Impact of a Moderately Hypocaloric Mediterranean Diet on the Gut Microbiota Composition of Italian Obese Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients Recruitment and Samples Collection
2.2. Anthropometric and Nutritional Assessment
2.3. Nutritional Intervention
2.4. Microbial DNA Extraction and 16S rRNA Gene-Based Illumina MiSeq Sequencing
2.5. Bioinformatics and Statistics
2.6. Availability of Data and Materials
3. Results
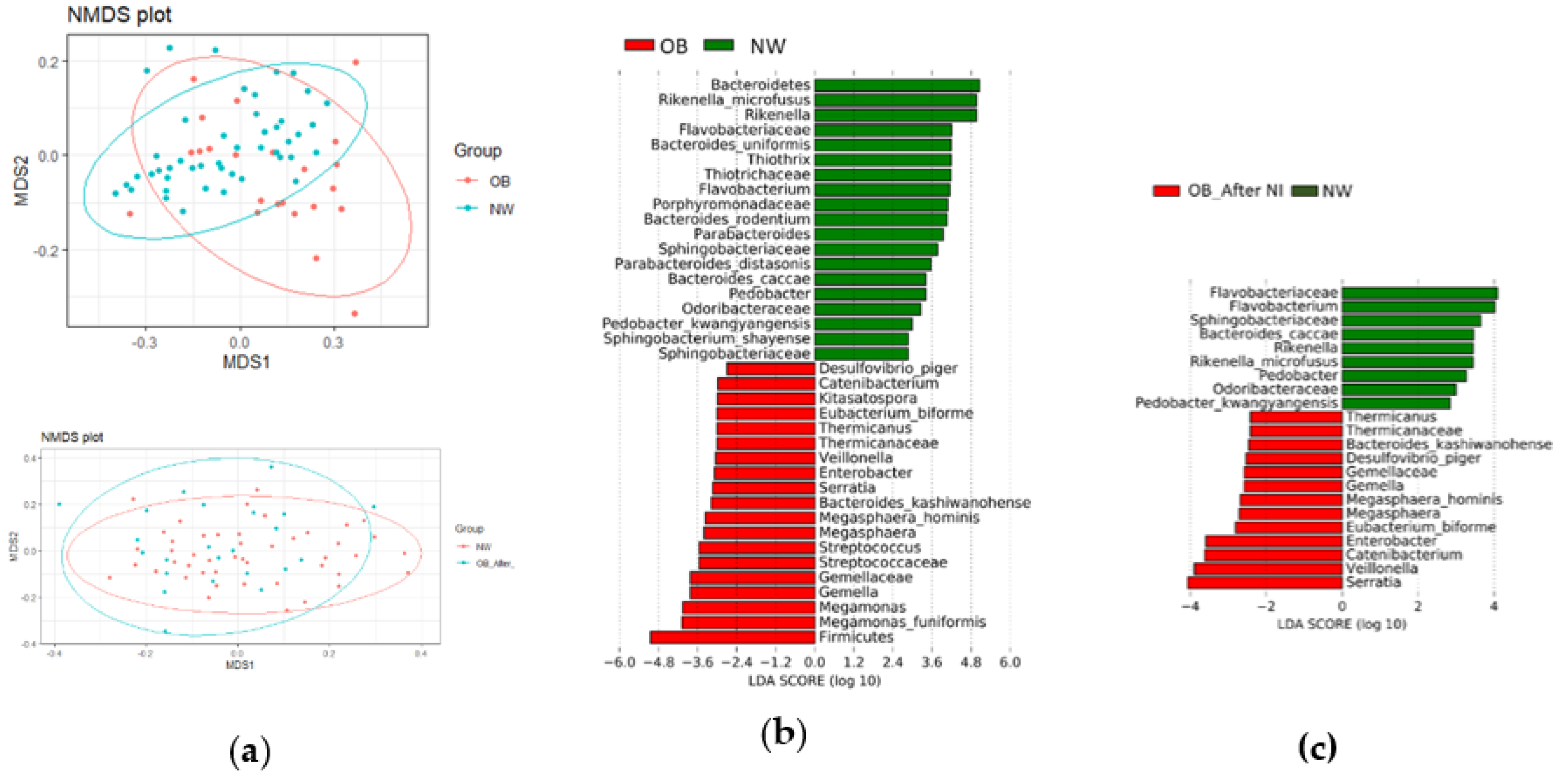
Gut Microbiota Diversity and Composition
3.1.1. Gut Microbiota Diversity
3.1.2. Gut Microbiota Composition
3.1.3. Predicted Metabolic Pathways
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 30 October 2019).
