An Observational Study Evaluating the Introduction of a Prolonged-Release Protein Substitute to the Dietary Management of Children with Phenylketonuria
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Study Product
2.4. Preparation of Study Product
2.5. Assessments
2.6. Ethical Permission
2.7. Statistical Analysis
3. Results
3.1. Subjects
3.2. Substudy Cohort Results
3.3. Administration of Study Product
3.4. Adherence with Study Product
3.5. Blood Phenylalanine and Tyrosine Control
3.6. Gastrointestinal Symptoms
3.7. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Wegberg, A.M.J.; MacDonald, A.; Ahring, K.; Belanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Gizewska, M.; et al. The complete European guidelines on phenylketonuria: Diagnosis and treatment. Orphanet J. Rare Dis. 2017, 12, 162. [Google Scholar] [CrossRef] [PubMed]
- Hood, A.; Grange, D.K.; Christ, S.E.; Steiner, R.; White, D.A. Variability in phenylalanine control predicts IQ and executive abilities in children with phenylketonuria. Mol. Genet. Metab. 2014, 111, 445–451. [Google Scholar] [CrossRef] [PubMed]
- DeRoche, K.; Welsh, M. Twenty-five years of research on neurocognitive outcomes in early-treated phenylketonuria: Intelligence and executive function. Dev. Neuropsychol. 2008, 33, 474–504. [Google Scholar] [CrossRef] [PubMed]
- Simon, E.; Schwarz, M.; Roos, J.; Dragano, N.; Geraedts, M.; Siegrist, J.; Kamp, G.; Wendel, U. Evaluation of quality of life and description of the sociodemographic state in adolescent and young adult patients with phenylketonuria (PKU). Health Qual. Life Outcomes 2008, 6, 25. [Google Scholar] [CrossRef]
- Al Hafid, N.; Christodoulou, J. Phenylketonuria: A review of current and future treatments. Transl. Pediatr. 2015, 4, 304–317. [Google Scholar]
- Van Spronsen, F.J.; van Wegberg, A.M.; Ahring, K.; Belanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Gizewska, M.; et al. Key European guidelines for the diagnosis and management of patients with phenylketonuria. Lancet Diabetes Endocrinol. 2017, 5, 743–756. [Google Scholar] [CrossRef]
- Macleod, E.L.; Ney, D.M. Nutritional Management of Phenylketonuria. Ann. Nestle 2010, 68, 58–69. [Google Scholar] [CrossRef]
- MacDonald, A.; Singh, R.H.; Rocha, J.C.; van Spronsen, F.J. Optimising amino acid absorption: Essential to improve nitrogen balance and metabolic control in phenylketonuria. Nutr. Res. Rev. 2019, 32, 70–78. [Google Scholar] [CrossRef]
- MaCdonald, A.; van Rijn, M.; Feillet, F.; Lund, A.M.; Bernstein, L.; Bosch, A.M.; Gizewska, M.; van Spronsen, F.J. Adherence issues in inherited metabolic disorders treated by low natural protein diets. Ann. Nutr. Metab. 2012, 61, 289–295. [Google Scholar] [CrossRef]
- Shaw, V. (Ed.) Clinical Paediatric Dietetics, 4th ed.; Wiley-Blackwell: Oxford, UK, 2014; p. 864. [Google Scholar]
