Intensive Running Enhances NF-κB Activity in the Mice Liver and the Intervention Effects of Quercetin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Animals Treatment and Exercise Protocol
2.3. Liver Pathomorphological Examination
2.4. Serum Assays by Biochemistry and ELISA
2.5. Liver RNA Extraction and qRT-PCR Analysis
2.6. Protein Detection by Immunohistochemistry and Western Blot
2.7. NF-κB Activation Assay by IFL, LSCM and EMSA
2.8. Statistical Analysis
3. Results
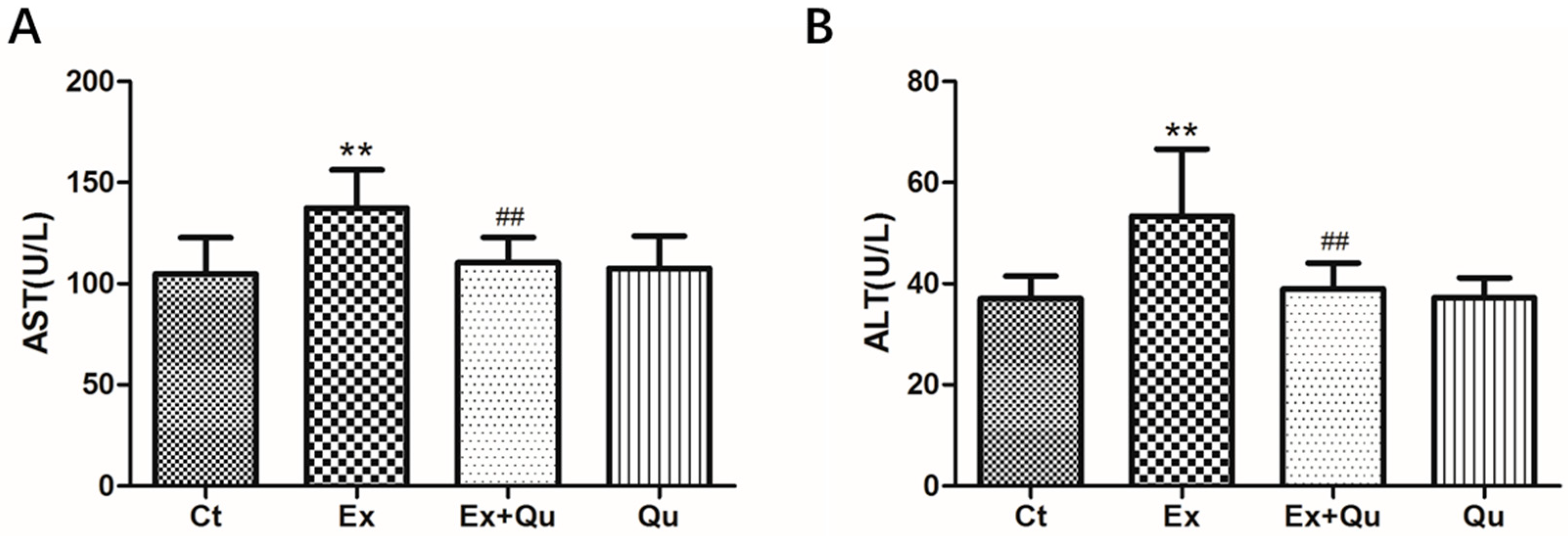
3.1. Quercetin Treatment Alleviated Liver Damage of Mice Exposed with Intensive Exercise
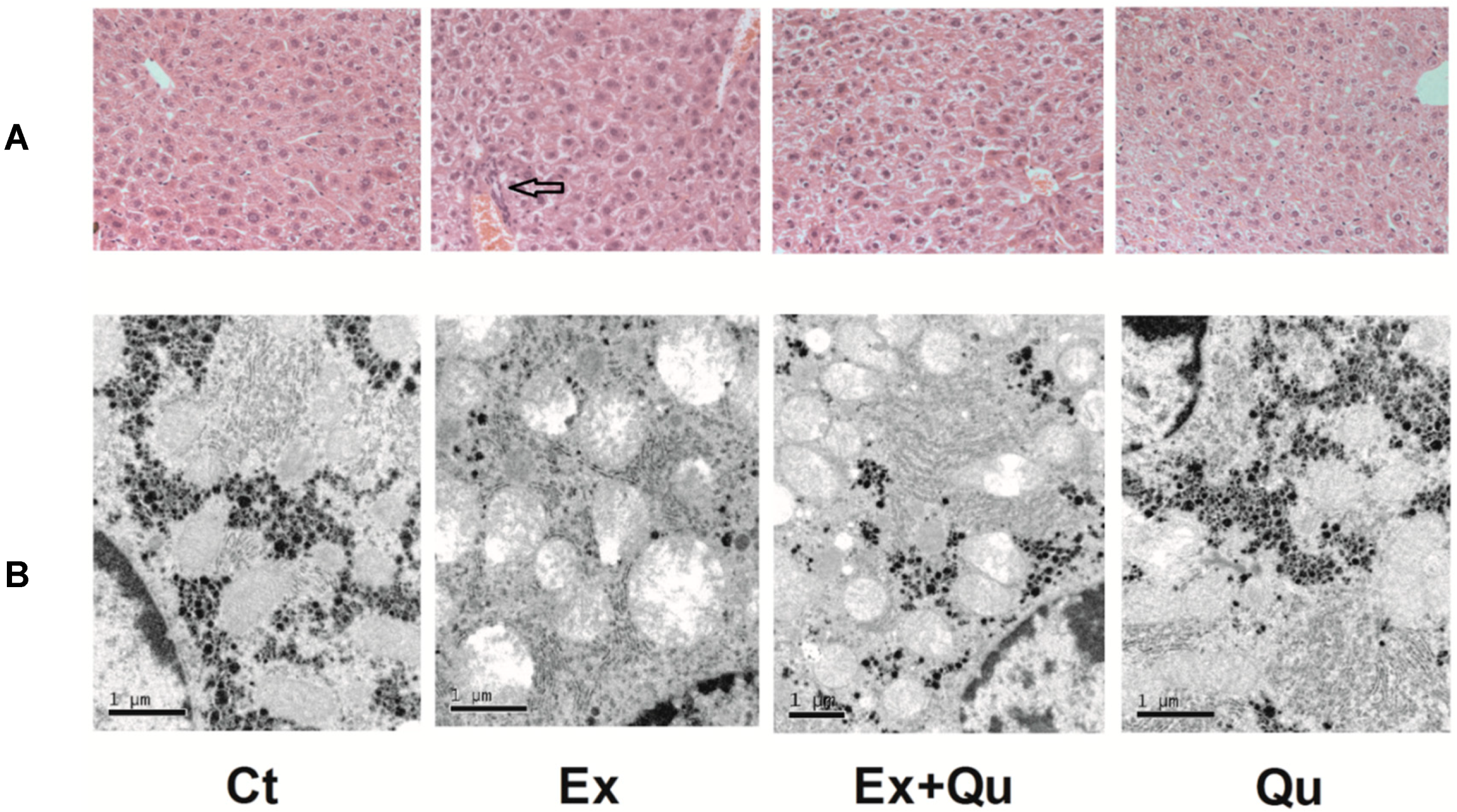
3.2. Quercetin Improved Intensive Exercise-Derived Hepatic Pathomorphological Abnormity
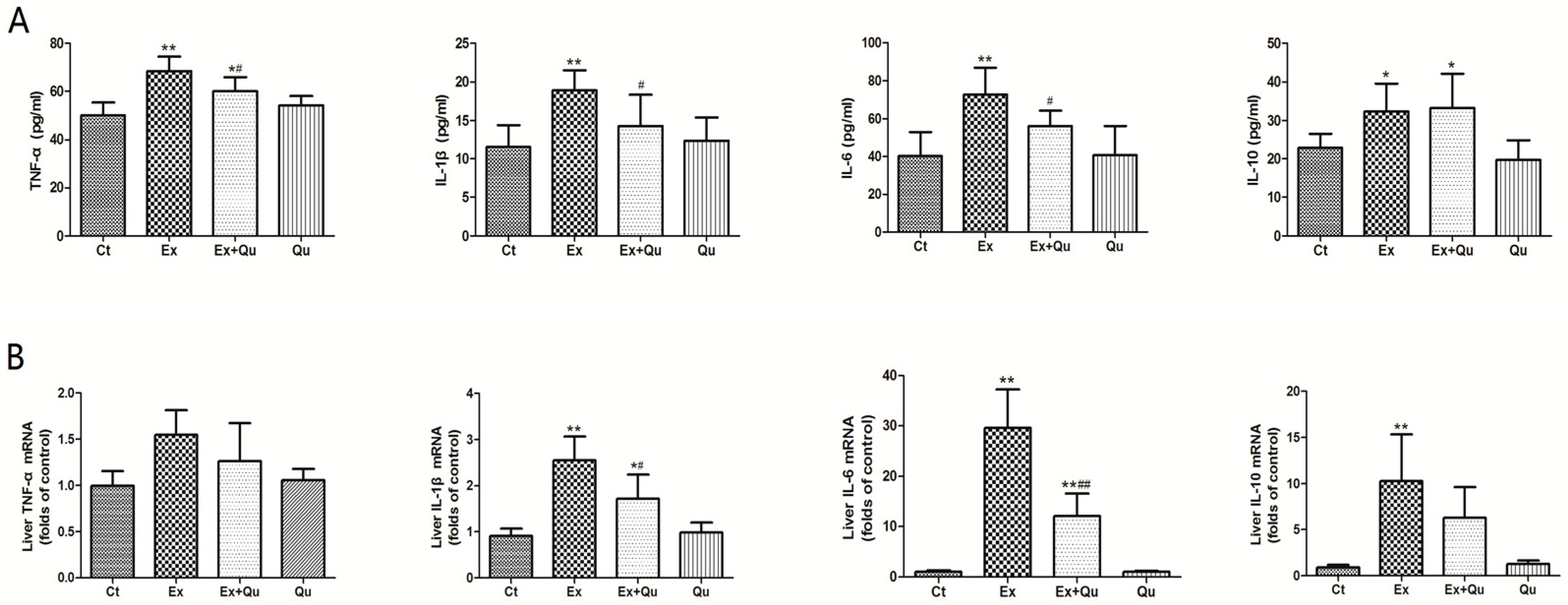
3.3. Quercetin Alleviated Intensive Exercise-Derived Inflammatory Stress
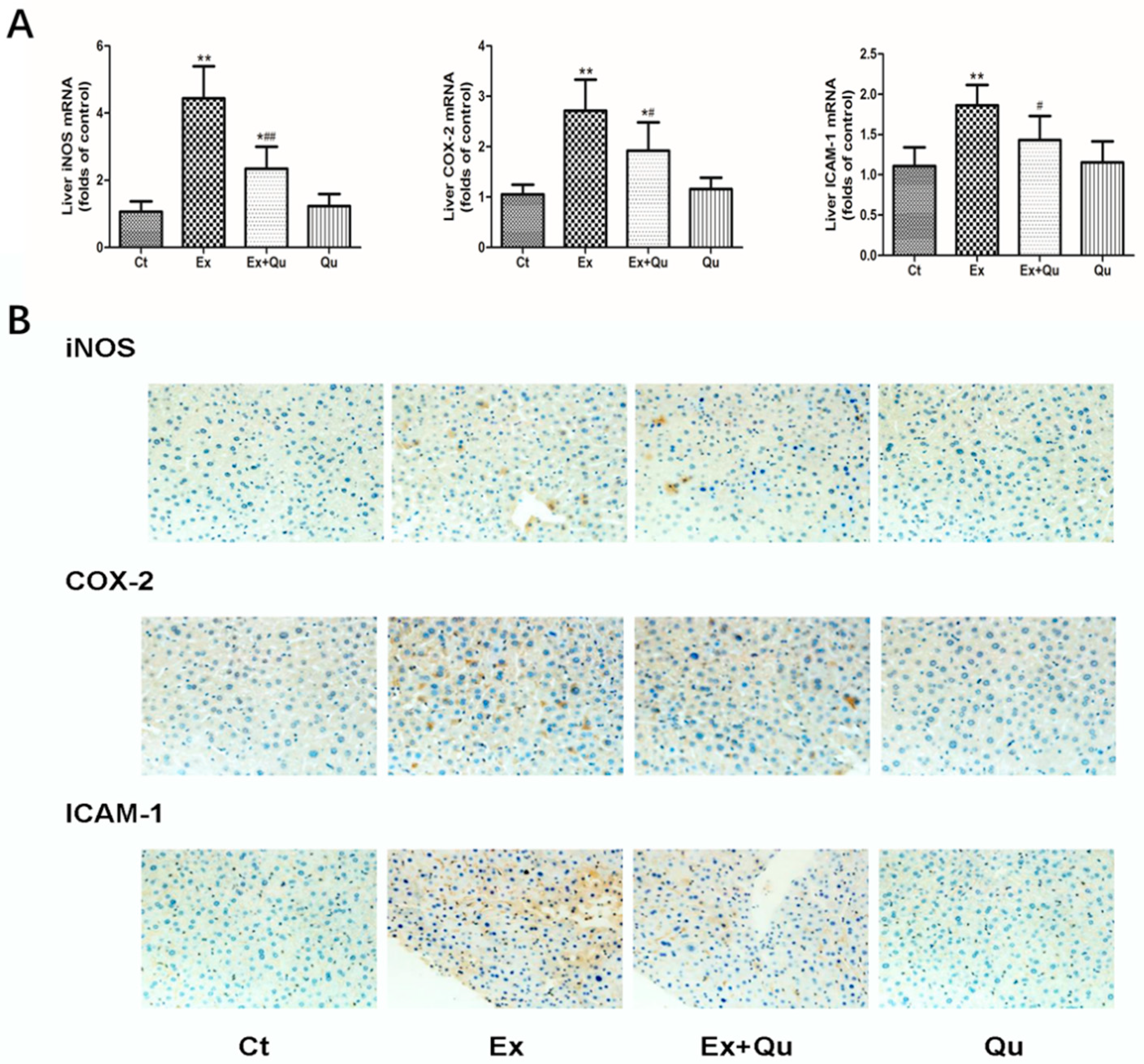
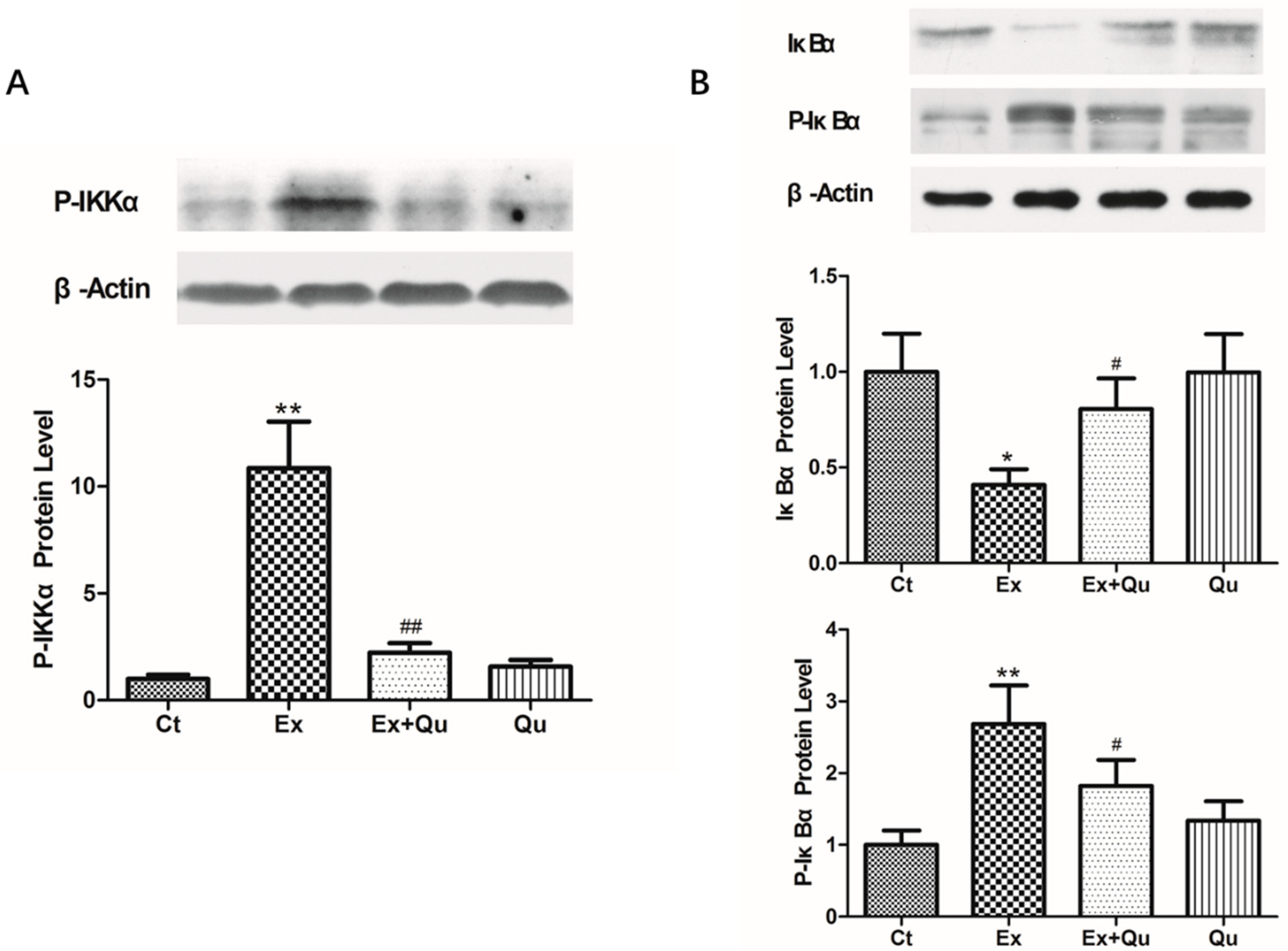
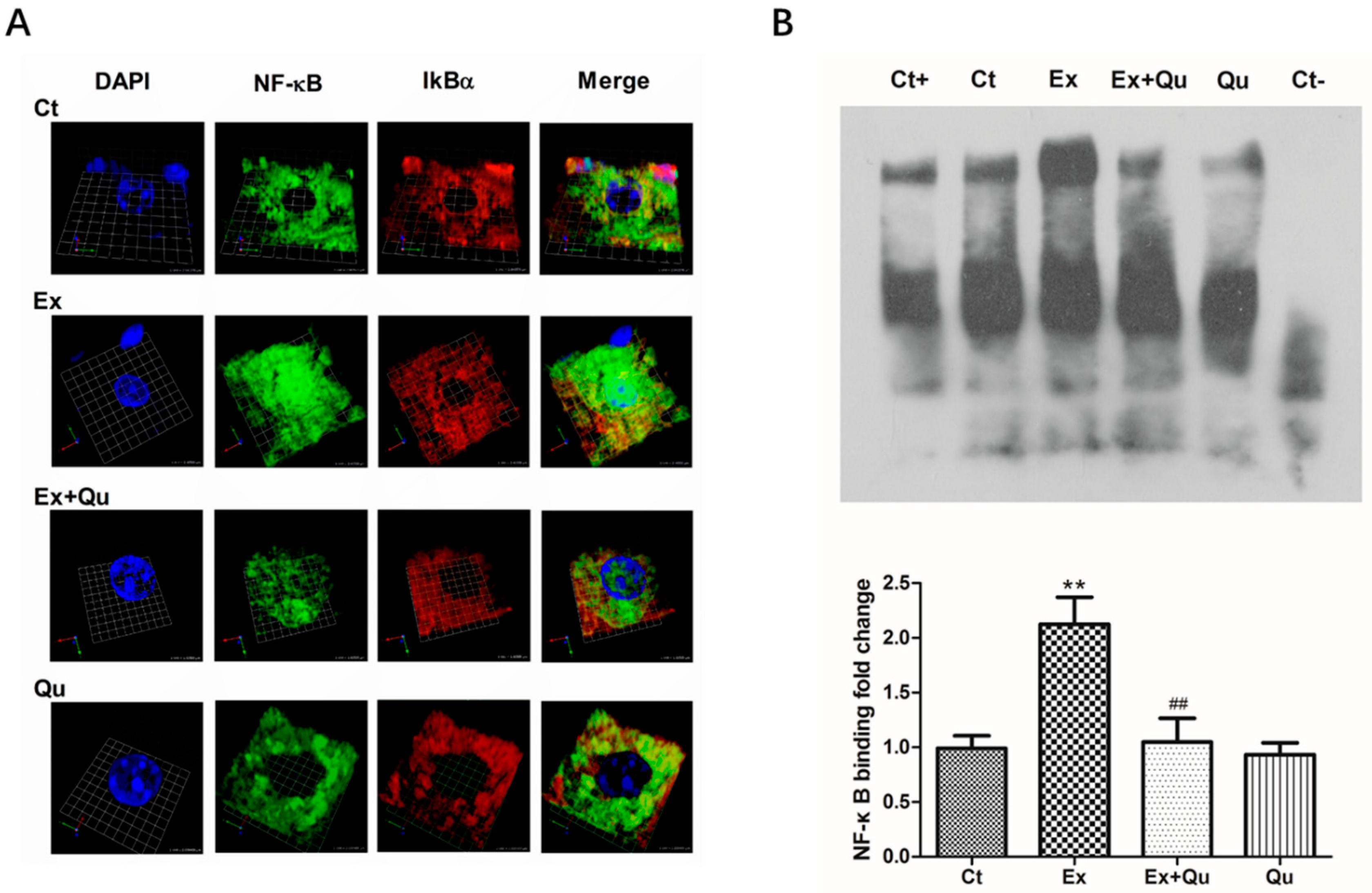
3.4. Quercetin Ameliorated Exercise-Induced Inflammatory Stress through Suppressing NF-κB Activation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Frodermann, V.; Rohde, D.; Courties, G.; Severe, N.; Schloss, M.J.; Amatullah, H. Exercise reduces inflammatory cell production and cardiovascular inflammation via instruction of hematopoietic progenitor cells. Nat. Med. 2019, 25, 1761–1771. [Google Scholar] [CrossRef] [PubMed]
