Impact of Storage Conditions on the Breast Milk Peptidome
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
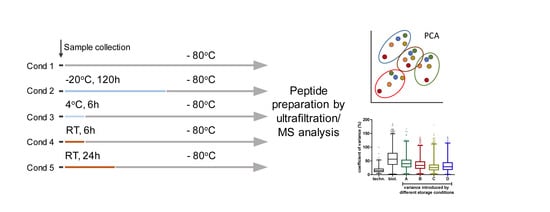
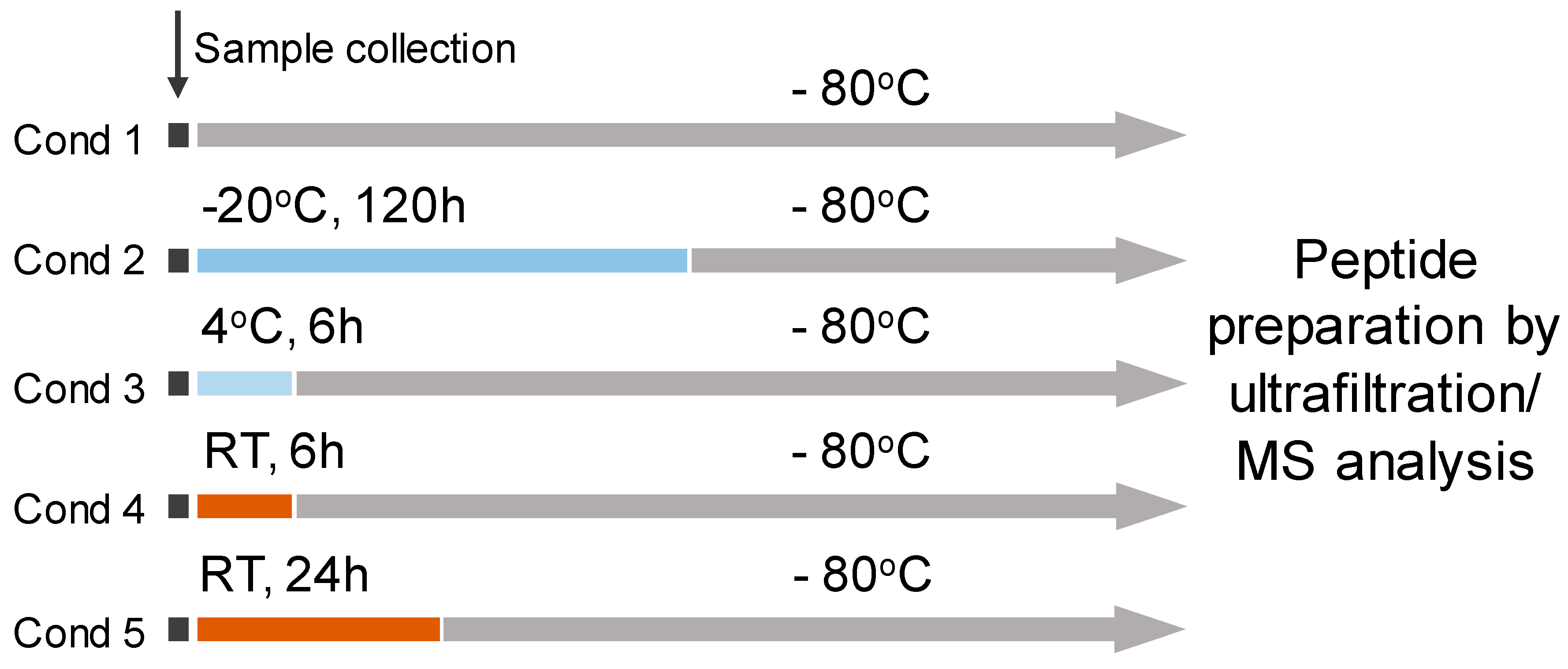
2.2. Storage Experiment
2.3. Sample Preparation
2.4. Tandem Mass Spectrometry
2.5. Data Analysis
3. Results
3.1. Impact of Storage Conditions on the Composition of the Milk Peptidome
3.2. Impact of Storage Conditions on Human Breast Milk Peptidome Variability
3.3. Identification of Cleavage Sites Dependent on Storage Condition
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Edmond, K.M.; Zandoh, C.; Quigley, M.A.; Amenga-Etego, S.; Owusu-Agyei, S.; Kirkwood, B.R. Delayed breastfeeding initiation increases risk of neonatal mortality. Pediatrics 2006, 117, e380–e386. [Google Scholar] [CrossRef]
- Sharp, J.A.; Modepalli, V.; Enjapoori, A.K.; Bisana, S.; Abud, H.E.; Lefevre, C.; Nicholas, K.R. Bioactive functions of milk proteins: A comparative genomics approach. J. Mammary Gland Biol. Neoplasia 2014, 19, 289–302. [Google Scholar] [CrossRef]
- Turfkruyer, M.; Verhasselt, V. Breast milk and its impact on maturation of the neonatal immune system. Curr. Opin. Infect. Dis. 2015, 28, 199–206. [Google Scholar] [CrossRef]
- Fengler, J.; Heckmann, M.; Lange, A.; Kramer, A.; Flessa, S. Cost analysis showed that feeding preterm infants with donor human milk was significantly more expensive than mother’s milk or formula. Acta Paediatr. 2020, 109, 959–966. [Google Scholar] [CrossRef]
- Miller, J.; Tonkin, E.; Damarell, R.A.; McPhee, A.J.; Suganuma, M.; Suganuma, H.; Middleton, P.F.; Makrides, M.; Collins, C.T. A systematic review and meta-analysis of human milk feeding and morbidity in very low birth weight infants. Nutrients 2018, 10, 707. [Google Scholar] [CrossRef]
- Spiegler, J.; Preuss, M.; Gebauer, C.; Bendiks, M.; Herting, E.; Gopel, W.; German Neonatal, N.; German Neonatal Network GNN. Does breastmilk influence the development of bronchopulmonary dysplasia? J. Pediatr. 2016, 169, 76–80.e74. [Google Scholar] [CrossRef]
- Quigley, M.; Embleton, N.D.; McGuire, W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst. Rev. 2019, 7, CD002971. [Google Scholar] [CrossRef]
