Omega-3 Polyunsaturated Fatty Acids EPA and DHA as an Adjunct to Non-Surgical Treatment of Periodontitis: A Randomized Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility and Exclusion Criteria
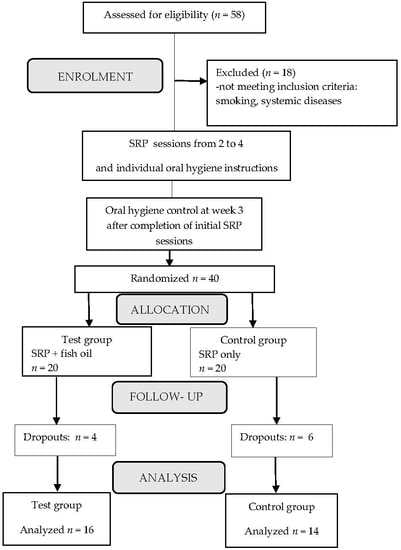
2.2. Study Design
2.3. Clinical Assessment
2.4. Saliva Sampling
2.5. Multiple Profiling Chemokine/Cytokine Assays
2.6. Statistical Analysis
3. Results
3.1. Dietary Supplementation with Omega-3 PUFA Resulted in the Improvement of CAL and BOP
3.2. Omega-3 PUFA Affected the Levels of Key Salivary Cytokines, Chemokines and Growth Factors
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Singhrao, S.K.; Olsen, I. Assessing the role of Porphyromonas gingivalis in periodontitis to determine a causative relationship with Alzheimer’s disease. J. Oral. Microbiol. 2019, 11, 1563405. [Google Scholar] [CrossRef] [Green Version]
- Kassebaum, N.J.; Smith, A.G.C.; Bernabé, E.; Fleming, T.D.; Reynolds, A.E.; Vos, T.; Murray, C.J.L.; Marcenes, W. Global, Regional, and National Prevalence, Incidence, and Disability-Adjusted Life Years for Oral Conditions for 195 Countries, 1990–2015: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors. J. Dent. Res. 2017, 96, 380–387. [Google Scholar] [CrossRef]
- Gutknecht, N.; Van Betteray, C.; Ozturan, S.; Vanweersch, L.; Franzen, R. Clinical Study Laser Supported Reduction of Specific Microorganisms in the Periodontal Pocket with the Aid of an Er,Cr:YSGG Laser: A Pilot Study. Sci. World J. 2015, 2015, 450258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis, C.; Da Costa, A.V.; Guimarães, J.T.; Tuna, D.; Braga, A.C.; Pacheco, J.J.; Arosa, F.A.; Salazar, F.; Cardoso, E.M. Clinical improvement following therapy for periodontitis: Association with a decrease in IL-1 and IL-6. Exp. Ther. Med. 2014, 8, 323–327. [Google Scholar] [CrossRef] [Green Version]
- Arron, J.R.; Choi, Y. Bone versus immune system. Nature 2000, 408, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Offenbacher, S.; Heasman, P.A.; Collins, J.G. Modulation of Host PGE 2 Secretion as a Determinant of Periodontal Disease Expression. J. Periodontol. 1993, 64, 432–444. [Google Scholar] [PubMed]
- Reddy, M.S.; Geurs, N.C.; Gunsolley, J.C. Periodontal host modulation with antiproteinase, anti-inflammatory, and bone-sparing agents. A systematic review. Ann. Periodontol. 2003, 8, 12–37. [Google Scholar] [CrossRef]
- Bhatavadekar, N.B.; Williams, R.C. New directions in host modulation for the management of periodontal disease: Commentary. J. Clin. Periodontol. 2009, 36, 124–126. [Google Scholar] [CrossRef]
- Salvi, G.E.; Lang, N.P. Host response modulation in the management of periodontal diseases. J. Clin. Periodontol. 2005, 32, 108–129. [Google Scholar] [CrossRef]