- Orlando, P.; Silvestri, S.; Galeazzi, R.; Antonicelli, R.; Marcheggiani, F.; Cirilli, I. Effect of ubiquinol supplementation on biochemical and oxidative stress indexes after intensive exercise in young athletes. Redox Rep. 2018, 23, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Lin, W.T.; Hsu, F.L.; Tsai, P.W.; Hou, C.C. Metabolomics investigation of exercise-modulated changes in metabolism in rat liver after exhaustive and endurance exercises. Eur. J. Appl. Physiol. 2010, 108, 557–566. [Google Scholar] [CrossRef]
- Kramer, H.F.; Goodyear, L.J. Exercise, MAPK, and NF-kappaB signaling in skeletal muscle. J. Appl. Physiol. (1985) 2007, 103, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L. Modulation of skeletal muscle antioxidant defense by exercise: Role of redox signaling. Free Radic. Biol. Med. 2008, 44, 142–152. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. NF-κB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef]
- Christian, F.; Smith, E.L.; Carmody, R.J. The regulation of NF-κB subunits by phosphorylation. Cells 2016, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Liu, M.; Zhang, Y.; Li, X. Trimetazidine Attenuates Exhaustive Exercise-Induced Myocardial Injury in Rats via Regulation of the Nrf2/NF-κB Signaling Pathway. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Bischoff, S.C. Quercetin: Potentials in the prevention and therapy of disease. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 733–740. [Google Scholar] [CrossRef]
- Mariee, A.D.; Abd-Allah, G.M.; El-Beshbishy, H.A. Protective effect of dietary flavonoid quercetin against lipemic-oxidative hepatic injury in hypercholesterolemic rats. Pharm. Biol. 2012, 50, 1019–1025. [Google Scholar] [CrossRef]
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D.; Davis, B. Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1071–R1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieman, D.C.; Williams, A.S.; Shanely, R.A.; Jin, F.; McAnulty, S.R.; Triplett, N.T. Quercetin’s influence on exercise performance and muscle mitochondrial biogenesis. Med. Sci. Sports Exerc. 2010, 42, 338–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, J.M.; Carlstedt, C.J.; Chen, S.; Carmichael, M.D.; Murphy, E.A. The dietary flavonoid quercetin increases VO(2max) and endurance capacity. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 56–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAnulty, S.R.; McAnulty, L.S.; Nieman, D.C.; Quindry, J.C.; Hosick, P.A.; Hudson, M.H. Chronic quercetin ingestion and exercise-induced oxidative damage and inflammation. Appl. Physiol. Nutr. Metab. 2008, 33, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Cureton, K.J.; Tomporowski, P.D.; Singhal, A.; Pasley, J.D.; Bigelman, K.A.; Lambourne, K. Dietary quercetin supplementation is not ergogenic in untrained men. J. Appl. Physiol. 2009, 107, 1095–1104. [Google Scholar] [CrossRef] [Green Version]
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D.; Zielinski, M.R.; Groschwitz, C.M.; Brown, A.S. Curcumin effects on inflammation and performance recovery following eccentric exercise-induced muscle damage. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R2168–R2173. [Google Scholar] [CrossRef] [Green Version]
- Nieman, D.C.; Stear, S.J.; Castell, L.M.; Burke, L.M. A-Z of nutritional supplements: Dietary supplements, sports nutrition foods and ergogenic aids for health and performance: Part 15. Br. J. Sports Med. 2010, 44, 1202–1205. [Google Scholar] [CrossRef] [Green Version]
- Nieman, D.C.; Henson, D.A.; Davis, J.M.; Angela Murphy, E.; Jenkins, D.P.; Gross, S.J. Quercetin’s influence on exercise-induced changes in plasma cytokines and muscle and leukocyte cytokine mRNA. J. Appl. Physiol. 2007, 103, 1728–1735. [Google Scholar] [CrossRef] [Green Version]
- Nieman, D.C. Immunonutrition support for athletes. Nutr. Rev. 2008, 66, 310–320. [Google Scholar] [CrossRef]