- Scafato, E.; Orsini, S. Fumo, Alcol, Alimentazione, Eccesso Ponderale e Prevenzione. Rapp. Oss. Available online: https://www.osservatoriosullasalute.it/osservasalute/rapporto-osservasalute-2016 (accessed on 2 February 2020).
- Aune, D.; Sen, A.; Prasad, M.; Norat, T.; Janszky, I.; Tonstad, S.; Romundstad, P.; Vatten, L.J. BMI and all cause mortality: Systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ 2016, 353, i2156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhaskaran, K.; dos-Santos-Silva, I.; Leon, D.A.; Douglas, I.J.; Smeeth, L. Association of BMI with overall and cause-specific mortality: A population-based cohort study of 3·6 million adults in the UK. Lancet Diabetes Endocrinol. 2018, 6, 944–953. [Google Scholar] [CrossRef] [Green Version]
- Romieu, I.; Dossus, L.; Barquera, S.; Blottière, H.M.; Franks, P.W.; Gunter, M.; Hwalla, N.; Hursting, S.D.; Leitzmann, M.; Margetts, B.; et al. Energy balance and obesity: What are the main drivers? Cancer Causes Control 2017, 28, 247–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, R.; Goh, W.-R.; Wu, R.; Yue, X.; Luo, X.; Khine, W.W.T.; Wu, J.; Lee, Y. Revisit gut microbiota and its impact on human health and disease. J. Food Drug Anal. 2019, 27, 623–631. [Google Scholar] [CrossRef]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Irritable bowel syndrome: A microbiome-gut-brain axis disorder? World J. Gastroenterol. 2014, 20, 14105–141025. [Google Scholar] [CrossRef]
- Castaner, O.; Goday, A.; Park, Y.-M.; Lee, S.-H.; Magkos, F.; Shiow, S.-A.; Schröder, H. The Gut Microbiome Profile in Obesity: A Systematic Review. Int. J. Endocrinol. 2018. [Google Scholar] [CrossRef]
- Davis, C.D. The Gut Microbiome and Its Role in Obesity. Nutr. Today 2016, 51, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Baothman, O.A.; Zamzami, M.A.; Taher, I.; Abubaker, J.; Abu-Farha, M. The role of Gut Microbiota in the development of obesity and Diabetes. Lipids Health Dis. 2016, 15, 108. [Google Scholar] [CrossRef] [Green Version]
- Seganfredo, F.B.; Blume, C.A.; Moehlecke, M.; Giongo, A.; Casagrande, D.S.; Spolidoro, J.V.N.; Padoin, A.V.; Schaan, B.D.; Mottin, C.C. Weight-loss interventions and gut microbiota changes in overweight and obese patients: A systematic review. Obes. Rev. J. Int. Assoc. Study Obes. 2017, 18, 832–851. [Google Scholar] [CrossRef]
- Hall, K.D.; Kahan, S. Maintenance of Lost Weight and Long-Term Management of Obesity. Med. Clin. North Am. 2018, 102, 183–197. [Google Scholar] [CrossRef]
- Lohman, T.J.; Roache, A.F.; Martorell, R. Anthropometric Standardization Reference Manual. Med. Sci. Sports Exerc. 1992, 24, 952. [Google Scholar] [CrossRef] [Green Version]
- Panagiotakos, D.B.; Pitsavos, C.; Arvaniti, F.; Stefanadis, C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev. Med. 2007, 44, 335–340. [Google Scholar] [CrossRef] [PubMed]
- LARN, 2014. Available online: https://sinu.it/tabelle-larn-2014/ (accessed on 29 October 2019).
