Effect of Konjac Glucomannan (KGM) on the Reconstitution of the Dermal Environment against UVB-Induced Condition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture and UVB Irradiation
2.3. Cell Viability Assay
2.4. SA-β-Galactosidase Assay and Staining
2.5. RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
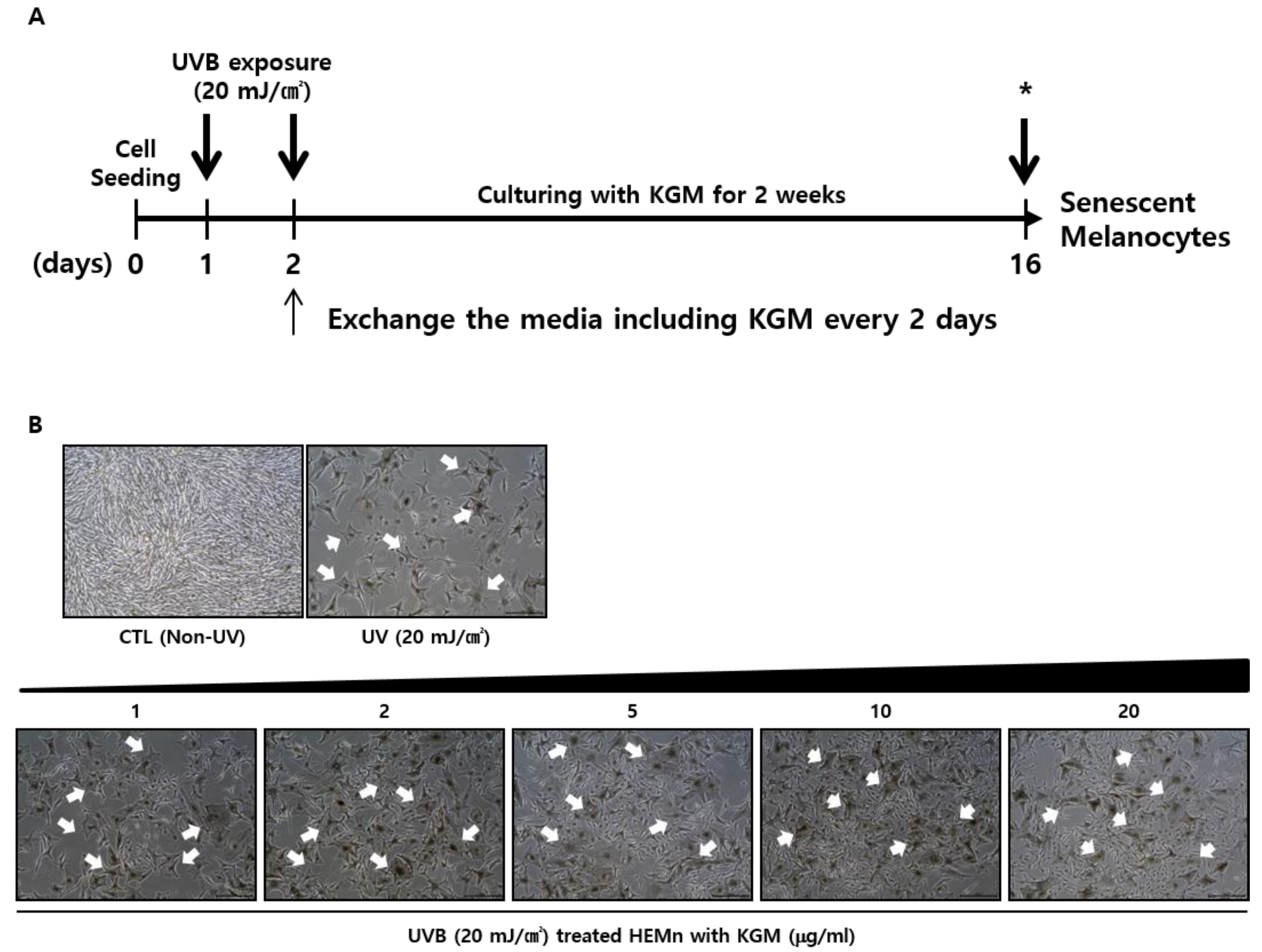
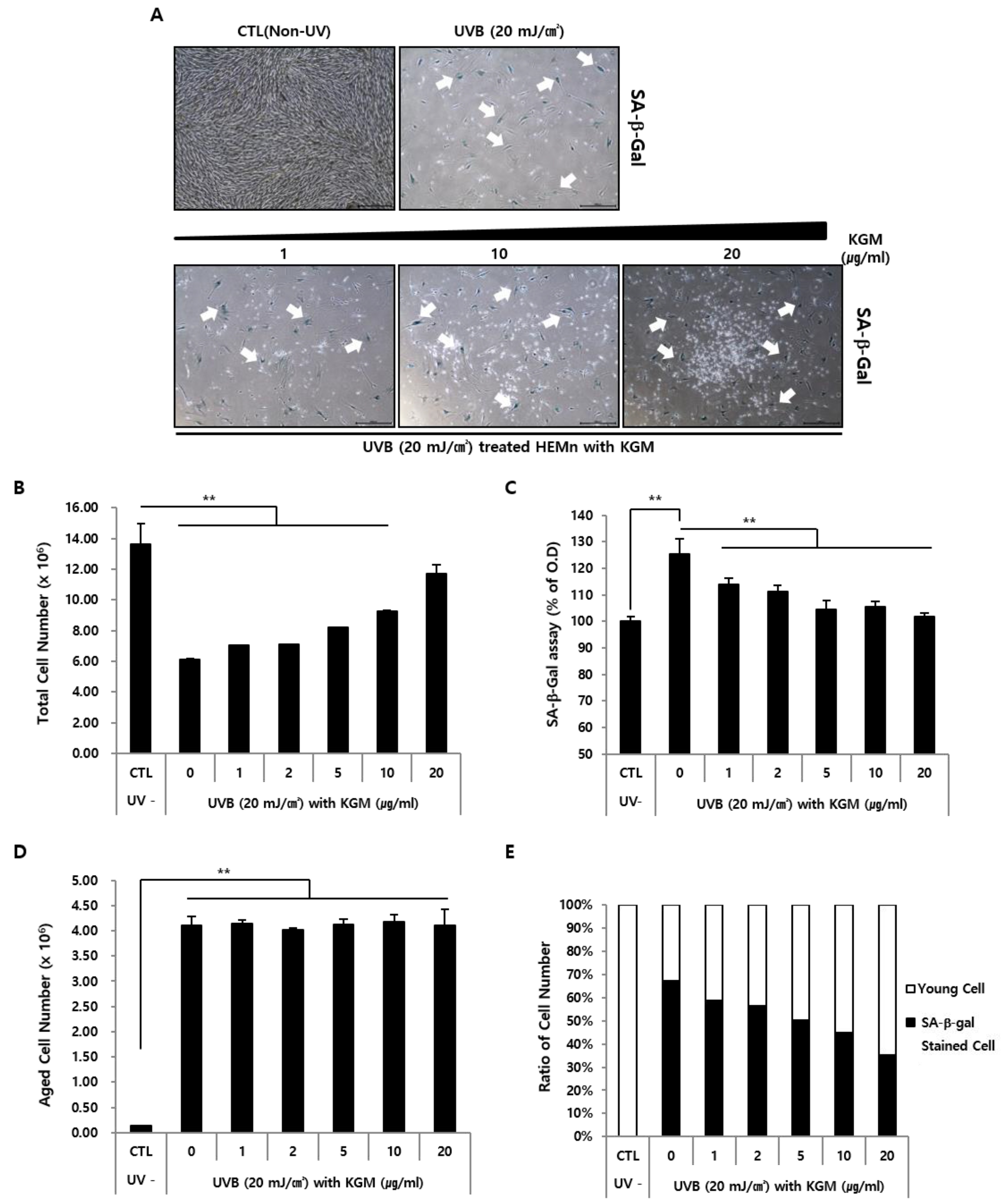
3.1. UVB-Induced Senescence in Human Epidermal Melanocytes Is Alleviated by KGM Treatment
3.2. KGM Promotes the Growth of UVB-Induced Senescent Human Melanocytes in a Dose-Dependent Manner, but Does Not Eliminate Dead Cells
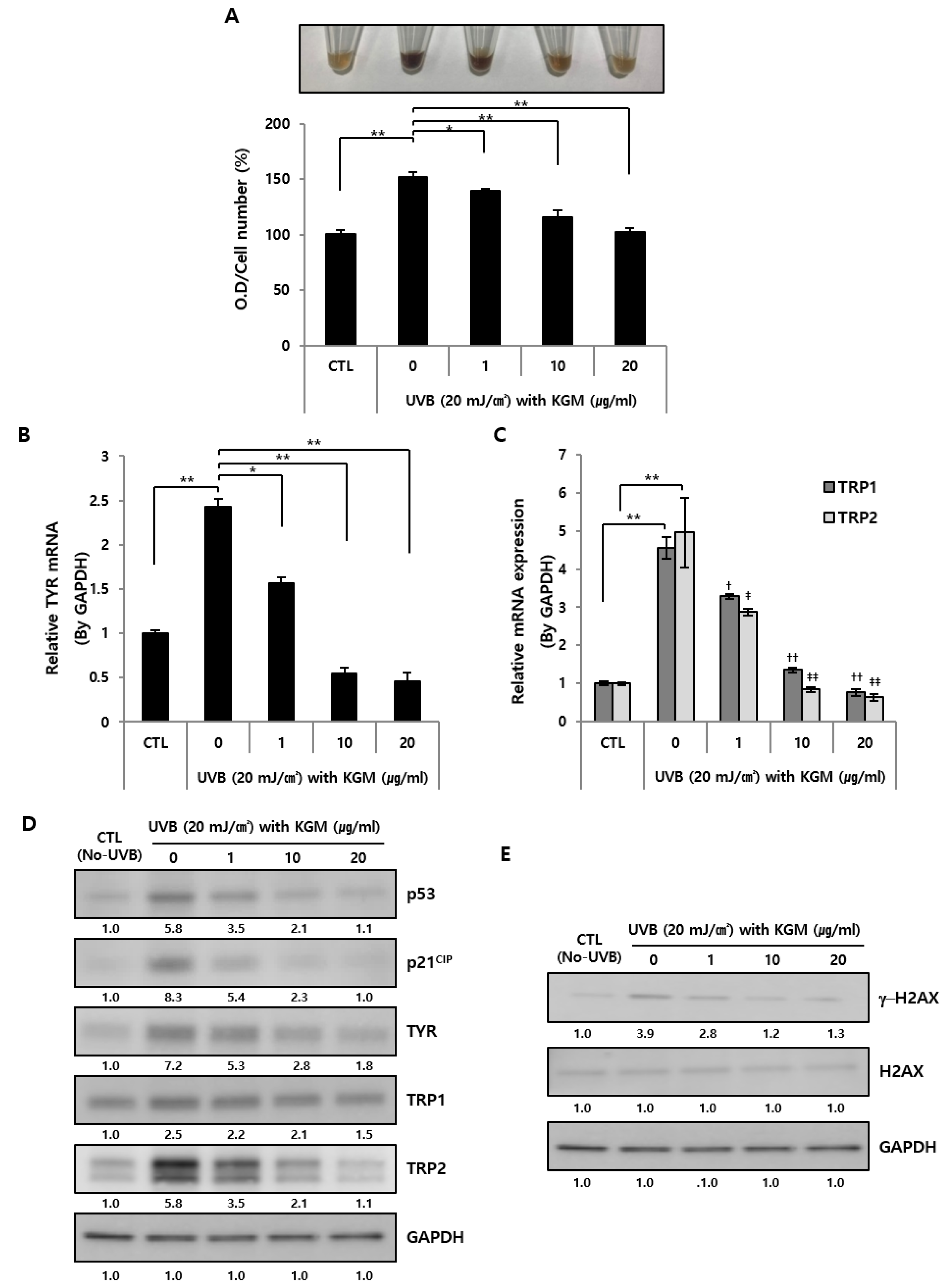
3.3. KGM Treatment Suppresses Hyperpigmentation in UVB-Induced Senescent Human Melanocytes
3.4. KGM Reconstitutes UVB-Damaged Human Primary Fibroblasts
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fuchs, E. Scratching the surface of skin development. Nature 2007, 445, 834–842. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.T.; Zmijewski, M.A.; Skobowiat, C.; Zbytek, B.; Slominski, R.M.; Steketee, J.D. Sensing the environment: Regulation of local and global homeostasis by the skin neuroendocrine system. In Advances in Anatomy, Embryology and Cell Biology; Springer: Berlin/Heidelberg, Germany, 2012; Volume 212. [Google Scholar]
- Sotiropoulou, P.A.; Blanpain, C. Development and homeostasis of the skin epidermis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008383. [Google Scholar] [CrossRef] [Green Version]
- Elwood, J.M.; Jopson, J. Melanoma and sun exposure: An overview of published studies. Int. J. Cancer 1997, 73, 198–203. [Google Scholar] [CrossRef]
- Lowe, N.J. An overview of ultraviolet radiation, sunscreens, and photo-induced dermatoses. Dermatol. Clin. 2006, 24, 9–17. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J. Neuroendocrinology of the skin. Endocr. Rev. 2000, 21, 457–487. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J.; Luger, T.; Paus, R.; Solomon, S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol. Rev. 2000, 80, 979–1020. [Google Scholar] [CrossRef] [Green Version]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [Green Version]