- Marra, S.; Burnett, M.; Hoffman-Goetz, L. Intravenous catecholamine administration affects mouse intestinal lymphocyte number and apoptosis. J. Neuroimmunol. 2005, 158, 76–85. [Google Scholar] [CrossRef]
- Gao, C.; Chen, X.; Li, J.; Li, Y.; Tang, Y.; Liu, L. Myocardial mitochondrial oxidative stress and dysfunction in intensive exercise: Regulatory effects of quercetin. Eur. J. Appl. Physiol. 2014, 114, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Gomez-Cabrera, M.C.; Steinhafel, N.; Vina, J. Acute exercise activates nuclear factor (NF)-kappaB signaling pathway in rat skeletal muscle. FASEB J. 2004, 18, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Pillon Barcelos, R.; Freire Royes, L.F.; Gonzalez-Gallego, J.; Bresciani, G. Oxidative stress and inflammation: Liver responses and adaptations to acute and regular exercise. Free Radic. Res. 2017, 51, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yu, J.; Xue, C.; Wang, Y.; Tang, Q.; Mao, X. Mechanism of neoagarotetraose protects against intensive exercise-induced liver injury based on molecular ecological network analysis. Biosci. Biotechnol. Biochem. 2019, 83, 1227–1238. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, J.; Gao, C.; Xu, Y.; Li, Y.; Yu, X. Hepatoprotective Effect of Quercetin on Endoplasmic Reticulum Stress and Inflammation after intensive Exercise in Mice through Phosphoinositide 3-Kinase and Nuclear Factor-Kappa B. Oxid. Med. Cell. Longev. 2016, 2016, 8696587. [Google Scholar] [CrossRef] [Green Version]