- Santoru, M.L.; Piras, C.; Murgia, A.; Palmas, V.; Camboni, T.; Liggi, S.; Ibba, I.; Lai, M.A.; Orrù, S.; Blois, S.; et al. Cross sectional evaluation of the gut-microbiome metabolome axis in an Italian cohort of IBD patients. Sci. Rep. 2017, 7, 9523. [Google Scholar] [CrossRef] [PubMed]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, D.; Hewlings, S.; Kalman, D. Body Composition Changes in Weight Loss: Strategies and Supplementation for Maintaining Lean Body Mass, a Brief Review. Nutrients 2018, 10, 1876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mantrana, I.; Selma-Royo, M.; Alcantara, C.; Collado, M.C. Shifts on Gut Microbiota Associated to Mediterranean Diet Adherence and Specific Dietary Intakes on General Adult Population. Front. Microbiol. 2018, 9, 890. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef] [Green Version]
- Del Chierico, F.; Vernocchi, P.; Dallapiccola, B.; Putignani, L. Mediterranean Diet and Health: Food Effects on Gut Microbiota and Disease Control. Int. J. Mol. Sci. 2014, 15, 11678–11699. [Google Scholar] [CrossRef]
- Foscolou, A.; Tyrovolas, S.; Soulis, G.; Mariolis, A.; Piscopo, S.; Valacchi, G.; Anastasiou, F.; Lionis, C.; Zeimbekis, A.; Tur, J.-A.; et al. The Impact of the Financial Crisis on Lifestyle Health Determinants among Older Adults Living in the Mediterranean Region: The Multinational MEDIS Study (2005–2015). J. Prev. Med. Pub. Health 2017, 50, 1–9. [Google Scholar] [CrossRef]
- Del Chierico, F.; Abbatini, F.; Russo, A.; Quagliariello, A.; Reddel, S.; Capoccia, D.; Caccamo, R.; Ginanni Corradini, S.; Nobili, V.; De Peppo, F.; et al. Gut Microbiota Markers in Obese Adolescent and Adult Patients: Age-Dependent Differential Patterns. Front. Microbiol. 2018, 9, 1210. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Díaz, I.; Fernández-Navarro, T.; Sánchez, B.; Margolles, A.; González, S. Mediterranean diet and faecal microbiota: A transversal study. Food Funct. 2016, 7, 2347–2356. [Google Scholar] [CrossRef] [PubMed]
- Mitsou, E.K.; Kakali, A.; Antonopoulou, S.; Mountzouris, K.C.; Yannakoulia, M.; Panagiotakos, D.B.; Kyriacou, A. Adherence to the Mediterranean diet is associated with the gut microbiota pattern and gastrointestinal characteristics in an adult population. Br. J. Nutr. 2017, 117, 1645–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semova, I.; Carten, J.D.; Stombaugh, J.; Mackey, L.C.; Knight, R.; Farber, S.A.; Rawls, J.F. Microbiota regulate intestinal absorption and metabolism of fatty acids in the zebrafish. Cell Host Microbe 2012, 12, 277–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborti, C.K. New-found link between microbiota and obesity. World J. Gastrointest Pathophysiol. 2015, 6, 110–119. [Google Scholar] [CrossRef]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Ross, R.P.; O’Toole, P.W.; Shanahan, F.; Cotter, P.D. Targeting the Microbiota to Address Diet-Induced Obesity: A Time Dependent Challenge. PLoS ONE 2013, 8, e65790. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.S.; Jeraldo, P.R.; Kurti, A.; Miller, M.E.B.; Cook, M.D.; Whitlock, K.; Goldenfeld, N.; Woods, J.A.; White, B.A.; Chia, N.; et al. Diet and exercise orthogonally alter the gut microbiome and reveal independent associations with anxiety and cognition. Mol. Neurodegener. 2014, 9, 36. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, W.; Wang, J.; et al. Parabacteroides distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell Rep. 2019, 26, 222–235.e5. [Google Scholar] [CrossRef] [Green Version]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [Green Version]