- Svobodová, A.; Psotová, J.; Walterová, D. Natural phenolics in the prevention of UV-induced skin damage. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech Repub. 2003, 147, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, M.; Niida, H.; Murakami, H.; Shimada, M. DNA damage responses in skin biology—Implications in tumor prevention and aging acceleration. J. Dermatol. Sci. 2009, 56, 76–81. [Google Scholar] [CrossRef]
- Godar, D.E. UV doses worldwide. Photochem. Photobiol. 2005, 81, 736–749. [Google Scholar] [CrossRef]
- Doré, J.F.; Chignol, M.C. Tanning salons and skin cancer. Photochem. Photobiol. Sci. 2012, 11, 30–37. [Google Scholar] [CrossRef]
- Choi, S.Y.; Bin, B.H.; Kim, W.; Lee, E.; Lee, T.R.; Cho, E.G. Exposure of human melanocytes to UVB twice and subsequent incubation leads to cellular senescence and senescence-associated pigmentation through the prolonged p53 expression. J. Dermatol. Sci. 2018, 90, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Baker, L.A.; Horbury, M.D.; Greenough, S.E.; Ashfold, M.N.; Stavros, V.G. Broadband ultrafast photoprotection by oxybenzone across the UVB and UVC spectral regions. Photochem. Photobiol. Sci. 2015, 14, 1814–1820. [Google Scholar] [CrossRef] [Green Version]
- Amaro-Ortiz, A.; Yan, B.; D’Orazio, J.A. Ultraviolet radiation, aging and the skin: Prevention of damage by topical cAMP manipulation. Molecules 2014, 19, 6202–6219. [Google Scholar] [CrossRef]
- Gonzaga, E.R. Role of UV light in photodamage, skin aging, and skin cancer: Importance of photoprotection. Am. J. Clin. Dermatol. 2009, 1, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Chua, M.; Baldwin, T.C.; Hocking, T.J.; Chan, K. Traditional uses and potential health benefits of Amorphophallus konjac K. Koch ex N.E.Br. J. Ethnopharmacol. 2010, 128, 268–278. [Google Scholar] [CrossRef]