- Urso, M.L. Anti-Inflammatory Interventions and Skeletal Muscle Injury: Benefit or Detriment? J. Appl. Physiol. 2013, 98, 1154–1162. [Google Scholar] [CrossRef] [Green Version]
- Pahl, H.L. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 1999, 18, 6853–6866. [Google Scholar] [CrossRef] [Green Version]
- Bakkar, N.; Guttridge, D.C. NF-kappaB signaling: A tale of two pathways in skeletal myogenesis. Physiol. Rev. 2010, 90, 495–511. [Google Scholar] [CrossRef] [Green Version]
- Gomez Cabrera, M.C.; Borrás, C.; Pallardó, F.V.; Sastre, J.; Ji, L.L.; Viña, J. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J. Physiol. 2005, 567, 113–120. [Google Scholar] [CrossRef]
- Vella, L.; Caldow, M.K.; Larsen, A.E.; Tassoni, D.; Della, G.P.; Gran, P. Resistance exercise increases NF-kappaB activity in human skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R667–R673. [Google Scholar] [CrossRef]
- Adams, V.; Nehrhoff, B.; Spate, U.; Linke, A.; Schulze, P.C.; Baur, A. Induction of iNOS expression in skeletal muscle by IL-1beta and NFkappaB activation: An in vitro and in vivo study. Cardiovasc. Res. 2002, 54, 95–104. [Google Scholar] [CrossRef]
