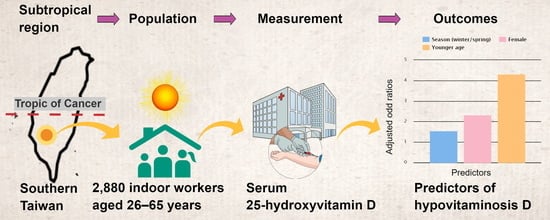
Age, Gender and Season Are Good Predictors of Vitamin D Status Independent of Body Mass Index in Office Workers in a Subtropical Region
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Study Parameters and Definitions
2.3. Subject Selection
2.4. Blood Collection and Determination of Serum 25(OH)D Levels
2.5. Blood Collection and Determination of Total Serum Calcium, Phosphate, Creatinine and Albumin
2.6. Statistical Analysis
3. Results
3.1. Demographic and Anthropometric Characteristics of the Study Population
3.2. Distribution of Serum Vitamin D among Indoor Office Workers
3.3. Associations of Risks of Hypovitaminosis D with Gender, Age, Season, and Body-Mass Index
3.4. Subgroup Analysis by Genders for Predictors of Hypovitaminosis D
3.5. Associations between Hypovitaminosis D and Corrected Total Serum Calcium Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Data Availability
Abbreviations
| AUC | Area under the receiver operating characteristic curve |
| 25(OH)D | 25-hydroxyvitamin D |
| BMI | Body mass index |
| ELISA | Enzyme linked immunosorbent assay |
| ICD-9 | International Classification of Diseases, Ninth Revision, Clinical Modification |
| ROC | Curve a receiver operating characteristic curve |
References
- Yang, C.Y.; Leung, P.S.; Adamopoulos, I.E.; Gershwin, M.E. The implication of vitamin D and autoimmunity: A comprehensive review. Clin. Rev. Allergy Immunol. 2013, 45, 217–226. [Google Scholar] [CrossRef]
- Feng, R.; Li, Y.; Li, G.; Li, Z.; Zhang, Y.; Li, Q.; Sun, C. Lower serum 25 (OH) D concentrations in type 1 diabetes: A meta-analysis. Diabetes Res. Clin. Pract. 2015, 108, e71–e75. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.Y.; Jeong, H.S.; Kim, D.H. Blood vitamin D status and metabolic syndrome in the general adult population: A dose-response meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Soderstrom, L.H.; Johnson, S.P.; Diaz, V.A.; Mainous, A.G., 3rd. Association between vitamin D and diabetic neuropathy in a nationally representative sample: Results from 2001–2004 NHANES. Diabet. Med. 2012, 29, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Lin, Y.T.; Wang, L.K.; Hung, K.C.; Lan, K.M.; Ho, C.H.; Chang, C.Y. Hypovitaminosis din postherpetic neuralgia-high prevalence and inverse association with pain: A retrospective study. Nutrients 2019, 11, 2787. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; van den Heuvel, E.G.; Schoemaker, R.J.; Preveraud, D.P.; Macdonald, H.M.; Arcot, J. 25-hydroxyvitamin D as a biomarker of vitamin D status and its modeling to inform strategies for prevention of vitamin D deficiency within the population. Adv. Nutr. 2017, 8, 947–957. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Vu, L.H.; Whiteman, D.C.; van der Pols, J.C.; Kimlin, M.G.; Neale, R.E. Serum vitamin D levels in office workers in a subtropical climate. Photochem. Photobiol. 2011, 87, 714–720. [Google Scholar] [CrossRef]
- Song, H.R.; Kweon, S.S.; Choi, J.S.; Rhee, J.A.; Lee, Y.H.; Nam, H.S.; Jeong, S.K.; Park, K.S.; Ryu, S.Y.; Choi, S.W.; et al. High prevalence of vitamin D deficiency in adults aged 50 years and older in Gwangju, Korea: The Dong-gu study. J. Korean Med. Sci. 2014, 29, 149–152. [Google Scholar] [CrossRef]