- Briones, A.; Coats, E.; Brinkman, C. Should We Build “Obese” or “Lean” Anaerobic Digesters? PLoS ONE 2014, 9, e97252. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, H.M.; Park, S.Y.; Lee, D.K.; Kim, J.R.; Cha, M.K.; Lee, S.W.; Lim, H.T.; Kim, K.J.; Ha, N.J. Antiobesity and lipid-lowering effects of Bifidobacterium spp. in high fat diet-induced obese rats. Lipids Health Dis. 2011, 10, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armour, C.R.; Nayfach, S.; Pollard, K.S.; Sharpton, T.J. A Metagenomic Meta-analysis Reveals Functional Signatures of Health and Disease in the Human Gut Microbiome. MSystems 2019, 4, e00332–e18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sze, M.A.; Schloss, P.D. Looking for a Signal in the Noise: Revisiting Obesity and the Microbiome. MBio 2016, 7, e01018–e16. [Google Scholar] [CrossRef] [Green Version]
- Finucane, M.M.; Sharpton, T.J.; Laurent, T.J.; Pollard, K.S. A Taxonomic Signature of Obesity in the Microbiome? Getting to the Guts of the Matter. PLoS ONE 2014, 9, e84689. [Google Scholar] [CrossRef] [Green Version]
- Walker, A.W.; Ince, J.; Duncan, S.H.; Webster, L.M.; Holtrop, G.; Ze, X.; Brown, D.; Stares, M.D.; Scott, P.; Bergerat, A.; et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011, 5, 220–230. [Google Scholar] [CrossRef]
- Stanislawski, M.A.; Dabelea, D.; Lange, L.A.; Wagner, B.D.; Lozupone, C.A. Gut microbiota phenotypes of obesity. NPJ Biofilms Microbiomes 2019, 5, 18. [Google Scholar] [CrossRef]
- Willis, H.J.; Slavin, J.L. The Influence of Diet Interventions Using Whole, Plant Food on the Gut Microbiome: A Narrative Review. J. Acad. Nutr. Diet. 2020, 120, 608–623. [Google Scholar] [CrossRef] [Green Version]
- Mackie, R.I.; Sghir, A.; Gaskins, H.R. Developmental microbial ecology of the neonatal gastrointestinal tract. Am. J. Clin. Nutr. 1999, 69, 1035S–1045S. [Google Scholar] [CrossRef]
- Gauffin Cano, P.; Santacruz, A.; Moya, Á.; Sanz, Y. Bacteroides uniformis CECT 7771 Ameliorates Metabolic and Immunological Dysfunction in Mice with High-Fat-Diet Induced Obesity. PLoS ONE 2012, 7, e41079. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Shen, D.; Fang, Z.; Jie, Z.; Qiu, X.; Zhang, C.; Chen, Y.; Ji, L. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS ONE 2013, 8, e71108. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, N.; Duncan, S.H.; Young, P.; Belenguer, A.; McWilliam Leitch, C.; Scott, K.P.; Flint, H.J.; Louis, P. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014, 8, 1323–1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Candela, M.; Biagi, E.; Soverini, M.; Consolandi, C.; Quercia, S.; Severgnini, M.; Peano, C.; Turroni, S.; Rampelli, S.; Pozzilli, P.; et al. Modulation of gut microbiota dysbioses in type 2 diabetic patients by macrobiotic Ma-Pi 2 diet. Br. J. Nutr. 2016, 116, 80–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, W.R.; Gratz, S.W.; Duncan, S.H.; Holtrop, G.; Ince, J.; Scobbie, L.; Duncan, G.; Johnstone, A.M.; Lobley, G.E.; Wallace, R.J.; et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am. J. Clin. Nutr. 2011, 93, 1062–1072. [Google Scholar] [CrossRef]