- Zhou, Y.; Cao, H.; Hou, M.; Nirasawa, S.; Tatsumi, E.; Foster, T.J.; Cheng, Y. Effect of konjac glucomannan on physical and sensory properties of noodles made from low-protein wheat flour. Food Res. Int. 2013, 51, 879–885. [Google Scholar] [CrossRef]
- Huang, C.Y.; Zhang, M.Y.; Peng, S.S.; Hong, J.R.; Wang, X.; Jiang, H.J.; Zhang, F.L.; Bai, Y.X.; Liang, J.Z.; Yu, Y.R.; et al. Effect of Konjac food on blood glucose level in patients with diabetes. Biomed. Environ. Sci. 1990, 3, 123–131. [Google Scholar]
- Wu, J.; Peng, S.S. Comparison of hypolipidemic effect of refined konjac meal with several common dietary fibers and their mechanisms of action. Biomed. Environ. Sci. 1997, 10, 27–37. [Google Scholar]
- Xiong, G.; Cheng, W.; Ye, L.; Du, X.; Zhou, M.; Lin, R.; Geng, S.; Chen, M.; Corke, H.; Cai, Y.Z. Effects of konjac glucomannan on physicochemical properties of myofibrillar protein and surimi gels from grass carp (Ctenopharyngodon idella). Food Chem. 2009, 116, 413–418. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, K.; Xiao, M.; Riffat, S.B.; Su, Y.; Jiang, F. Thermal conductivity, structure and mechanical properties of konjac glucomannan/starch based aerogel strengthened by wheat straw. Carbohydr. Polym. 2018, 197, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, J.D.; Yang, F.Q. Konjac glucomannan, a promising polysaccharide for OCDDS. Carbohydr. Polym. 2014, 104, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Katsuraya, K.; Okuyama, K.; Hatanaka, K.; Oshima, R.; Sato, T.; Matsuzaki, K. Constitution of konjac glucomannan: Chemical analysis and 13C NMR spectroscopy. Carbohydr. Polym. 2003, 53, 183–189. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Xie, B.; Gan, X. Advance in the applications of konjac glucomannan and its derivatives. Carbohydr. Polym. 2005, 60, 27–31. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C. Nutritional and potential health benefits of konjac glucomannan, a promising polysaccharide of elephant foot yam, Amorphophallus konjac K. Koch: Review. Food Rev. Int. 2017, 33, 22–43. [Google Scholar] [CrossRef]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef] [Green Version]