- Kung, A.W.; Lee, K.K. Knowledge of vitamin D and perceptions and attitudes toward sunlight among Chinese middle-aged and elderly women: A population survey in Hong Kong. BMC Public Health 2006, 6, 226. [Google Scholar] [CrossRef] [PubMed]
- Mithal, A.; Wahl, D.A.; Bonjour, J.P.; Burckhardt, P.; Dawson-Hughes, B.; Eisman, J.A.; El-Hajj Fuleihan, G.; Josse, R.G.; Lips, P.; Morales-Torres, J. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos. Int. 2009, 20, 1807–1820. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Li, H.L.; Hung, T.H.; Chang, H.Y.; Yang, F.L.; Wahlqvist, M.L. Vitamin D intake and its food sources in Taiwanese. Asia Pac. J. Clin. Nutr. 2008, 17, 397–407. [Google Scholar] [PubMed]
- Ginde, A.A.; Liu, M.C.; Camargo, C.A., Jr. Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch. Intern. Med. 2009, 169, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Man, R.E.; Li, L.J.; Cheng, C.Y.; Wong, T.Y.; Lamoureux, E.; Sabanayagam, C. Prevalence and determinants of suboptimal vitamin D levels in a multiethnic Asian population. Nutrients 2017, 9, 313. [Google Scholar] [CrossRef]
- Lagunova, Z.; Porojnicu, A.C.; Lindberg, F.; Hexeberg, S.; Moan, J. The dependency of vitamin D status on body mass index, gender, age and season. Anticancer Res. 2009, 29, 3713–3720. [Google Scholar] [CrossRef]
- OliaiAraghi, S.; van Dijk, S.C.; Ham, A.C.; Brouwer-Brolsma, E.M.; Enneman, A.W.; Sohl, E.; Swart, K.M.; van der Zwaluw, N.L.; van Wijngaarden, J.P.; Dhonukshe-Rutten, R.A.; et al. BMI and body fat mass is inversely associated with vitamin D levels in older individuals. J. Nutr. Health Aging 2015, 19, 980–985. [Google Scholar] [CrossRef]
- Wanner, M.; Richard, A.; Martin, B.; Linseisen, J.; Rohrmann, S. Associations between objective and self-reported physical activity and vitamin D serum levels in the US population. Cancer Causes Control 2015, 26, 881–891. [Google Scholar] [CrossRef]
- Coppeta, L.; Papa, F.; Magrini, A. Are shiftwork and indoor work related to D3 vitamin deficiency? A systematic review of current evidences. J. Environ. Public Health 2018, 2018, 8468742. [Google Scholar] [CrossRef]
- Choi, E.Y. 25(OH)D status and demographic and lifestyle determinants of 25(OH)D among Korean adults. Asia Pac. J. Clin. Nutr. 2012, 21, 526–535. [Google Scholar]
- Huang, C.H.; Huang, Y.A.; Lai, Y.C.; Sun, C.K. Prevalence and predictors of hypovitaminosis D among the elderly in subtropical region. PLoS ONE 2017, 12, e0181063. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schleicher, R.L.; Sternberg, M.R.; Looker, A.C.; Yetley, E.A.; Lacher, D.A.; Sempos, C.T.; Taylor, C.L.; Durazo-Arvizu, R.A.; Maw, K.L.; Chaudhary-Webb, M.; et al. National estimates of serum total 25-hydroxyvitamin D and metabolite concentrations measured by liquid chromatography-tandem mass spectrometry in the US population during 2007–2010. J. Nutr. 2016, 146, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.J.; Chang, H.Y.; Pan, W.H. Time trend of obesity, the metabolic syndrome and related dietary pattern in Taiwan: From NAHSIT 1993–1996 to NAHSIT 2005–2008. Asia Pac. J. Clin. Nutr. 2011, 20, 292–300. [Google Scholar] [PubMed]