- Keskiner, I.; Saygun, I.; Bal, V.; Serdar, M.; Kantarci, A. Dietary supplementation with low-dose omega-3 fatty acids reduces salivary tumor necrosis factor-α levels in patients with chronic periodontitis: A randomized controlled clinical study. J. Periodontal Res. 2017, 52, 695–703. [Google Scholar] [CrossRef]
- Elwakeel, N.M.; Hazaa, H.H. Effect of omega 3 fatty acids plus low-dose aspirin on both clinical and biochemical profiles of patients with chronic periodontitis and type 2 diabetes: A randomized double blind placebo-controlled study. J. Periodontal Res. 2015, 50, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Deore, G.D.; Gurav, A.N.; Patil, R.; Shete, A.R.; NaikTari, R.S.; Inamdar, S.P. Omega 3 fatty acids as a host modulator in chronic periodontitis patients: A randomised, double-blind, palcebo-controlled, clinical trial. J. Periodontal Implant Sci. 2014, 44, 25–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, G.L.; Koury, J.C.; Martins, M.A.; Nogueira, F.; Fischer, R.G.; Gustafsson, A.; Figueredo, C.M.S. Serum level changes of long chain-polyunsaturated fatty acids in patients undergoing periodontal therapy combined with one year of omega-3 supplementation: A pilot randomized clinical trial. J. Periodontal Implant Sci. 2014, 44, 169–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elkhouli, A.M. The efficacy of host response modulation therapy (omega-3 plus low-dose aspirin) as an adjunctive treatment of chronic periodontitis (Clinical and biochemical study). J. Periodontal Res. 2011, 46, 261–268. [Google Scholar] [CrossRef]
- Naqvi, A.Z.; Hasturk, H.; Mu, L.; Phillips, R.S.; Davis, R.B.; Halem, S.; Campos, H. Docosahexaenoic Acid and Periodontitis in Adults: A Randomized Controlled Trial. J. Dent. Res. 2014, 93, 767–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef]
- Stańdo, M.; Lewkowicz, N. Omega-3 Polyunsaturated Fatty Acids as an Adjunct to Non-Surgical Treatment of Periodontitis. Eur. J. Lipid Sci. Tech. 2019, 121, 1800345. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef] [Green Version]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89, 1475. [Google Scholar] [CrossRef] [Green Version]
- McGrory, K.; Flaitz, C.M.; Klein, J.R. Chemokine changes during oral wound healing. Biochem. Biophys. Res. Commun. 2004, 324, 317–320. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-6/omega-3 essential fatty acids: Biological effects. World Rev. Nutr. Diet. 2009, 99, 1–16. [Google Scholar] [PubMed]
- Russo, G.L. Dietary n-6 and n-3 polyunsaturated fatty acids: From biochemistry to clinical implications in cardiovascular prevention. Biochem. Pharmacol. 2009, 77, 937–946. [Google Scholar] [CrossRef]