- Szczepanik, M. Melatonin and its influence on immune system. J. Physiol. Pharmacol. 2007, 58 (Suppl. S6), 115–124. [Google Scholar]
- Li, J.H.; Yu, J.P.; Yu, H.G.; Xu, X.M.; Yu, L.L.; Liu, J. Melatonin reduces inflammatory injury through inhibiting NF-kappaB activation in rats with colitis. Mediators Inflamm. 2005, 2005, 185–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira, A.J.; Fraga, C.; Alonso, M.; Collado, P.S.; Zetller, C.; Marroni, C. Quercetin prevents oxidative stress and NF-kappaB activation in gastric mucosa of portal hypertensive rats. Biochem. Pharmacol. 2004, 68, 1939–1946. [Google Scholar] [CrossRef]
- Garcia-Mediavilla, V.; Crespo, I.; Collado, P.S.; Esteller, A.; Sanchez-Campos, S.; Tunon, M.J. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur. J. Pharmacol. 2007, 557, 221–229. [Google Scholar] [CrossRef]
- Comalada, M.; Camuesco, D.; Sierra, S.; Ballester, I.; Xaus, J.; Galvez, J. In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-kappaB pathway. Eur. J. Immunol. 2005, 35, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.P.; Mahajan, S.; Reynolds, J.L.; Aalinkeel, R.; Nair, H.; Schwartz, S.A. The flavonoid quercetin inhibits proinflammatory cytokine (tumor necrosis factor alpha) gene expression in normal peripheral blood mononuclear cells via modulation of the NF-kappa beta system. Clin. Vaccine Immunol. 