- Victora, C.G.; Bahl, R.; Barros, A.J.; Franca, G.V.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- WHO. Guidelines on Optimal Feeding of Low Birth-Weight Infants in Low- and Middle-Income Countries; UNICEF and WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Nutrition, E.C.; Arslanoglu, S.; Corpeleijn, W.; Moro, G.; Braegger, C.; Campoy, C.; Colomb, V.; Decsi, T.; Domellof, M.; Fewtrell, M.; et al. Donor human milk for preterm infants: Current evidence and research directions. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 535–542. [Google Scholar]
- Campanhon, I.B.; da Silva, M.R.S.; de Magalhaes, M.T.Q.; Zingali, R.B.; Bezerra, F.F.; Torres, A.G. Protective factors in mature human milk: A look into the proteome and peptidome of adolescent mothers’ breast milk. Br. J. Nutr. 2019, 122, 1377–1385. [Google Scholar] [CrossRef]
- Wang, X.; Yan, X.; Zhang, L.; Cai, J.; Zhou, Y.; Liu, H.; Hu, Y.; Chen, W.; Xu, S.; Liu, P.; et al. Identification and peptidomic profiling of exosomes in preterm human milk: Insights into necrotizing enterocolitis prevention. Mol. Nutr. Food Res. 2019, 63, e1801247. [Google Scholar] [CrossRef]
- Nielsen, S.D.; Beverly, R.L.; Dallas, D.C. Milk proteins are predigested within the human mammary gland. J. Mammary Gland Biol. Neoplasia 2017, 22, 251–261. [Google Scholar] [CrossRef]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Dallas, D.C.; Guerrero, A.; Khaldi, N.; Castillo, P.A.; Martin, W.F.; Smilowitz, J.T.; Bevins, C.L.; Barile, D.; German, J.B.; Lebrilla, C.B. Extensive in vivo human milk peptidomics reveals specific proteolysis yielding protective antimicrobial peptides. J. Proteome Res. 2013, 12, 2295–2304. [Google Scholar] [CrossRef]
- Wada, Y.; Lonnerdal, B. Bioactive peptides derived from human milk proteins--mechanisms of action. J. Nutr. Biochem. 2014, 25, 503–514. [Google Scholar] [CrossRef]
- Zhu, J.; Dingess, K.A. The functional power of the human milk proteome. Nutrients 2019, 11, 1834. [Google Scholar] [CrossRef]
- Tonolo, F.; Moretto, L.; Ferro, S.; Folda, A.; Scalcon, V.; Sandre, M.; Fiorese, F.; Marin, O.; Bindoli, A.; Rigobello, M.P. Insight into antioxidant properties of milk-derived bioactive peptides in vitro and in a cellular model. J. Pept. Sci. 2019, 25, e3162. [Google Scholar] [CrossRef]
- Raikos, V.; Dassios, T. Health-promoting properties of bioactive peptides derived from milk proteins in infant food: A review. Dairy Sci. Technol. 2014, 94, 91–101. [Google Scholar] [CrossRef]