- Gómez Candela, C.; Bermejo López, L.M.; Loria Kohen, V. Importance of a balanced omega 6/omega 3 ratio for the maintenance of health. Nutritional recommendations. Nutr. Hosp. 2011, 26, 323–329. [Google Scholar] [PubMed]
- Fetterman, J.W.; Zdanowicz, M.M. Therapeutic potential of n-3 polyunsaturated fatty acids in disease. Am. J. Health Syst. Pharm. 2009, 66, 1169–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 2002, 21, 495–505. [Google Scholar] [CrossRef]
- Vane, J.R.; Botting, R.M. Mechanism of action of aspirin-like drugs. Semin. Arthritis Rheum. 1997, 26, 2–10. [Google Scholar] [CrossRef]
- Hassan, S.H.S.; El-Refai, M.I.; Ghallab, N.A.; Kasem, R.F.; Shaker, O.G. Effect of periodontal surgery on osteoprotegerin levels in gingival crevicular fluid, saliva, and gingival tissues of chronic periodontitis patients. Dis. Markers 2015, 2015, 341259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Sharkawy, H.; Aboelsaad, N.; Eliwa, M.; Darweesh, M.; Alshahat, M.; Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Adjunctive Treatment of Chronic Periodontitis With Daily Dietary Supplementation With Omega-3 Fatty Acids and Low-Dose Aspirin. J. Periodontol. 2010, 81, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Stuntz, M.; Bernstein, B. Recent trends in the prevalence of low-dose aspirin use for primary and secondary prevention of cardiovascular disease in the United States, 2012–2015. Prev. Med. Rep. 2017, 5, 183–186. [Google Scholar] [CrossRef]
- Chojnowska, S.; Baran, T.; Wilińska, I.; Sienicka, P.; Cabaj-Wiater, I.; Knaś, M. Human saliva as a diagnostic material. Adv. Med. Sci. 2018, 63, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Henson, B.S.; Camargo, P.M.; Wong, D.T. The clinical value of salivary biomarkers for periodontal disease. Periodontol. 2000 2009, 51, 25–37. [Google Scholar] [CrossRef]
- Meghil, M.M.; Hutchens, L.; Raed, A.; Multani, N.A.; Rajendran, M.; Zhu, H.; Looney, S.; Elashiry, M.; Arce, R.M.; Peacock, M.E.; et al. The influence of vitamin D supplementation on local and systemic inflammatory markers in periodontitis patients: A pilot study. Oral. Dis. 2019, 25, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Özcan, E.; Işıl Saygun, N.; Serdar, M.A.; Umut Bengi, V.; Kantarcı, A. Non-Surgical Periodontal Therapy Reduces Saliva Adipokine and Matrix Metalloproteinase Levels in Periodontitis. J. Periodontol. 2016, 87, 934–943. [Google Scholar] [CrossRef]
- Beiler, T.F.C.S.B.; de Mello Neto, J.M.; Alves, J.C.; Hamlet, S.; Ipe, D.; da Silva Figueredo, C.M. Impact of non-surgical periodontal treatment on salivary expression of cytokines related to bone metabolism. Odontology 2020, 108, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Öngöz Dede, F.; Balli, U.; Bozkurt Doğan, Ş.; Güven, B. Interleukin-32 levels in gingival crevicular fluid and saliva of patients with chronic periodontitis after periodontal treatment. J. Periodontal Res. 2017, 52, 397–407. [Google Scholar] [CrossRef]