2006, 13, 319–328. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.W.; Bode, A.M.; Dong, Z. Molecular targets of phytochemicals for cancer prevention. Nat. Rev. Cancer 2011, 11, 211–218. [Google Scholar] [CrossRef]
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D. Effects of the dietary flavonoid quercetin upon performance and health. Curr. Sport. Med. Rep. 2009, 8, 206–213. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, C.; Liu, Y.; Jiang, C.; Liu, L.; Li, J.; Li, D.; Guo, X.; Wang, Z.; Yang, Y.; Liu, L.; et al. Intensive Running Enhances NF-κB Activity in the Mice Liver and the Intervention Effects of Quercetin. Nutrients 2020, 12, 2770. https://doi.org/10.3390/nu12092770
Gao C, Liu Y, Jiang C, Liu L, Li J, Li D, Guo X, Wang Z, Yang Y, Liu L, et al. Intensive Running Enhances NF-κB Activity in the Mice Liver and the Intervention Effects of Quercetin. Nutrients. 2020; 12(9):2770. https://doi.org/10.3390/nu12092770
Chicago/Turabian StyleGao, Chao, Yang Liu, Chunjie Jiang, Liang Liu, Juan Li, Dan Li, Xiaoping Guo, Zhu Wang, Yuexin Yang, Liegang Liu, and et al. 2020. "Intensive Running Enhances NF-κB Activity in the Mice Liver and the Intervention Effects of Quercetin" Nutrients 12, no. 9: 2770. https://doi.org/10.3390/nu12092770