- Peters, M.D.; McArthur, A.; Munn, Z. Safe management of expressed breast milk: A systematic review. Women Birth 2016, 29, 473–481. [Google Scholar] [CrossRef]
- Ten-Domenech, I.; Ramos-Garcia, V.; Pineiro-Ramos, J.D.; Gormaz, M.; Parra-Llorca, A.; Vento, M.; Kuligowski, J.; Quintas, G. Current practice in untargeted human milk metabolomics. Metabolites 2020, 10, 43. [Google Scholar] [CrossRef]
- Eglash, A.; Simon, L.; Academy of Breastfeeding. ABM clinical protocol #8: Human milk storage information for home use for full-term infants, revised 2017. Breastfeed. Med. 2017, 12, 390–395. [Google Scholar]
- Wada, Y.; Lonnerdal, B. Bioactive peptides released from in vitro digestion of human milk with or without pasteurization. Pediatr. Res. 2015, 77, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Nebbia, S.; Giribaldi, M.; Cavallarin, L.; Bertino, E.; Coscia, A.; Briard-Bion, V.; Ossemond, J.; Henry, G.; Menard, O.; Dupont, D.; et al. Differential impact of holder and high temperature short time pasteurization on the dynamic in vitro digestion of human milk in a preterm newborn model. Food Chem. 2020, 328, 127126. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, L.; Yu, Z.; Zhang, A.; Wu, W.; Chen, W.; Yan, X.; Liu, H.; Hu, Y.; Jiang, C.; et al. Peptidomic analysis reveals multiple protection of human breast milk on infants during different stages. J. Cell Physiol. 2019, 234, 15510–15526. [Google Scholar] [CrossRef]
- Nielsen, S.D.; Beverly, R.L.; Underwood, M.A.; Dallas, D.C. Release of functional peptides from mother’s milk and fortifier proteins in the premature infant stomach. PLoS ONE 2018, 13, e0208204. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J.; Thomas, P.D.; Huang, X.; Bateman, A.; Finn, R.D. The merops database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the panther database. Nucleic Acids Res. 2018, 46, D624–D632. [Google Scholar] [CrossRef]
- Crooks, G.E.; Hon, G.; Chandonia, J.M.; Brenner, S.E. Weblogo: A sequence logo generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef]