- Yue, Y.; Liu, Q.; Xu, C.; Loo, W.T.Y.; Wang, M.; Wen, G.; Cheung, M.N.B.; Bai, L.J.; Dou, Y.D.; Chow, L.W.C.; et al. Comparative evaluation of cytokines in gingival crevicular fluid and saliva of patients with aggressive periodontitis. Int. J. Biol Markers 2013, 28, 108–112. [Google Scholar] [CrossRef]
- Eivazi, M.; Falahi, N.; Eivazi, N.; Eivazi, M.A.; Raygani, A.V.; Rezaei, F. The Effect of Scaling and Root Planning on Salivary TNF-α and IL-1α Concentrations in Patients with Chronic Periodontitis. Open Dent. J. 2017, 11, 573–580. [Google Scholar] [CrossRef]
- Prakasam, S.; Srinivasan, M. Evaluation of salivary biomarker profiles following non-surgical management of chronic periodontitis. Oral. Dis. 2014, 20, 171–177. [Google Scholar] [CrossRef]
- Maekawa, T.; Hosur, K.; Abe, T.; Kantarci, A.; Ziogas, A.; Wang, B.; Van Dyke, T.E.; Chavakis, T.; Hajishengallis, G. Antagonistic effects of IL-17 and D-resolvins on endothelial Del-1 expression through a GSK-3β-CEBPβ pathway. Nat. Commun. 2015, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Mizraji, G.; Heyman, O.; Van Dyke, T.E.; Wilensky, A. Resolvin D2 restrains Th1 immunity and prevents alveolar bone loss in murine periodontitis. Front. Immunol. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Girnary, M.; Wang, L.; Jiao, Y.; Zeng, E.; Mercer, K.; Zhang, J.; Marchesan, J.T.; Yu, N.; Moss, K.; et al. IL-10 Dampens an IL-17–Mediated Periodontitis-Associated Inflammatory Network. J. Immunol. 2020, 204, 2177–2191. [Google Scholar] [CrossRef] [PubMed]
- Lewkowicz, N.; Mycko, M.P.; Przygodzka, P.; Ćwiklińska, H.; Cichalewska, M.; Matysiak, M.; Selmaj, K.; Lewkowicz, P. Induction of human IL-10-producing neutrophils by LPS-stimulated Treg cells and IL-10. Mucosal Immunol. 2016, 9, 364–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, H.; Balto, K.; Kawashima, N.; Eastcott, J.; Hoshino, K.; Akira, S.; Stashenko, P. Gamma Interferon (IFN-γ) and IFN-γ-Inducing Cytokines Interleukin-12 (IL-12) and IL-18 Do Not Augment Infection- Stimulated Bone Resorption In Vivo. Clin. Diagn. Lab. Immunol. 2004, 11, 106–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; Khattak, B.P.; Naagtilak, S.; Singh, G.; Bano, T. Effect of Periodontal Therapy on Salivary Interleukin-12 Levels in Chronic Periodontitis. J. Clin. Diagn. Res. 2014, 8, 90–93. [Google Scholar] [CrossRef]
- Issaranggun Na Ayuthaya, B.; Everts, V.; Pavasant, P. Interleukin-12 Induces Receptor Activator of Nuclear Factor-Kappa B Ligand Expression by Human Periodontal Ligament Cells. J. Periodontol. 2017, 88, e109–e119. [Google Scholar] [CrossRef]