- Pataky, Z.; Genton, L.; Spahr, L.; Lazarevic, V.; Terraz, S.; Gaïa, N.; Rubbia-Brandt, L.; Golay, A.; Schrenzel, J.; Pichard, C. Impact of Hypocaloric Hyperproteic Diet on Gut Microbiota in Overweight or Obese Patients with Nonalcoholic Fatty Liver Disease: A Pilot Study. Dig. Dis. Sci. 2016, 61, 2721–2731. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- Tamanai-Shacoori, Z.; Smida, I.; Bousarghin, L.; Loreal, O.; Meuric, V.; Fong, S.B.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Roseburia spp.: A marker of health? Future Microbiol. 2017, 12, 157–170. [Google Scholar] [CrossRef]
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.T.; et al. Human genetics shape the gut microbiome. Cell 2014, 159, 789–799. [Google Scholar] [CrossRef] [Green Version]
- Kong, L.-C.; Tap, J.; Aron-Wisnewsky, J.; Pelloux, V.; Basdevant, A.; Bouillot, J.-L.; Zucker, J.-D.; Doré, J.; Clément, K. Gut microbiota after gastric bypass in human obesity: Increased richness and associations of bacterial genera with adipose tissue genes. Am. J. Clin. Nutr. 2013, 98, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, B.M.; Kvach, E.; Eckel, R.H. Treatment of Obesity: Weight Loss and Bariatric Surgery. Circ. Res. 2016, 118, 1844–1855. [Google Scholar] [CrossRef]
- Zhang, H.; DiBaise, J.K.; Zuccolo, A.; Kudrna, D.; Braidotti, M.; Yu, Y.; Parameswaran, P.; Crowell, M.D.; Wing, R.; Rittmann, B.E.; et al. Human gut microbiota in obesity and after gastric bypass. Proc. Natl. Acad. Sci. USA 2009, 106, 2365–2370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Cuesta-Zuluaga, J.; Mueller, N.T.; Álvarez-Quintero, R.; Velásquez-Mejía, E.P.; Sierra, J.A.; Corrales-Agudelo, V.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Higher Fecal Short-Chain Fatty Acid Levels Are Associated with Gut Microbiome Dysbiosis, Obesity, Hypertension and Cardiometabolic Disease Risk Factors. Nutrients 2018, 11, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.N.; Yao, Y.; Ju, S.Y. Short Chain Fatty Acids and Fecal Microbiota Abundance in Humans with Obesity: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 2512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ang, Z.; Ding, J.L. GPR41 and GPR43 in Obesity and Inflammation—Protective or Causative? Front. Immunol. 2016, 7, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konikoff, T.; Gophna, U. Oscillospira: A Central, Enigmatic Component of the Human Gut Microbiota. Trends Microbiol. 2016, 24, 523–524. [Google Scholar] [CrossRef]
- Tripathi, C.K.M.; Banga, J.; Mishra, V. Microbial heparin/heparan sulphate lyases: Potential and applications. Appl. Microbiol. Biotechnol. 2012, 94, 307–321. [Google Scholar] [CrossRef]
- Cancello, R.; Turroni, S.; Rampelli, S.; Cattaldo, S.; Candela, M.; Cattani, L.; Mai, S.; Vietti, R.; Scacchi, M.; Brigidi, P.; et al. Effect of Short-Term Dietary Intervention and Probiotic Mix Supplementation on the Gut Microbiota of Elderly Obese Women. Nutrients 2019, 11, 3011. [Google Scholar] [CrossRef] [Green Version]
- Furet, J.-P.; Kong, L.-C.; Tap, J.; Poitou, C.; Basdevant, A.; Bouillot, J.-L.; Mariat, D.; Corthier, G.; Doré, J.; Henegar, C.; et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: Links with metabolic and low-grade inflammation markers. Diabetes 2010, 59, 3049–3057. [Google Scholar] [CrossRef] [Green Version]