- MacDonald, A. Diet and compliance in phenylketonuria. Eur. J. Pediatr. 2000, 159 (Suppl. S2), S136–S141. [Google Scholar] [CrossRef]
- Ford, S.; O’Driscoll, M.; MacDonald, A. Living with Phenylketonuria: Lessons from the PKU community. Mol. Genet. Metab. Rep. 2018, 17, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.H.; White, F.J. Blood phenylalanine control in adolescents with phenylketonuria. Int. J. Adolesc. Med. Health 2004, 16, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Green, B.; Browne, R.; Firman, S.; Hill, M.; Rahman, Y.; Kaalund Hansen, K.; Adam, S.; Skeath, R.; Hallam, P.; Herlihy, I.; et al. Nutritional and metabolic characteristics of UK adult phenylketonuria patients with varying dietary adherence. Nutrients 2019, 11, 2459. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A.; Harris, G.; Rylance, G.; Asplin, D.; Booth, I.w. Abnormal feeding behaviours in phenylketonuria. J. Hum. Nutr. Diet. 1997, 10, 163–170. [Google Scholar] [CrossRef]
- Giarratana, N.; Gallina, G.; Panzeri, V.; Frangi, A.; Canobbio, A.; Reiner, G. A new Phe-free protein substitute engineered to allow a physiological absorption of free amino acids for phenylketonuria. J. Inborn Errors Metab. 2018, 6, 1–9. [Google Scholar] [CrossRef]
- Pena, M.J.; de Almeida, M.F.; van Dam, E.; Ahring, K.; Belanger-Quintana, A.; Dokoupil, K.; Gokmen-Ozel, H.; Lammardo, A.M.; MacDonald, A.; Robert, M.; et al. Protein substitutes for phenylketonuria in Europe: Access and nutritional composition. Eur. J. Clin. Nutr. 2016, 70, 785–789. [Google Scholar] [CrossRef]
- Paul, D.B.; Brosco, J.P. The PKU Paradox. A Short History of a Genetic Disease; Johns Hopkins University Press: Baltimore, MD, USA, 2013. [Google Scholar]
- Tiele, A.; Daly, A.; Hattersley, J.; Pinto, A.; Evans, S.; Ashmore, C.; MacDonald, A.; Covington, J.A. Investigation of paediatric PKU breath malodour, comparing glycomacropeptide with phenylalanine free L-amino acid supplements. J. Breath Res. 2019, 14, 016001. [Google Scholar] [CrossRef]
- Lim, K.; van Calcar, S.C.; Nelson, K.L.; Gleason, S.T.; Ney, D.M. Acceptable low-phenylalanine foods and beverages can be made with glycomacropeptide from cheese whey for individuals with PKU. Mol. Genet. Metab. 2007, 92, 176–178. [Google Scholar] [CrossRef]
- MacDonald, A.; Gokmen-Ozel, H.; van Rijn, M.; Burgard, P. The reality of dietary compliance in the management of phenylketonuria. J. Inherit. Metab. Dis. 2010, 33, 665–670. [Google Scholar] [CrossRef]
- Duran, G.P.; Rohr, F.J.; Slonim, A.; Guttler, F.; Levy, H.L. Necessity of complete intake of phenylalanine-free amino acid mixture for metabolic control of phenylketonuria. J. Am. Diet. Assoc. 1999, 99, 1559–1563. [Google Scholar] [CrossRef]