- Tesmer, L.A.; Lundy, S.K.; Sarkar, S.; Fox, D.A. Th17 cells in human disease. Immunol. Rev. 2008, 223, 87–113. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H.; Ogasawara, K.; Hida, S.; Chiba, T.; Murata, S.; Sato, K.; Takaoka, A.; Yokochi, T.; Oda, H.; Tanaka, K.; et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-γ. Nature 2000, 408, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yu, F.; Xu, X.; Li, C.; Huang, D.; Zhou, X.; Ye, L.; Zheng, L. Evaluation of recombinant human FGF-2 and PDGF-BB in periodontal regeneration: A systematic review and meta-Analysis. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Joseph, A.; Nalini, H.; Kumar, A.; Devi, R. Pharmacotherapy for host modulation in periodontal disease: A review. J. Indian Acad. Dent. Spec. Res. 2015, 2, 35. [Google Scholar] [CrossRef]
- Mori, T.A. Fitoterapia Marine OMEGA-3 fatty acids in the prevention of cardiovascular disease. Fitoterapia 2017, 123, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, M.F.; van der Weijden, G.A. Risk factors for periodontitis. Int. J. Dent. Hyg. 2006, 4, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Genco, R.J. Current View of Risk Factors for Periodontal Diseases. J. Periodontol. 1996, 67, 1041–1049. [Google Scholar] [PubMed]
- Holm-Pedersen, P.; Löe, H. Wound healing in the gingiva of young and old individuals. Eur. J. Oral Res. 1971, 79, 40–53. [Google Scholar] [CrossRef]
- Lindhe, J.; Socransky, S.; Nyman, S.; Westfelt, E.; Haffajee, A. Effect of age on healing following periodontal therapy. J. Clin. Periodontol. 1985, 12, 774–787. [Google Scholar] [CrossRef]
- Lewkowicz, P.; Banasik, M.; Głowacka, E.; Lewkowicz, N.; Tchórzewski, H. Effect of high doses of shark liver oil supplementation on T cell polarization and peripheral blood polymorphonuclear cell function. Pol. Merkur. Lek. 2005, 18, 686–692. [Google Scholar]
- Iannitti, T.; Palmieri, B. An update on the therapeutic role of alkylglycerols. Mar. Drugs 2010, 8, 2267–2300. [Google Scholar] [CrossRef] [Green Version]

| SRP Plus Fish Oil | SRP Alone | p Value | |
|---|---|---|---|
| Age (years; mean ± SD) | 45 ± 8 | 54 ± 11 | 0.005 |
| Sex (% of females) | 50 | 43 | 0.349 |
| Variables | SRP Plus Fish Oil n = 16 | SRP Alone n = 14 | Inter-Group Comparisons p Value |
|---|---|---|---|
| Number of PD ≥ 4 mm | |||
| Baseline | 55 ± 29 | 52 ± 17 | 0.337 |
| 3 months | 32 ± 21 | 32 ± 17 | 0.461 |
| Δ baseline–3 months | 24 ± 18 | 20 ± 7 | 0.222 |
| PD (mm) | |||
| Baseline | 5.0 ± 0.5 | 5.1 ± 0.8 | 0.426 |
| 3 months | 3.7 ± 0.7 | 4.0 ± 0.7 | 0.461 |
| Δ baseline–3 months | 1.3 ± 0.7 | 1.1 ± 0.4 | 0.124 |