- Mansueto, P.; Seidita, A.; Vitale, G.; Gangemi, S.; Iaria, C.; Cascio, A. Vitamin D deficienczy in HIV infection: Not only a bone disorder. Biomed. Res. Int. 2015, 2015, 735615. [Google Scholar] [CrossRef]
- Stein, E.M.; Shane, E. Vitamin D in organ transplantation. Osteoporos. Int. 2011, 22, 2107–2118. [Google Scholar] [CrossRef]
- Lee, M.J.; Hsu, H.J.; Wu, I.W.; Sun, C.Y.; Ting, M.K.; Lee, C.C. Vitamin D deficiency in northern Taiwan: A community-based cohort study. BMC Public Health 2019, 19, 337. [Google Scholar] [CrossRef]
- Levis, S.; Gomez, A.; Jimenez, C.; Veras, L.; Ma, F.; Lai, S.; Hollis, B.; Roos, B.A. Vitamin D deficiency and seasonal variation in an adult South Florida population. J. Clin. Endocrinol. Metab. 2005, 90, 1557–1562. [Google Scholar] [CrossRef]
- Floege, J.; Tsirtsonis, K.; Iles, J.; Drueke, T.B.; Chertow, G.M.; Parfrey, P. Incidence, predictors and therapeutic consequences of hypocalcemia in patients treated with cinacalcet in the EVOLVE trial. Kidney Int. 2018, 93, 1475–1482. [Google Scholar] [CrossRef]
- Bushinsky, D.A.; Monk, R.D. Electrolyte quintet: Calcium. Lancet 1998, 352, 306–311. [Google Scholar] [CrossRef]
- Gutierrez, O.; Isakova, T.; Rhee, E.; Shah, A.; Holmes, J.; Collerone, G.; Juppner, H.; Wolf, M. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J. Am. Soc. Nephrol. 2005, 16, 2205–2215. [Google Scholar] [CrossRef]
- Creswell, J.W. Research Design: Qualitative, Quantitative and Mixed Methods Approaches; Sage: London, UK, 2004; Volume 12, pp. 82–83. [Google Scholar] [CrossRef]
- Koivula, M.K.; Matinlassi, N.; Laitinen, P.; Risteli, J. Four automated 25-OH total vitamin D immunoassays and commercial liquid chromatography tandem-mass spectrometry in Finnish population. Clin. Lab. 2013, 59, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Janssen, J.W.; Helbing, A.R. ArsenazoIII: An improvement of the routine calcium determination in serum. Eur. J. Clin. Chem. Clin. Biochem. 1991, 29, 197–201. [Google Scholar] [PubMed]
- Gamst, O.; Try, K. Determination of serum-phosphate without deproteinization by ultraviolet spectrophotometry of the phosphomolybdic acid complex. Scand. J. Clin. Lab. Investig. 1980, 40, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Garcia Moreira, V.; BeridzeVaktangova, N.; Martinez Gago, M.D.; Laborda Gonzalez, B.; Garcia Alonso, S.; Fernandez Rodriguez, E. Overestimation of albumin measured by bromocresol green vs bromocresol purple method: Influence of acute-phase globulins. Lab. Med. 2018, 49, 355–361. [Google Scholar] [CrossRef]
- Choi, H.S.; Oh, H.J.; Choi, H.; Choi, W.H.; Kim, J.G.; Kim, K.M.; Kim, K.J.; Rhee, Y.; Lim, S.K. Vitamin D insufficiency in Korea—A greater threat to younger generation: The Korea National Health And Nutrition Examination Survey (KNHANES) 2008. J. Clin. Endocrinol. Metab. 2011, 96, 643–651. [Google Scholar] [CrossRef]
- Chailurkit, L.O.; Aekplakorn, W.; Ongphiphadhanakul, B. Regional variation and determinants of vitamin D status in sunshine-abundant Thailand. BMC Public Health 2011, 11, 853. [Google Scholar] [CrossRef]