- Scheinin, M.; Barassi, A.; Junnila, J.; Lovro, Z.; Reiner, G.; Sarkkinen, E.; MacDonald, A. Amino acid plasma profiles from a prolonged-release protein substitute for phenylketonuria: A randomized, single-dose, four-way crossover trial in healthy volunteers. Nutrients 2020, 12, 1653. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.; Daly, A.; Chahal, S.; MacDonald, J.; MacDonald, A. Food acceptance and neophobia in children with phenylketonuria: A prospective controlled study. J. Hum. Nutr. Diet. 2016, 29, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.; Daly, A.; Chahal, S.; Ashmore, C.; MacDonald, J.; MacDonald, A. The influence of parental food preference and neophobia on children with phenylketonuria (PKU). Mol. Genet. Metab. Rep. 2018, 14, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, L.E.; Helm, J.R.; Rocha, J.C.; Almeida, M.F.; Feillet, F.; Link, R.M.; Gizewska, M. Nutrition education tools used in phenylketonuria: Clinician, parent and patient perspectives from three international surveys. J. Hum. Nutr. Diet. 2014, 27 (Suppl. S2), 4–11. [Google Scholar] [CrossRef]
- Giovannini, M.; Verduci, E.; Salvatici, E.; Paci, S.; Riva, E. Phenylketonuria: Nutritional advances and challenges. Nutr. Metab. 2012, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Ford, S.; O’Driscoll, M.; MacDonald, A. Reproductive experience of women living with phenylketonuria. Mol. Genet. Metab. Rep. 2018, 17, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, K.; Winnicka, K. Ethylcellulose—A pharmaceutical excipient with multidirectional application in drug dosage forms development. Materials (Basel) 2019, 12, 3386. [Google Scholar] [CrossRef]
- PBMK, A. Nutritional Management of Patients with Inherited Disorders of Aromatic Amino Acid Metabolism; Jones and Bartlett: Boston, MA, USA, 2010. [Google Scholar]
- Arica, B.; Calis, S.; Atilla, P.; Durlu, N.T.; Cakar, N.; Kas, H.S.; Hincal, A.A. In vitro and in vivo studies of ibuprofen-loaded biodegradable alginate beads. J. Microencapsul. 2005, 22, 153–165. [Google Scholar] [CrossRef]
- Rocha, J.C.; MacDonald, A. Dietary intervention in the management of phenylketonuria: Current perspectives. Pediatr. Health Med. Ther. 2016, 7, 155–163. [Google Scholar] [CrossRef]
- Lacroix, M.; Bos, C.; Leonil, J.; Airinei, G.; Luengo, C.; Dare, S.; Benamouzig, R.; Fouillet, H.; Fauquant, J.; Tome, D.; et al. Compared with casein or total milk protein, digestion of milk soluble proteins is too rapid to sustain the anabolic postprandial amino acid requirement. Am. J. Clin. Nutr. 2006, 84, 1070–1079. [Google Scholar] [CrossRef]
- Bujko, J.; Schreurs, V.V.; Nolles, J.A.; Verreijen, A.M.; Koopmanschap, R.E.; Verstegen, M.W. Application of a [13CO2] breath test to study short-term amino acid catabolism during the postprandial phase of a meal. Br. J. Nutr. 2007, 97, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Metges, C.C.; Barth, C.A. Metabolic consequences of a high dietary-protein intake in adulthood: Assessment of the available evidence. J. Nutr. 2000, 130, 886–889. [Google Scholar] [CrossRef] [PubMed]