- Weaver, G.; Bertino, E.; Gebauer, C.; Grovslien, A.; Mileusnic-Milenovic, R.; Arslanoglu, S.; Barnett, D.; Boquien, C.Y.; Buffin, R.; Gaya, A.; et al. Recommendations for the establishment and operation of human milk banks in Europe: A consensus statement from the European milk bank association (emba). Front. Pediatr. 2019, 7, 53. [Google Scholar] [CrossRef]
- Dallas, D.C.; Murray, N.M.; Gan, J. Proteolytic systems in milk: Perspectives on the evolutionary function within the mammary gland and the infant. J. Mammary Gland Biol. Neoplasia 2015, 20, 133–147. [Google Scholar] [CrossRef]
- Demers-Mathieu, V.; Nielsen, S.D.; Underwood, M.A.; Borghese, R.; Dallas, D.C. Analysis of milk from mothers who delivered prematurely reveals few changes in proteases and protease inhibitors across gestational age at birth and infant postnatal age. J. Nutr. 2017, 147, 1152–1159. [Google Scholar] [CrossRef]
- Daniels, J.R.; Cao, Z.; Maisha, M.; Schnackenberg, L.K.; Sun, J.; Pence, L.; Schmitt, T.C.; Kamlage, B.; Rogstad, S.; Beger, R.D.; et al. Stability of the human plasma proteome to pre-analytical variability as assessed by an aptamer-based approach. J. Proteome Res. 2019, 18, 3661–3670. [Google Scholar] [CrossRef] [PubMed]
- Witkowska-Zimny, M.; Kaminska-El-Hassan, E. Cells of human breast milk. Cell Mol. Biol. Lett. 2017, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Bottger, R.; Hoffmann, R.; Knappe, D. Differential stability of therapeutic peptides with different proteolytic cleavage sites in blood, plasma and serum. PLoS ONE 2017, 12, e0178943. [Google Scholar] [CrossRef] [PubMed]
- Dingess, K.A.; de Waard, M.; Boeren, S.; Vervoort, J.; Lambers, T.T.; van Goudoever, J.B.; Hettinga, K. Human milk peptides differentiate between the preterm and term infant and across varying lactational stages. Food Funct. 2017, 8, 3769–3782. [Google Scholar] [CrossRef]
- Dallas, D.C.; Smink, C.J.; Robinson, R.C.; Tian, T.; Guerrero, A.; Parker, E.A.; Smilowitz, J.T.; Hettinga, K.A.; Underwood, M.A.; Lebrilla, C.B.; et al. Endogenous human milk peptide release is greater after preterm birth than term birth. J. Nutr. 2015, 145, 425–433. [Google Scholar] [CrossRef]
- Cui, X.; Li, Y.; Yang, L.; You, L.; Wang, X.; Shi, C.; Ji, C.; Guo, X. Peptidome analysis of human milk from women delivering macrosomic fetuses reveals multiple means of protection for infants. Oncotarget 2016, 7, 63514–63525. [Google Scholar] [CrossRef]