| REC (mm) | |||
| Baseline | 0.8 ± 0.6 | 1.1 ± 0.9 | 0.121 |
| 3 months | 0.8 ± 0.5 | 1.3 ± 0.7 | 0.023 |
| Δ baseline–3 months | 0.1 ± 0.3 | 0.2 ± 0.4 | 0.115 |
| CAL (mm) | |||
| Baseline | 5.8 ± 0.8 | 6.1 ± 1.1 | 0.144 |
| 3 months | 4.4 ± 1.1 | 5.3 ± 1.0 | 0.017 |
| Δ baseline–3 months | 1.3 ± 0.7 | 0.9 ± 0.4 | 0.021 |
| BOP (%) | |||
| Baseline | 28 ± 16 | 36 ± 19 | 0.069 |
| 3 months | 14 ± 6 | 21 ± 7 | 0.004 |
| Δ baseline–3 months | 13 ± 15 | 16 ± 21 | 0.335 |
| FMPI (%) | |||
| Baseline | 35 ± 21 | 49 ± 19 | 0.031 |
| 3 months | 17 ± 9 | 34 ± 16 | 0.0004 |
| Δ baseline–3 months | 18 ± 19 | 15 ± 23 | 0.345 |
| Closed pockets (%) with PD ≤4 mm and no BOP | |||
| Baseline | 0 | 0 | - |
| 3 months | 58 ± 17 | 49 ± 11 | 0.042 |
| Δ baseline–3 months | 58 ± 17 | 49 ± 11 | 0.042 |
| SRP Plus Fish Oil | SRP Alone | |||
|---|---|---|---|---|
| Baseline | 3 Months | Baseline | 3 Months | |
| Cytokines | ||||
| IL-1β | 223 ± 147 | 206 ± 144 | 330 ± 273 | 199 ± 176 † |
| IL-1RA | 5764 ± 2186 | 6804 ± 3095 | 5555 ± 3851 | 5671 ± 3235 |
| IL-2 | 21 ± 12 | 13 ± 4 † | 15 ± 5 | 15 ± 8 |
| IL-4 | 22 ± 12 | 18 ± 5 | 17 ± 10 | 15 ± 4 |
| IL-5 | 7.6 ± 5.7 | 9.4 ± 5.8 | 5.4 ± 4.8 | 7.1 ± 5.5 |
| IL-6 | 110 ± 82 | 139 ± 115 | 193 ± 150 | 133 ± 137 |
| IL-7 | 31 ± 20 | 26 ± 15 | 20 ± 10 | 48 ± 13 |
| IL-9 | 11 ± 6 | 14 ± 8 | 10 ± 4 | 12 ± 6 |
| IL-10 | 149 ± 51 | 211 ± 65 *,† | 133 ± 34 | 129 ± 44 |
| IL-12 | 1.7 ± 1.3 | 3.2 ± 1.8 *,† | 0.9 ± 0.5 | 1.6 ± 0.9 † |
| IL-13 | 0.5 ± 0.2 | 0.5 ± 0.4 | 0.5 ± 0.2 | 0.5 ± 0.6 |
| IL-15 | nd | 44 ± 37 | nd | 49 ± 13 |
| IL-16 | 2600 ± 2680 | 1228 ± 872 | 1163 ± 754 | 1556 ± 1596 |
| IL-17 | 17 ± 8 | 8 ± 3 *,† | 12 ± 3 | 11 ± 2 |
| IFN-γ | 38 ± 17 | 46 ± 29 | 46 ± 21 | 38 ± 16 |
| MIF | 677,488 ± 645,516 | 445,004 ± 302,624 | 345,344 ± 289,914 | 280,090 ± 287,976 |
| TNF-α | 54 ± 29 | 41 ± 26 † | 37 ± 13 | 33 ± 10 |
| Chemokines | ||||
| CCL1/I-309 | 38 ± 12 | 34 ± 9 | 29 ± 10 | 27 ± 9 |
| CCL2/MCP-1 | 1205 ± 870 | 1515 ± 1795 | 1194 ± 689 | 1642 ± 1579 |
| CCL3/MIP-1 α | 33 ± 31 | 47 ± 63 | 16 ± 10 | 17 ± 16 |
| CCL4/MIP-1 β | 8.7 ± 5.8 | 18 ± 16 † | 7.3 ± 1.7 | 9.4 ± 5.1 |
| CCL5/RANTES | 4.8 ± 2.3 | 7.2 ± 1.6 *,† | 4.2 ± 3.5 | 4.2 ± 2.3 |
| CCL7/MCP-3 | 33 ± 27 | 28 ± 23 | 24 ± 10 | 26 ± 11 |
| CCL8/MCP-2 | 3.9 ± 5.0 | 2.4 ± 1.5 | 1.6 ± 1.0 | 2.1 ± 1.4 |
| CCL11/Eotaxin | 28 ± 8 | 28 ± 8 | 24 ± 7 | 23 ± 5 |
| CCL13/MCP-4 | 98 ± 63 | 96 ± 68 | 57 ± 34 | 70 ± 42 |
| CCL15/MIP-1delta | 127 ± 87 | 268 ± 196 † | 271 ± 319 | 181 ± 166 |
| CCL17/TARC | 7.2 ± 7.4 | 2.3 ± 2.9 | 7.3 ± 4.6 | 2.8 ± 3.1 |
| CCL19/MIP-3 β | 124 ± 51 | 119 ± 52 | 99 ± 34 | 153 ± 101 |
| CCL20/MIP-3 α | 6.7 ± 6.0 | 10 ± 20 | 3.8 ± 3.2 | 3.6 ± 3.4 |