- Nimitphong, H.; Holick, M.F. Vitamin D status and sun exposure in Southeast Asia. Dermatoendocrinology 2013, 5, 34–37. [Google Scholar] [CrossRef]
- MacLaughlin, J.; Holick, M.F. Aging decreases the capacity of human skin to produce vitamin D3. J. Clin. Investig. 1985, 76, 1536–1538. [Google Scholar] [CrossRef]
- Malacova, E.; Cheang, P.R.; Dunlop, E.; Sherriff, J.L.; Lucas, R.M.; Daly, R.M.; Nowson, C.A.; Black, L.J. Prevalence and predictors of vitamin D deficiency in a nationally representative sample of adults participating in the 2011–2013 Australian Health Survey. Br. J. Nutr. 2019, 121, 894–904. [Google Scholar] [CrossRef]
- Hayashi, F.; Takimoto, H.; Yoshita, K.; Yoshiike, N. Perceived body size and desire for thinness of young Japanese women: A population-based survey. Br. J. Nutr. 2006, 96, 1154–1162. [Google Scholar] [CrossRef]
- Okabe, H.; Shimizu, C.; Yamamoto, M.; Kikuchi, R.; Minami, A.; Chen, Y.F.; Imai, H.; Mizuta, M.; Chen, Z.; Chiba, H.; et al. Determination of serum 25-hydroxyvitamin D3 by LC/MS/MS and its monthly variation in sapporo indoor workers. Anal. Sci. 2018, 34, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H.; Sugimoto, K.; Inatomi, S.; Maeda, T.; Osanai, M.; Uchiyama, Y.; Yamamoto, Y.; Wada, T.; Kojima, T.; Yokozaki, H.; et al. Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol. Biol. Cell 2008, 19, 1912–1921. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Vitamin D and calcium interactions: Functional outcomes. Am. J. Clin. Nutr. 2008, 88, 541S–544S. [Google Scholar] [CrossRef]
- Lamberg-Allardt, C. The relationship between serum 25-hydroxy-vitamin D levels and other variables related to calcium and phosphorus metabolism in the elderly. ActaEndocrinol. (Copenhagen) 1984, 105, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Timalsina, S.; Sigdel, M.R.; Baniya, S.; Subedee, S. Status of vitamin D and parameters of calcium homeostasis in renal transplant recipients in Nepal: A cross sectional study. BMC Nephrol. 2018, 19, 290. [Google Scholar] [CrossRef]
- Haarburger, D.; Hoffman, M.; Erasmus, R.T.; Pillay, T.S. Relationship between vitamin D, calcium and parathyroid hormone in Cape Town. J. Clin. Pathol. 2009, 62, 567–569. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Bhandari, A.; Yang, F.; Zhang, W.; Xue, L.J.; Liu, H.G.; Zhang, X.H.; Chen, C.Z. Risk factors for hypocalcemia and hypoparathyroidism following thyroidectomy: A retrospective Chinese population study. Cancer Manag. Res. 2017, 9, 627–635. [Google Scholar] [CrossRef]
- Rude, R.K. Renal cortical adenylate cyclase: Characterization of magnesium activation. Endocrinology 1983, 113, 1348–1355. [Google Scholar] [CrossRef]
- Hutchinson, K.; Healy, M.; Crowley, V.; Louw, M.; Rochev, Y. Verification of Abbott 25-OH-vitamin D assay on the architect system. Pract. Lab. Med. 2017, 7, 27–35. [Google Scholar] [CrossRef]


| Total (n = 2880) | Female (n = 1440) | Male (n = 1440) | p | |
|---|---|---|---|---|
| Age, mean (SD), years | 46 (11) | 46 (11) | 46 (11) | 1.0 |
| BMI, mean (SD) | 24 (4) | 22 (4) | 25 (4) | 0.75 |
| Vitamin D status, n (%) | ||||