- Ombra, M.N.; Musumeci, M.; Simpore, J.; Palano, G.M.; Musumeci, S. Beta-endorphin concentration in colostrums of burkinabe and sicilian women. Nutrition 2008, 24, 31–36. [Google Scholar] [CrossRef]
- Arslanoglu, S.; Boquien, C.Y.; King, C.; Lamireau, D.; Tonetto, P.; Barnett, D.; Bertino, E.; Gaya, A.; Gebauer, C.; Grovslien, A.; et al. Fortification of human milk for preterm infants: Update and recommendations of the European milk bank association (emba) working group on human milk fortification. Front. Pediatr. 2019, 7, 76. [Google Scholar] [CrossRef]
- Buhrer, C.; Fischer, H.S.; Wellmann, S. Nutritional interventions to reduce rates of infection, necrotizing enterocolitis and mortality in very preterm infants. Pediatr. Res. 2020, 87, 371–377. [Google Scholar] [CrossRef]
- Wada, Y.; Lonnerdal, B. Bioactive peptides derived from human milk proteins: An update. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 217–222. [Google Scholar] [CrossRef]
- Sun, H.; Cao, Y.; Han, S.; Cheng, R.; Liu, L.; Liu, J.; Xia, S.; Zhang, J.; Li, Z.; Cheng, X.; et al. A randomized controlled trial protocol comparing the feeds of fresh versus frozen mother’s own milk for preterm infants in the nicu. Trials 2020, 21, 170. [Google Scholar] [CrossRef]
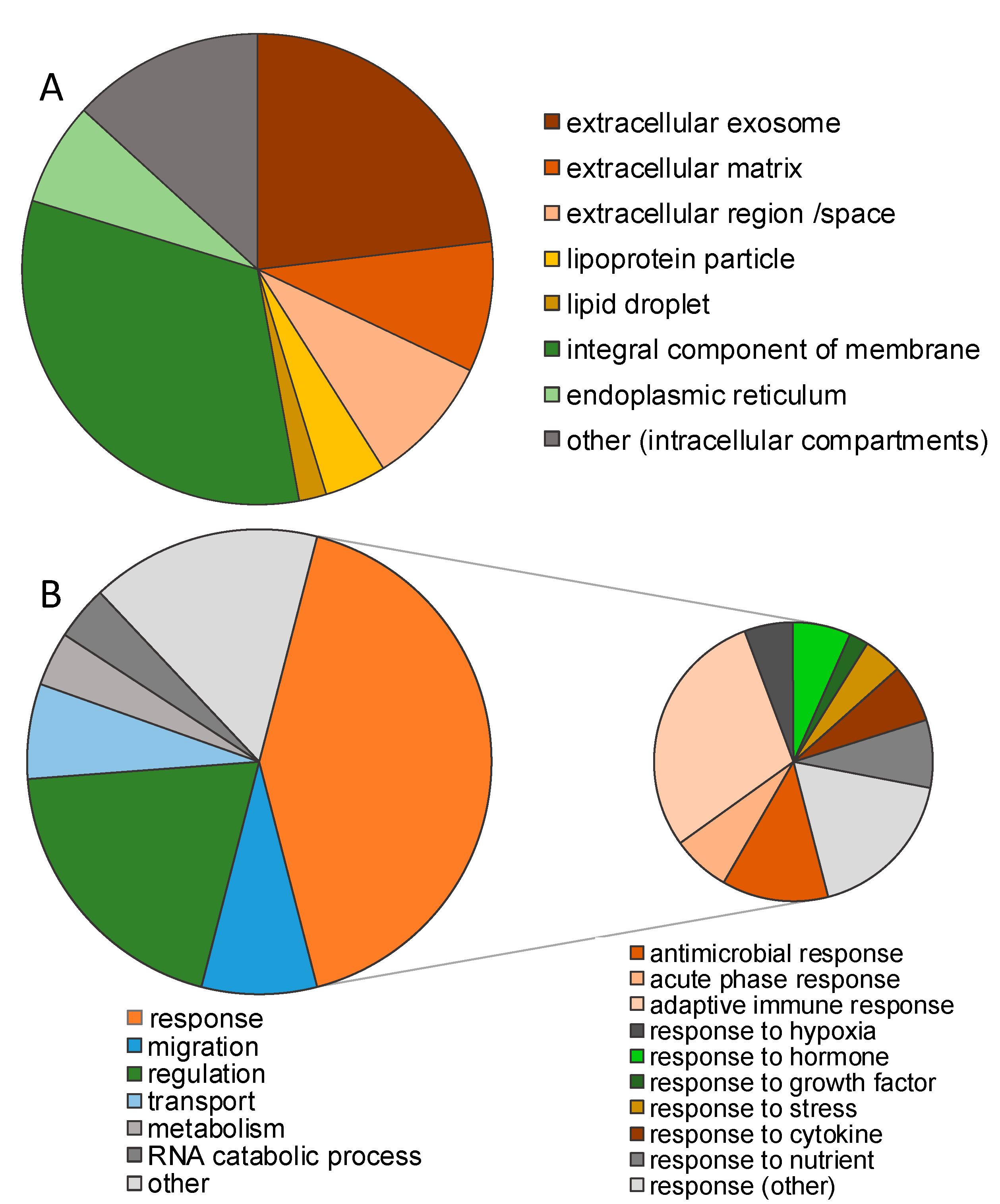




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Howland, V.; Klaedtke, M.; Ruhnau, J.; Dhople, V.M.; Grabe, H.J.; Völker, U.; Heckmann, M.; Hammer, E. Impact of Storage Conditions on the Breast Milk Peptidome. Nutrients 2020, 12, 2733. https://doi.org/10.3390/nu12092733
Howland V, Klaedtke M, Ruhnau J, Dhople VM, Grabe HJ, Völker U, Heckmann M, Hammer E. Impact of Storage Conditions on the Breast Milk Peptidome. Nutrients. 2020; 12(9):2733. https://doi.org/10.3390/nu12092733
Chicago/Turabian StyleHowland, Vanessa, Maik Klaedtke, Johanna Ruhnau, Vishnu M. Dhople, Hans J. Grabe, Uwe Völker, Matthias Heckmann, and Elke Hammer. 2020. "Impact of Storage Conditions on the Breast Milk Peptidome" Nutrients 12, no. 9: 2733. https://doi.org/10.3390/nu12092733
APA StyleHowland, V., Klaedtke, M., Ruhnau, J., Dhople, V. M., Grabe, H. J., Völker, U., Heckmann, M., & Hammer, E. (2020). Impact of Storage Conditions on the Breast Milk Peptidome. Nutrients, 12(9), 2733. https://doi.org/10.3390/nu12092733