| CCL21/6Ckine | 2329 ± 1891 | 1297 ± 746 † | 1370 ± 861 | 1750 ± 1119 |
| CCL22/MDC | 32 ± 12 | 33 ± 16 * | 25 ± 10 | 21 ± 7 |
| CCL23/MPIF-1 | 30 ± 170 | 26 ± 14 | 25 ± 10 | 17 ± 7 |
| CCL24/Eotaxin-2 | 25 ± 31 | 20 ± 8 | 18 ± 6 | 23 ± 5 † |
| CCL25/TECK | 827 ± 316 | 843 ± 408*,† | 533 ± 126 | 523 ± 142 |
| CCL26/Eotaxin-3 | 24 ± 10 | 15 ± 7 † | 13 ± 5 | 12 ± 4 |
| CCL27/CTACK | 18 ± 12 | 11 ± 7 † | 14 ± 9 | 12 ± 9 |
| CX3CL1/Factalkine | 1858 ± 1336 | 2625 ± 1791*,† | 1422 ± 874 | 1187 ± 828 |
| CXCL1/Gro-alpha | 10,812 ± 11,390 | 5212 ± 5106 † | 3113 ± 1802 | 3626 ± 3493 |
| CXCL2/Gro-beta | 1115 ± 1329 | 669 ± 705 | 591 ± 371 | 439 ± 369 |
| CXCL5/ENA-78 | 20,513 ± 21,340 | 19,718 ± 24,877 | 12,199 ± 14,653 | 16,399 ± 18,018 |
| CXCL6/GCP-2 | 163 ± 200 | 188 ± 339 | 22 ± 18 | 50 ± 60 |
| CXCL8/IL-8 | 20,811 ± 18,099 | 8580 ± 10,551 *,† | 41,658 ± 37,582 | 29,557 ± 28,905 |
| CXCL9/MIG | 562 ± 470 | 625 ± 509 | 384 ± 416 | 330 ± 350 |
| CXCL10/IP-10 | 287 ± 267 | 173 ± 216† | 153 ± 78 | 140 ± 95 |
| CXCL11/I-TAC | 4.0 ± 3.3 | 3.2 ± 3.2 | 2.3 ± 2.4 | 1.7 ± 1.0 |
| CXCL12/SDF-1 α | 81 ± 34 | 84 ± 40 | 78 ± 23 | 68 ± 21 |
| CXCL13/BCA-1 | 5.1 ± 4.8 | 4.4 ± 5.0 | 3.1 ± 2.8 | 4.3 ± 8.4 |
| CXCL16/SCYB16 | 158 ± 136 | 88 ± 74 † | 100 ± 93 | 107 ± 147 |
| Growth factors | ||||
| FGF2 | 35 ± 10 | 40 ± 11 * | 29 ± 12 | 29 ± 9 |
| G-CSF | 358 ± 196 | 222 ± 84 *,† | 299 ± 142 | 410 ± 308 |
| GM-CSF | 22 ± 9 | 24 ± 19 | 20 ± 7 | 17 ± 5 |
| PDGF-BB | 13 ± 6 | 18 ± 13 | 10 ± 9 | 12 ± 9 |
| VEGF | 317 ± 228 | 330 ± 199 | 293 ± 147 | 282 ± 219 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stańdo, M.; Piatek, P.; Namiecinska, M.; Lewkowicz, P.; Lewkowicz, N. Omega-3 Polyunsaturated Fatty Acids EPA and DHA as an Adjunct to Non-Surgical Treatment of Periodontitis: A Randomized Clinical Trial. Nutrients 2020, 12, 2614. https://doi.org/10.3390/nu12092614
Stańdo M, Piatek P, Namiecinska M, Lewkowicz P, Lewkowicz N. Omega-3 Polyunsaturated Fatty Acids EPA and DHA as an Adjunct to Non-Surgical Treatment of Periodontitis: A Randomized Clinical Trial. Nutrients. 2020; 12(9):2614. https://doi.org/10.3390/nu12092614
Chicago/Turabian StyleStańdo, Mirella, Paweł Piatek, Magdalena Namiecinska, Przemysław Lewkowicz, and Natalia Lewkowicz. 2020. "Omega-3 Polyunsaturated Fatty Acids EPA and DHA as an Adjunct to Non-Surgical Treatment of Periodontitis: A Randomized Clinical Trial" Nutrients 12, no. 9: 2614. https://doi.org/10.3390/nu12092614
APA StyleStańdo, M., Piatek, P., Namiecinska, M., Lewkowicz, P., & Lewkowicz, N. (2020). Omega-3 Polyunsaturated Fatty Acids EPA and DHA as an Adjunct to Non-Surgical Treatment of Periodontitis: A Randomized Clinical Trial. Nutrients, 12(9), 2614. https://doi.org/10.3390/nu12092614