| Optimal (≥30 ng/mL) | 1098 (38.1) | 405 (28.1) | 693 (48.1) | <0.001 |
| Hypovitaminosis D (<30 ng/mL) | 1782 (61.9) | 1035 (71.9) | 747 (51.9) | |
| 25(OH)D, mean (SD), ng/mL | 29 (9) | 26 (8) | 31 (10) | <0.001 |
| 25(OH)D in age groups, mean (SD), ng/mL, n | ||||
| 26–35 years | 26 (7), 720 | 23 (7), 360 | 28 (7), 360 | <0.001 |
| 36–45 years | 27 (8), 720 | 25 (7), 360 | 29 (9), 360 | <0.001 |
| 46–55 years | 30 (9), 720 | 28 (8), 360 | 31 (9), 360 | <0.001 |
| 56–65 years | 33 (10), 720 | 30 (8), 360 | 35 (12), 360 | <0.001 |
| 25(OH)D in seasons, mean (SD), ng/mL, n | ||||
| Shorter daylight (Winter/Spring) | 28 (9), 1440 | 26 (7), 720 | 30 (10), 720 | <0.001 |
| Longer daylight (Summer/Autumn) | 30 (9), 1440 | 27 (7), 720 | 32 (10), 720 | <0.001 |
| 25(OH)D in BMI groups, mean (SD), ng/mL, n | ||||
| Obese (≥30.0) | 28 (9), 203 | 27 (8), 57 | 28 (9), 146 | 0.63 |
| Overweight (25 ≤ BMI < 30) | 29 (9), 741 | 28 (8), 239 | 30 (9), 502 | 0.01 |
| Normal (20 ≤ BMI < 25) | 29 (10), 1431 | 26 (8), 741 | 31 (10), 690 | <0.001 |
| Under weight (<20.0) | 27 (8), 505 | 26 (8), 403 | 32 (10), 102 | <0.001 |
| Corrected total serum calcium concentration, mean (SD), mg/ml | 8 (1) | 8 (0) | 8 (1) | 0.33 |
| Serumphosphate concentration, mg/ml | 7 (2) | 7 (2) | 7 (2) | 0.19 |
| eGFR | 83 (15) | 85 (14) | 82 (15) | 0.65 |
| Vitamin D Status | Hypovitaminosis D (<30 ng/mL), n = 1782 (61.9%), n (%) | Optimal (≥30 ng/mL), n = 1098 (38.1%), n (%) | Crude OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|---|---|
| Gender | ||||
| Female (n = 1440) | 1035 (71.9) | 405 (28.1) | 2.37 (2.03, 2.77) | 2.33 (1.75, 3.09) * |
| Male (n = 1440) | 747 (52.7) | 693 (47.3) | 1.0 | 1.0 |
| Age group, years | ||||
| 26–35 (n = 720) | 543 (75.4) | 177 (24.6) | 3.65 (2.91, 4.56) | 4.32 (2.98, 6.24) * |
| 36–45 (n = 720) | 506 (70.3) | 214 (29.7) | 2.81 (2.26, 3.49) | 2.82 (1.93, 4.12) * |
| 46–55 (n = 720) | 404 (56.1) | 316 (43.9) | 1.52 (1.23, 1.87) | 1.50 (1.03, 2.17) * |
| 56–65 (n = 720) | 329 (45.7) | 391 (54.3) | 1.0 | 1.0 |
| Season | ||||
| (Winter/Spring) (n = 1440) | 952 (66.1) | 488 (33.9) | 1.43 (1.23, 1.67) | 1.55 (1.08, 2.22) * |
| (Summer/Autumn) (n = 1440) | 830 (57.6) | 610 (42.4) | 1.0 | 1.0 |
| BMI (kg/m2) | ||||
| Obese (≥30.0) | 133 (65.5) | 70 (34.5) | 1.17 (0.86, 1.59) | |
| Overweight (25 ≤ BMI < 30) | 427 (57.6) | 314 (42.4) | 0.84 (0.70, 1.00) | |
| Normal (20 ≤ BMI < 25) | 886 (61.9) | 545 (38.1) | 1.0 | |
| Under weight (<20.0) | 336 (66.5) | 169 (33.5) | 1.22 (0.99, 1.51) |
| Females | (n = 1440) | |||
|---|---|---|---|---|
| Vitamin D Status | Hypovitaminosis D, n = 1035, n (%) | Optimal, n = 405, n (%) | Crude OR (95% CI) | Adjusted OR (95% CI) |
| Age group, years | ||||
| 26–35 (n = 360) | 308 (86.1) | 52 (13.9) | 4.74 (3.31, 6.79) | 4.05 * (2.35, 6.98) |
| 36–45 (n = 360) | 292 (81.1) | 68 (18.9) | 3.44 (2.46, 4.81) | 3.22 * (1.81, 5.71) |
| 46–55 (n = 360) | 235 (65.3) | 125 (34.7) | 1.50 (1.11, 2.03) | 1.67 (1.00, 2.80) |
| 56–65 (n = 360) | 200 (55.6) | 160 (44.4) | 1.0 | 1.0 |
| Season | ||||
| (Winter/Spring) (n = 720) | 538 (74.7) | 182 (25.3) | 1.33 (1.05, 1.67) | 1.12 (0.78, 1.62) |