| Component | Per 100 g | Per Sachet of 24 g |
|---|---|---|
| Energy | 280 kcal/1187 kJ | 67 kcal/286 kJ |
| Fat | 0 g | 0 g |
| of which saturated | 0 g | 0 g |
| Carbohydrate | 4.3 g | 1.0 g |
| of which sugars | 0 g | 0 g |
| Fibre | 7.1 g | 1.7 g |
| Protein equivalent 1 | 62.2 g | 15 g |
| Salt | 0.06 g | 0.015 g |
| Amino Acids | ||
| L-serine | 2.5 g | 0.6 g |
| L-threonine | 3.8 g | 0.9 g |
| L-leucine | 8.6 g | 2.1 g |
| Glycine | 3.8 g | 0.9 g |
| L-alanine | 2.3 g | 0.5 g |
| L-arginine | 3.0 g | 0.7 g |
| L-cysteine | 1.5 g | 0.4 g |
| L-glutamine | 15.0 g | 3.6 g |
| L-histidine | 2.1 g | 0.5 g |
| L-aspartic acid | 4.5 g | 1.1 g |
| L-proline | 4.5 g | 1.1 g |
| L-isoleucine | 4.1 g | 1.0 g |
| L-lysine | 5.3 g | 1.3 g |
| L-tryptophan | 1.5 g | 0.4 g |
| L-valine | 3.8 g | 0.9 g |
| L-methionine | 1.0 g | 0.3 g |
| L-tyrosine | 7.5 g | 1.8 g |
| Vitamins | ||
| Vitamin A (RE) | 1295 mcg | 311 mcg |
| Vitamin D | 25 mcg | 6.0 mcg |
| Vitamin E (αTE) | 13 mg | 3.2 mg |
| Vitamin K | 100 mcg | 24 mcg |
| Vitamin C | 135 mg | 32.31 mg |
| Thiamine | 2.0 mg | 0.5 mg |
| Riboflavin | 1.9 mg | 0.5 mg |
| Niacin | 27 mg | 6.4 mg |
| Vitamin B6 | 2.6 mg | 0.6 mg |
| Folic acid | 267 mcg | 64.1 mcg |
| Vitamin B12 | 4.2 mcg | 1.0 mcg |
| Biotin | 54 mcg | 13 mcg |
| Pantothenic acid | 11 mg | 2.6 mg |
| Minerals | ||
| Potassium | 1250 mg | 300 mg |
| Calcium | 1339 mg | 321 mg |
| Magnesium | 304 mg | 72.9 mg |
| Phosphorus | 1060 mg | 254 mg |
| Chloride | 0.75 mg | 0.18 mg |
| Sodium | 25 mg | 5.9 mg |
| Iron | 23 mg | 5.6 mg |
| Zinc | 14 mg | 3.4 mg |
| Copper | 1.4 mg | 0.3 mg |
| Manganese | 2.5 mg | 0.6 mg |
| Selenium | 58 mcg | 14 mcg |
| Chromium | 46 mcg | 11 mcg |
| Molybdenum | 88 mcg | 21 mcg |
| Iodine | 225 mcg | 54.0 mcg |
| Other Nutrients | ||
| Carnitine | 0.08 g | 0.02 g |
| Taurine | 0.21 g | 0.05 g |
| Choline | 321 mg | 77.1 mg |
| Inositol | 214 mg | 51.4 mg |
| Baseline Demographics | |||||||
| Subject | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Age (years) | 11 | 11 | 12 | 9 | 7 | 11 | 15 |
| Weight (kg) | 60.3 | 53.3 | 45.9 | 25.8 | 26.6 | 45.6 | 55.8 |
| Height (cm) | 152.3 | 147.5 | 155.8 | 124.5 | 119.7 | 154.8 | 174.4 |
| PKU classification | Classical | Classical | Moderate | Classical | Classical | Classical | Classical |
| Blood Phe on diagnosis (µmol/L) | 1700 | 1680 | 900 | 1390 | 1590 | 2520 | 2690 |
| Gender | Female | Female | Male | Male | Male | Male | Male |
| Ethnicity | Pakistani | Pakistani | White European | White British | Mixed race | White British | White British |
| Diet and Protein-Substitute Profile | |||||||
| Natural protein allowance (g/day) | 4.0 | 4.0 | 7.0 | 3.0 | 6.5 | 7.5 | 18.0 |
| Protein equivalent from usual protein substitutes g/day (g/kg/day) | 60.0 (1.0) | 60.0 (1.1) | 60.0 (1.3) | 80.0 (3.1) | 60.0 (2.3) | 80.0 (1.7) | 60.0 (1.2) |