- Pan, T.; Peng, S.; Xu, Z.; Xiong, B.; Wen, C.; Yao, M.; Pang, J. Synergetic degradation of konjac glucomannan by γ-ray irradiation and hydrogen peroxide. Carbohydr. Polym. 2013, 93, 761–767. [Google Scholar] [CrossRef]
- Chen, H.L.; Cheng, H.C.; Wu, W.T.; Liu, Y.J.; Liu, S.Y. Supplementation of konjac glucomannan into a low-fiber Chinese diet promoted bowel movement and improved colonic ecology in constipated adults: A placebo-controlled, diet-controlled trial. J. Am. Coll. Nutr. 2008, 27, 102–108. [Google Scholar] [CrossRef]
- Chen, M.; Wang, S.; Liang, X.; Ma, D.; He, L.; Liu, Y. Effect of dietary acidolysis-oxidized Konjac glucomannan supplementation on serum immune parameters and intestinal immune-related gene expression of Schizothorax prenanti. Int. J. Mol. Sci. 2017, 18, 2258. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Tian, J.; Zou, J.; Yuan, X.; Li, J.; Liang, H.; Zhan, F.; Li, B. Partial removal of acetyl groups in konjac glucomannan significantly improved the rheological properties and texture of konjac glucomannan and κ-carrageenan blends. Int. J. Biol. Macromol. 2019, 123, 1165–1171. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, J.; Ran, L.; Yu, K.; Lu, B.; Lan, G.; Dai, F.; Lu, F. An injectable self-healing hydrogel with adhesive and antibacterial properties effectively promotes wound healing. Carbohydr. Polym. 2018, 201, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Zhao, X.; Guo, B.; Ma, P.X. Self-healing conductive injectable hydrogels with antibacterial activity as cell delivery carrier for cardiac cell therapy. ACS Appl. Mater. Interfaces 2016, 8, 17138–17150. [Google Scholar] [CrossRef] [PubMed]
- Park, P.J.; Lee, T.R.; Cho, E.G. Substance P stimulates endothelin 1 secretion via endothelin-converting enzyme 1 and promotes melanogenesis in human melanocytes. J. Investig. Dermatol. 2015, 135, 551–559. [Google Scholar] [CrossRef] [Green Version]