- Tremaroli, V.; Karlsson, F.; Werling, M.; Ståhlman, M.; Kovatcheva-Datchary, P.; Olbers, T.; Fändriks, L.; le Roux, C.W.; Nielsen, J.; Bäckhed, F. Roux-en-Y Gastric Bypass and Vertical Banded Gastroplasty Induce Long-Term Changes on the Human Gut Microbiome Contributing to Fat Mass Regulation. Cell Metab. 2015, 22, 228–238. [Google Scholar] [CrossRef] [Green Version]
- Palleja, A.; Kashani, A.; Allin, K.H.; Nielsen, T.; Zhang, C.; Li, Y.; Brach, T.; Liang, S.; Feng, Q.; Jørgensen, N.B.; et al. Roux-en-Y gastric bypass surgery of morbidly obese patients induces swift and persistent changes of the individual gut microbiota. Genome Med. 2016, 8, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graessler, J.; Qin, Y.; Zhong, H.; Zhang, J.; Licinio, J.; Wong, M.-L.; Xu, A.; Chavakis, T.; Bornstein, A.B.; Ehrhart-Bornstein, M.; et al. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: Correlation with inflammatory and metabolic parameters. Pharm. J. 2013, 13, 514–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaakoush, N.O. Sutterella Species, IgA-degrading Bacteria in Ulcerative Colitis. Trends Microbiol. 2020, 28, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-P.; He, Q.-Q.; Ouyang, H.-M.; Peng, H.-S.; Wang, Q.; Li, J.; Lv, X.-F.; Zheng, Y.-N.; Li, S.-C.; Liu, H.-L.; et al. Human Gut Microbiota Associated with Obesity in Chinese Children and Adolescents. BioMed Res. Int. 2017, 2017, 7585989. [Google Scholar] [CrossRef]
- Louis, S.; Tappu, R.-M.; Damms-Machado, A.; Huson, D.H.; Bischoff, S.C. Characterization of the Gut Microbial Community of Obese Patients Following a Weight-Loss Intervention Using Whole Metagenome Shotgun Sequencing. PLoS ONE 2016, 11, e0149564. [Google Scholar] [CrossRef]
- Everard, A.; Geurts, L.; Caesar, R.; Van Hul, M.; Matamoros, S.; Duparc, T.; Denis, R.G.P.; Cochez, P.; Pierard, F.; Castel, J.; et al. Intestinal epithelial MyD88 is a sensor switching host metabolism towards obesity according to nutritional status. Nat. Commun. 2014, 5, 5648. [Google Scholar] [CrossRef] [Green Version]
- Chávez-Carbajal, A.; Nirmalkar, K.; Pérez-Lizaur, A.; Hernández-Quiroz, F.; Ramírez-del-Alto, S.; García-Mena, J.; Hernández-Guerrero, C. Gut Microbiota and Predicted Metabolic Pathways in a Sample of Mexican Women Affected by Obesity and Obesity Plus Metabolic Syndrome. Int. J. Mol. Sci. 2019, 20, 438. [Google Scholar] [CrossRef] [Green Version]
- Gao, R.; Zhu, C.; Li, H.; Yin, M.; Pan, C.; Huang, L.; Kong, C.; Wang, X.; Zhang, Y.; Qu, S.; et al. Dysbiosis Signatures of Gut Microbiota Along the Sequence from Healthy, Young Patients to Those with Overweight and Obesity. Obesity 2018, 26, 351–361. [Google Scholar] [CrossRef]
- Peters, B.; Shapiro, J.; Church, T.; Miller, G.; Trinh-Shevrin, C.; Yuen, E.; Friedlander, C.; Hayes, R.; Ahn, J. A taxonomic signature of obesity in a large study of American adults. Sci. Rep. 2018, 8, 9749. [Google Scholar] [CrossRef] [Green Version]


| Clinical Parameter | OB | NW |
|---|---|---|
| Sex, (Females/Males) | 20/3 | 40/6 |
| Age, M (SD) | 53 (9) | 49 (11) |
| Overweight, N (%) | 3 (13) | 0 |
| Class 1 obesity, N (%) | 8 (35) | 0 |
| Class 2 obesity, N (%) | 9 (39) | 0 |