| Number of different protein substitutes/day | 2 | 2 | 2 | 1 | 2 | 2 | 1 |
| Number of doses/day | 3 | 3 | 4 | 4 | 4 | 4 | 3 |
| Study Product Treatment Schedule and Preparation | |||||||
| Daily dose (g) | 24.0 | 24.0 | 24.0 | 32.0 | 24.0 | 24.0 | 24.0 |
| Protein equivalent from study product (g) | 15.0 | 15.0 | 15.0 | 20.0 | 15.0 | 15.0 | 15.0 |
| Total daily protein equivalent from all protein substitutes (g) 1 | 55.0 | 55.0 | 55.0 | 80.0 | 60.0 | 75.0 | 55.0 |
| Protein equivalent from study product (% of daily intake) | 27.3 | 27.3 | 27.3 | 25.0 | 25.0 | 20.0 | 27.3 |
| Method of administration | In fruit juice | In fruit juice | Food and drinks | In fruit juice | In fruit juice and food | Fruit smoothie | Smoothie |
| Timing of administration of study product | Evening | Evening | Evening | Evening | Morning midday and bedtime | Morning or evening | Morning or evening |
| Comments on study product | Left some bits behind on cup | Last bit was hard to take | No comments | No comments | No comments | Required blender to mix | No comments |
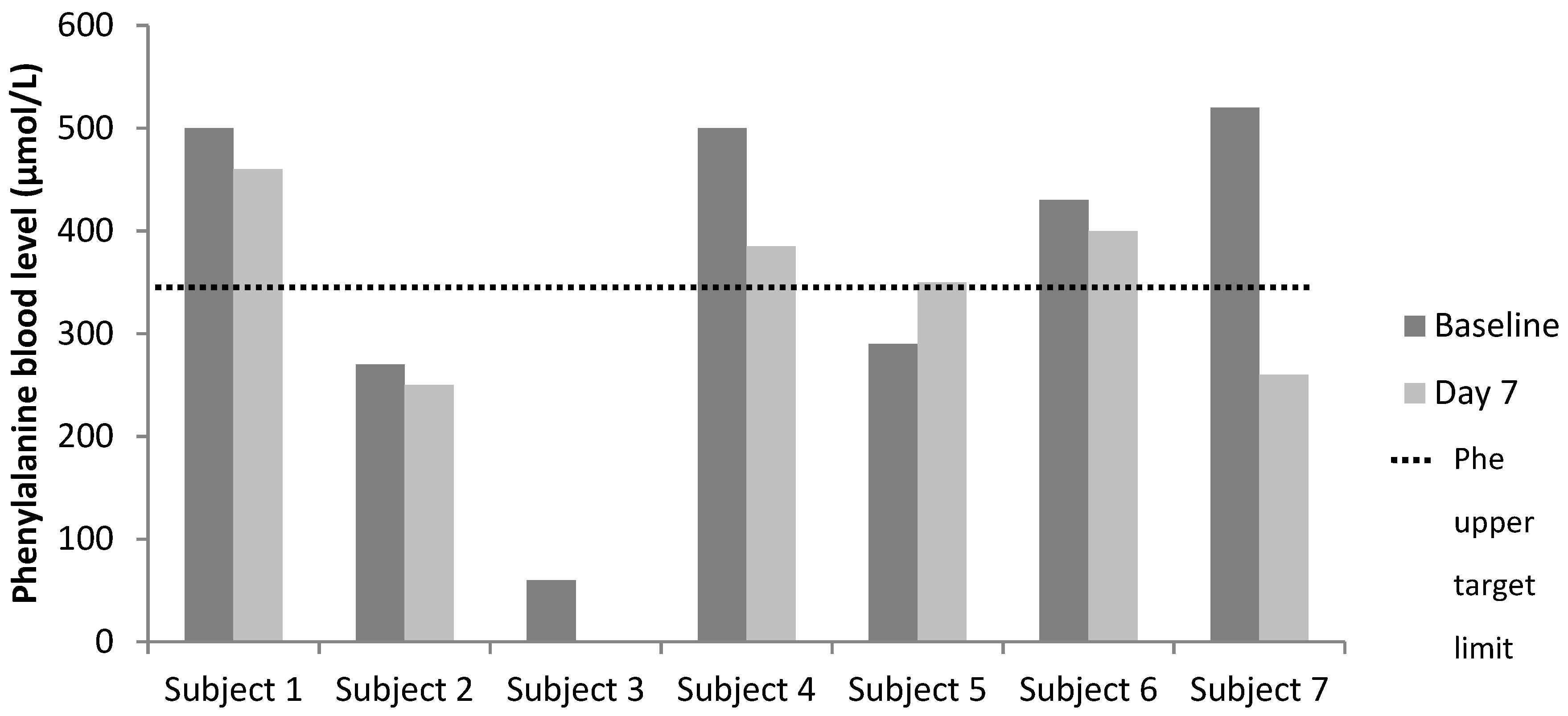
| Subject | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Target Phe levels (µmol/L) | 120–360 | 120–360 | 120–360 | 120–360 | 120–360 | 120–360 | 120–600 |
| Phenylalanine Levels (µmol/L) | |||||||
| Baseline | 500 | 270 | 60 | 500 | 290 | 430 | 520 |
| Day 7 | 460 | 250 | NA 1 | 385 | 350 | 400 | 260 |
| Tyrosine Levels (µmol/L) | |||||||
| Baseline | 110 | 120 | 50 | 30 | 60 | 50 | 70 |
| Day 7 | 150 | 160 | NA 1 | NA 1 | 40 | 60 | 50 |
| Phenylalanine/Tyrosine Ratio | |||||||
| Baseline | 4.5 | 2.3 | 1.2 | 16.7 | 4.8 | 8.6 | 7.4 |
| Day 7 | 3.1 | 1.6 | NC 1 | NC 1 | 8.8 | 6.7 | 5.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
MacDonald, A.; Ashmore, C.; Daly, A.; Pinto, A.; Evans, S. An Observational Study Evaluating the Introduction of a Prolonged-Release Protein Substitute to the Dietary Management of Children with Phenylketonuria. Nutrients 2020, 12, 2686. https://doi.org/10.3390/nu12092686
MacDonald A, Ashmore C, Daly A, Pinto A, Evans S. An Observational Study Evaluating the Introduction of a Prolonged-Release Protein Substitute to the Dietary Management of Children with Phenylketonuria. Nutrients. 2020; 12(9):2686. https://doi.org/10.3390/nu12092686
Chicago/Turabian StyleMacDonald, Anita, Catherine Ashmore, Anne Daly, Alex Pinto, and Sharon Evans. 2020. "An Observational Study Evaluating the Introduction of a Prolonged-Release Protein Substitute to the Dietary Management of Children with Phenylketonuria" Nutrients 12, no. 9: 2686. https://doi.org/10.3390/nu12092686
APA StyleMacDonald, A., Ashmore, C., Daly, A., Pinto, A., & Evans, S. (2020). An Observational Study Evaluating the Introduction of a Prolonged-Release Protein Substitute to the Dietary Management of Children with Phenylketonuria. Nutrients, 12(9), 2686. https://doi.org/10.3390/nu12092686






