protein Expression HUVEC | p21 pg/mL PE/CA-PJ49 | n-fold p21 Protein Expression PE/CA-PJ49 |
|---|---|---|---|---|
| Control | 183 | 1 | 625 | 1 |
| 5 μM CisPt | 238 | 1.3 | 1157 | 1.85 |
| 20 μM CisPt | 195 | 1.07 | 915 | 1.46 |
| 40 μM RSV | 148 | 0.81 | 1550 | 2.48 |
| 5 μM CisPt + 40 μM RSV | 196 | 1.07 | 1429 | 2.29 |
| 20 μM CisPt + 40 μM RSV | 253 | 1.38 | 1124 | 1.80 |
| 15 μM CRM | 221 | 1.21 | 941 | 1.51 |
| 5 μM CisPt + 15 μM CRM | 272 | 1.49 | 2335 | 3.74 |
| 20 μM CisPt + 15 μM CRM | 164 | 0.90 | 2675 | 4.28 |
| Treatment | HUVEC | PE/CA-PJ49 |
|---|---|---|
| Control | 1 | 1 |
| 5 μM CisPt | 1.64 | 2.0 |
| 20 μM CisPt | 1.54 | 1.46 |
| 40 μM RSV | 0.36 | 3.1 |
| 5 μM CisPt + 40 μM RSV | 0.97 | 2.68 |
| 20 μM CisPt + 40 μM RSV | 1.34 | 2.31 |
| 15 μM CRM | 1.39 | 1.2 |
| 5 μM CisPt + 15 μM CRM | 2.26 | 5.24 |
| 20 μM CisPt + 15 μM CRM | 0.54 | 6.03 |
| Treatment | Necrosis = Q1 (%) | Early Apoptosis = Q4 (%) | Late Apoptosis = Q2 (%) | Total Apoptosis = Q2 + Q4 (%) |
|---|---|---|---|---|
| Control | 0.2 | 1.8 | 0.3 | 2.1 |
| 5 μM CisPt | 2.1 | 11.3 | 1.2 | 12.5 |
| 20 μM CisPt | 1.2 | 15.3 | 2.1 | 17.4 |
| 40 μM RSV | 5.4 | 17.7 | 9.5 | 27.2 |
| 5 μM CisPt + 40 μM RSV | 9.4 | 19.9 | 11.2 | 31.1 |
| 20 μM CisPt + 40 μM RSV | 6.5 | 21.2 | 13.7 | 34.9 |
| 15 μM CRM | 6.7 | 19.1 | 9.1 | 28.2 |
| 5 μM CisPt + 15 μM CRM | 7.7 | 26.4 | 12.3 | 38.7 |
| 20 μM CisPt + 15 μM CRM | 6.8 | 28.4 | 11.9 | 40.3 |
| Treatment | Necrosis = Q1 (%) | Early Apoptosis = Q4 (%) | Apoptoza Tarzie = Q2 (%) | Apoptoza Totală = Q2+Q4 (%) |
|---|---|---|---|---|
| Control | 1.1 | 4.5 | 0.2 | 4.7 |
| 5 μM CisPt | 1.6 | 5.6 | 0.5 | 6.1 |
| 20 μM CisPt | 1.5 | 7.7 | 0.5 | 8.2 |
| 40 μM RSV | 1.2 | 4.8 | 0.3 | 5.1 |
| 5 μM CisPt + 40 μM RSV | 0.4 | 12.8 | 1.2 | 14 |
| 20 μM CisPt + 40 μM RSV | 2.5 | 14.9 | 6.7 | 21.6 |
| 15 μM CRM | 2.6 | 6.7 | 0.5 | 7.2 |
| 5 μM CisPt + 15 μM CRM | 5.7 | 13.4 | 6.3 | 19.7 |
| 20 μM CisPt + 15 μM CRM | 3.3 | 19.5 | 4.4 | 23.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bostan, M.; Petrică-Matei, G.G.; Radu, N.; Hainarosie, R.; Stefanescu, C.D.; Diaconu, C.C.; Roman, V. The Effect of Resveratrol or Curcumin on Head and Neck Cancer Cells Sensitivity to the Cytotoxic Effects of Cisplatin. Nutrients 2020, 12, 2596. https://doi.org/10.3390/nu12092596
Bostan M, Petrică-Matei GG, Radu N, Hainarosie R, Stefanescu CD, Diaconu CC, Roman V. The Effect of Resveratrol or Curcumin on Head and Neck Cancer Cells Sensitivity to the Cytotoxic Effects of Cisplatin. Nutrients. 2020; 12(9):2596. https://doi.org/10.3390/nu12092596
Chicago/Turabian StyleBostan, Marinela, Georgiana Gabriela Petrică-Matei, Nicoleta Radu, Razvan Hainarosie, Cristian Dragos Stefanescu, Carmen Cristina Diaconu, and Viviana Roman. 2020. "The Effect of Resveratrol or Curcumin on Head and Neck Cancer Cells Sensitivity to the Cytotoxic Effects of Cisplatin" Nutrients 12, no. 9: 2596. https://doi.org/10.3390/nu12092596