| Class 3 obesity, N (%) | 3 (13) | 0 |
| Hypertension, N (%) | 7 (30) | 0 |
| Dyslipidemia, N (%) | 6 (26) | 0 |
| Insulin Resistance, N (%) | 3 (13) | 0 |
| Type II Diabetes, N (%) | 2 (9) | 0 |
| Current Smoking status (Yes), N (%) | 6 (26) | 7 (15) |
| Former Smoking status (Yes), N (%) | 5 (22) | 0 |
| Current Alcohol consumption (None), N (%) | 9 (39) | 10 (22) |
| Current Alcohol consumption (Rare), N (%) | 8 (35) | 24 (52) |
| Current Alcohol consumption (Moderate), N (%) | 6 (26) | 12 (26) |
| NW | OB | |||
|---|---|---|---|---|
| Clinical Parameter | T0 | T0 | T3 | p * |
| Weight (Kg), M (SD) | 54.9 (11.0) | 89.5 (19.3) | 82.8 (17.0) | 0.015 |
| Waist circumference (cm), M (SD) | 74 (6) | 108 (14) | 102 (16) | 0.040 |
| Body mass index, M (SD) | 21.6 (2.0) | 35.2 (4.3) | 33.6 (4.5) | 0.001 |
| Fat mass (Kg) | NA | 37.8 (10.2) | 32.7 (8.2) | 0.0002 |
| Muscle mass (Kg) | NA | 47.2 (14.0) | 47.6 (9.8) | 0.493 |
| Daily caloric intake (Kcal), M (SD) | 1468 (160) | 1779 (534) | 1341 (298) | 0.007 |
| Carbohydrates intake (%), M (SD) | 51 (3) | 50 (6) | 50 (8) | 0.578 |
| Lipids intake (%), M (SD) | 27 (4) | 33 (6) | 29 (9) | 0.196 |
| Saturated lipids intake/Total lipids intake (%), M (SD) | 28 (4) | 39 (5) | 35 (8) | 0.139 |
| Daily proteins intake (grams/day), M (SD) | 62 (9) | 73 (23) | 64 (13) | 0.384 |
| Daily fibers intake (grams/day), M(SD) | 20 (3) | 14 (6) | 17 (6) | 0.234 |
| MedDietScore ** | 33 (4) | 29 (5) | 32 (5) | 0.665 |
| Phylum | Family | Genus | Species | Median (IQR) at T0 | Median (IQR) at T3 | Prevalent Direction of Change (N) | p | q |
|---|---|---|---|---|---|---|---|---|
| Actinobacteria | Bifidobacteriaceae | Bifidobacterium | B. bifidum | 0 (0.002) | 0.006 (0.073) | ↑ (15) | 0.043 | 0.196 |
| Bacteroidetes | Bacteroidaceae | Bacteroides | B. cellulosilyticus | 0.019 (0.050) | 0.036 (0.238) | ↑ (20) | 0.006 | 0.039 |
| B. rodentium | 0.706 (0.643) | 1.431 (2.603) | ↑ (16) | 0.012 | 0.059 | |||
| B. stercorirosoris | 0.226 (0.199) | 0.289 (0.400) | ↑ (16) | 0.016 | 0.091 | |||
| B. uniformis | 0.803 (0.970) | 1.967 (2.808) | ↑ (17) | 0.005 | 0.036 | |||
| Tannerellaceae | Parabacteroides | 0.742 (0.689) | 1.485 (1.646) | ↑ (16) | 0.016 | 0.093 | ||
| P. distasonis | 0.211 (0.367) | 0.309 (0.613) | ↑ (17) | 0.019 | 0.097 | |||
| Prevotellaceae | Prevotella | P. stercorea | 0 (0) | 0 (0.001) | ≡ (14), ↑ (8) | 3.09 × 10−5 | 0.001 | |
| Sphingobacteriaceae | 0.302 (0.389) | 0.442 (0.454) | ↑ (13) | 1.62 × 10−4 | 0.003 | |||
| Sphingobacteriaceae | Sphingobacterium | 0.088 (0.119) | 0.115 (0.308) | ↑ (12) | 1.26 × 10−3 | 0.011 | ||
| S. shayense | 0.039 (0.042) | 0.055 (0.083) | ↑ (16) | 1.62 × 10−4 | 0.003 | |||
| Chloroflexi | Caldilineaceae | Caldilinea | 0.042 (0.097) | 0.063 (0.096) | ↑ (16) | 0.045 | 0.196 | |
| C. tarbellica | 0.042 (0.097) | 0.063 (0.096) | ↑ (14) | 0.045 | 0.196 | |||
| Firmicutes | Acidaminococcaceae | Acidaminococcus | A. fermentans | 0.003 (0.009) | 0.018 (0.025) | ↑ (14) | 0.036 | 0.099 |
| Erysipelotrichaceae | Catenibacterium | 0.002 (0.113) | 0.005 (0.366) | ↑ (19) | 0.007 | 0.049 | ||
| Lachnospiraceae | 15.329 (10.160) | 11.358 (13.241) | ↓ (14) | 0.042 | 0.194 | |||
| Lachnospiraceae | Coprococcus | C. eutactus | 0.004 (0.089) | 0.018 (0.399) | ↑ (20) | 0.001 | 0.005 | |