| (Summer/Autumn) (n = 720) | 497 (69.0) | 223 (31.0) | 1.0 | 1.0 |
| BMI (kg/m2), n (%) | ||||
| Obese (≥30.0) | 39 (68.4) | 18 (31.6) | 0.84 (0.47, 1.50) | |
| Overweight (25 ≤ BMI < 30) | 169 (70.7) | 70 (29.3) | 0.94 (0.68, 1.29) | |
| Normal (20 ≤ BMI < 25) | 534 (72.1) | 207 (27.9) | 1.0 | |
| Under weight (<20.0) | 293 (72.7) | 110 (27.3) | 1.0 (0.79,1.35) |
| Males | (n = 1440) | |||
|---|---|---|---|---|
| Vitamin D Status | Hypovitaminosis D, n = 747, n (%) | Optimal, n = 693, n (%) | Crude OR (95% CI) | Adjusted OR (95% CI) |
| Age group, years | ||||
| 26–35 (n = 360) | 235 (65.8) | 125 (34.2) | 3.37 (2.48, 4.57) | 4.73 * (2.76, 8.09) |
| 36–45 (n = 360) | 214 (60.6) | 146 (39.4) | 2.62 (1.94, 3.55) | 2.30 * (1.34, 3.92) |
| 46–55 (n = 360) | 169 (47.5) | 191 (52.5) | 1.58 (1.18, 2.14) | 1.22 (0.70, 2.13) |
| 56–65 (n = 360) | 129 (36.9) | 231 (63.1) | 1.0 | 1.0 |
| Season | ||||
| (Winter/Spring) (n = 720) | 414 (57.5) | 306 (42.5) | 1.57 (1.28, 1.94) | 1.99 * (1.40, 2.83) |
| (Summer/Autumn) (n = 720) | 333 (46.2) | 387 (53.8) | 1.0 | 1.0 |
| BMI (kg/m2), n (%) | ||||
| Obese (≥30.0) | 94 (64.4) | 52 (35.6) | 1.74 (1.20, 2.51) | 1.27 (0.89, 1.94) |
| Overweight (2530) | 258 (51.4) | 244 (48.6) | 1.02 (0.81, 1.28) | 1.06 (0.85, 1.23) |
| Normal (20 ≤ BMI < 25) | 352 (51.0) | 338 (49.0) | 1.0 | 1.0 |
| Under weight (<20.0) | 43 (42.2) | 59 (57.8) | 0.70 (0.46, 1.07) | 0.78 (0.57, 1.10) |
| Corrected Total Serum Calcium Status * | Hypocalcemia (<8.4 mg/mL) (n = 1413) | Normal Calcium Levels (8.4~10.4 mg/mL) (n = 1467) | p | Calcium Levels Mean (SD), mg/mL |
|---|---|---|---|---|
| Vitamin D status (serum 25(OH)D concentrations), n (%) | <0.001 | |||
| Deficiency (<20 ng/mL) (n = 408) | 214 (15.1) | 194 (13.2) | 8.39 (0.41) | |
| Insufficiency (20–29.9 ng/mL) (n = 1374) | 716 (50.7) | 658 (44.9) | 8.39 (0.40) | |
| Optimal (≥30 ng/mL) (n = 1098) | 483 (34.2) | 615 (41.9) | 8.47 (0.39) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.-K.; Hung, K.-C.; Lin, Y.-T.; Chang, Y.-J.; Wu, Z.-F.; Ho, C.-H.; Chen, J.-Y. Age, Gender and Season Are Good Predictors of Vitamin D Status Independent of Body Mass Index in Office Workers in a Subtropical Region. Nutrients 2020, 12, 2719. https://doi.org/10.3390/nu12092719
Wang L-K, Hung K-C, Lin Y-T, Chang Y-J, Wu Z-F, Ho C-H, Chen J-Y. Age, Gender and Season Are Good Predictors of Vitamin D Status Independent of Body Mass Index in Office Workers in a Subtropical Region. Nutrients. 2020; 12(9):2719. https://doi.org/10.3390/nu12092719
Chicago/Turabian StyleWang, Li-Kai, Kuo-Chuan Hung, Yao-Tsung Lin, Ying-Jen Chang, Zhi-Fu Wu, Chung-Han Ho, and Jen-Yin Chen. 2020. "Age, Gender and Season Are Good Predictors of Vitamin D Status Independent of Body Mass Index in Office Workers in a Subtropical Region" Nutrients 12, no. 9: 2719. https://doi.org/10.3390/nu12092719
APA StyleWang, L.-K., Hung, K.-C., Lin, Y.-T., Chang, Y.-J., Wu, Z.-F., Ho, C.-H., & Chen, J.-Y. (2020). Age, Gender and Season Are Good Predictors of Vitamin D Status Independent of Body Mass Index in Office Workers in a Subtropical Region. Nutrients, 12(9), 2719. https://doi.org/10.3390/nu12092719