| Pseudobutyrivibrio | P. xylanivorans | 0.410 (0.665) | 0.216 (0.402) | ↓ (17) | 5.23 × 10−5 | 0.001 | ||
| Roseburia | 1.904 (2.995) | 1.379 (2.298) | ↓ (19) | 0.004 | 0.026 | |||
| Roseburia | R. faecis | 0.489 (0.594) | 0.264 (0.360) | ↓ (16) | 2.70 × 10−5 | 0.001 | ||
| Firmicutes | Selenomonadaceae | Megamonas | 0 (0.002) | 0 (0) | ≡ (14), ↓ (9) | 0.007 | 0.046 | |
| Megamonas | M. funiformis | 0 (0.002) | 0 (0) | ≡ (13), ↓ (10) | 0.005 | 0.038 | ||
| Ruminococcaceae | 15.395 (23.822) | 13.491 (14.593) | ↓ (12) | 2.62 × 10−4 | 0.003 | |||
| Ruminococcaceae | Oscillospira | O. eae | 0.440 (0.632) | 0.568 (0.825) | ↑ (14) | 0.048 | 0.196 | |
| Ruminococcus | 3.561 (4.819) | 2.284 (2.619) | ↓ (12) | 2.70 × 10−5 | 0.001 | |||
| R. albus | 0.001 (0.102) | 0.005 (0.045) | ↓ (13) | 0.003 | 0.012 | |||
| R. bromii | 0.056 (0.252) | 0.154 (0.491) | ↑ (12) | 0.039 | 0.186 | |||
| R. callidus | 0.011 (0.116) | 0.013 (0.074) | ↓ (12) | 0.001 | 0.008 | |||
| R. gnavus | 0.312 (0.607) | 0.215 (0.433) | ↓ (15) | 0.042 | 0.196 | |||
| unclassified Tissierellia | Sedimentibacter | S. hydroxybenzoicus | 0.075 (0.078) | 0.073 (0.082) | ↓ (13) | 3.33 × 10−4 | 0.004 | |
| Streptococcaceae | 0.218 (0.329) | 0.114 (0.136) | ↓ (17) | 0.009 | 0.051 | |||
| Streptococcus | 0.215 (0.332) | 0.114 (0.134) | ↓ (16) | 0.015 | 0.073 | |||
| Streptococcus | S. vestibularis | 0.038 (0.060) | 0.014 (0.029) | ↓ (20) | 3.09 × 10−5 | 0.001 | ||
| Veillonellaceae | 3.338 (6.828) | 3.492 (6.288) | ↓ (12) | 2.95 × 10−4 | 0.004 | |||
| Veillonellaceae | Veillonella | 0.059 (0.123) | 0.150 (0.185) | ↑ (17) | 3.73 × 10−4 | 0.004 | ||
| V. montpellierensis | 0.024 (0.047) | 0.038 (0.048) | ↑ (15) | 1.27 × 10−4 | 0.001 | |||
| Proteobacteria | 3.492 (3.670) | 3.502 (5.981) | ↓ (12) | 2.70 × 10−5 | 0.001 | |||
| Sutterellaceae | Sutterella | 0.108 (0.187) | 0.204 (0.420) | ↓ (12) | 0.001 | 0.012 | ||
| S. wadsworthensis | 0.002 (0.033) | 0.003 (0.029) | ↑ (10) | 0.018 | 0.093 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisanu, S.; Palmas, V.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Vascellari, S.; Boi, F.; Loviselli, A.; et al. Impact of a Moderately Hypocaloric Mediterranean Diet on the Gut Microbiota Composition of Italian Obese Patients. Nutrients 2020, 12, 2707. https://doi.org/10.3390/nu12092707
Pisanu S, Palmas V, Madau V, Casula E, Deledda A, Cusano R, Uva P, Vascellari S, Boi F, Loviselli A, et al. Impact of a Moderately Hypocaloric Mediterranean Diet on the Gut Microbiota Composition of Italian Obese Patients. Nutrients. 2020; 12(9):2707. https://doi.org/10.3390/nu12092707
Chicago/Turabian StylePisanu, Silvia, Vanessa Palmas, Veronica Madau, Emanuela Casula, Andrea Deledda, Roberto Cusano, Paolo Uva, Sarah Vascellari, Francesco Boi, Andrea Loviselli, and et al. 2020. "Impact of a Moderately Hypocaloric Mediterranean Diet on the Gut Microbiota Composition of Italian Obese Patients" Nutrients 12, no. 9: 2707. https://doi.org/10.3390/nu12092707
APA StylePisanu, S., Palmas, V., Madau, V., Casula, E., Deledda, A., Cusano, R., Uva, P., Vascellari, S., Boi, F., Loviselli, A., Manzin, A., & Velluzzi, F. (2020). Impact of a Moderately Hypocaloric Mediterranean Diet on the Gut Microbiota Composition of Italian Obese Patients. Nutrients, 12(9), 2707. https://doi.org/10.3390/nu12092707