- Bennett, D.C. Human melanocyte senescence and melanoma susceptibility genes. Oncogene 2003, 22, 3063–3069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef] [Green Version]
- Yamaba, H.; Haba, M.; Kunita, M.; Sakaida, T.; Tanaka, H.; Yashiro, Y.; Nakata, S. Morphological change of skin fibroblasts induced by UV Irradiation is involved in photoaging. Exp. Dermatol. 2016, 3, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.J.; Lee, E.H.; Cho, E.B.; Kim, D.H.; Kim, B.O.; Kang, I.K.; Jung, H.Y.; Cho, Y.J. Protective effects of galangin against UVB irradiation-induced photo-aging in CCD-986sk human skin fibroblasts. Appl. Biol. Chem. 2019, 62, 40. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.Y.; Han, J.A.; Im, J.S.; Morrone, A.; Johung, K.; Goodwin, E.C.; Kleijer, W.J.; DiMaio, D.; Hwang, E.S. Senescence-associated β-galactosidase is lysosomal β-galactosidase. Aging Cell 2006, 5, 187–195. [Google Scholar] [CrossRef]
- Szolnoky, G.; Bata-Csörgö, Z.; Kenderessy, A.S.; Kiss, M.; Pivarcsi, A.; Novák, Z.; Nagy, N.K.; Michel, G.; Ruzicka, T.; Maródi, L.; et al. A mannose-binding receptor is expressed on human keratinocytes and mediates killing of Candida albicans. J. Investig. Dermatol. 2001, 117, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, H.; Yarwood, H.; Ashworth, A.; Isacke, C.M. Share. Endo180, an endocytic recycling glycoprotein related to the macrophage mannose receptor is expressed on fibroblasts, endothelial cells and macrophages and functions as a lectin receptor. J. Cell Sci. 2000, 113, 1021–1032. [Google Scholar]
- Graf, S.A.; Busch, C.; Bosserhoff, A.K.; Besch, R.; Berking, C. SOX10 promotes melanoma cell invasion by regulating melanoma inhibitory activity. J. Investig. Dermatol. 2014, 134, 2212–2220. [Google Scholar] [CrossRef] [Green Version]
- Sellheyer, K. Pathogenesis of solar elastosis: Synthesis or degradation? J. Cutan. Pathol. 2003, 30, 123–127. [Google Scholar] [CrossRef]
- Heng, J.K.; Aw, D.C.; Tan, K.B. Solar elastosis in its papular form: Uncommon, mistakable. Case Rep. Dermatol. 2014, 6, 124–128. [Google Scholar] [CrossRef]
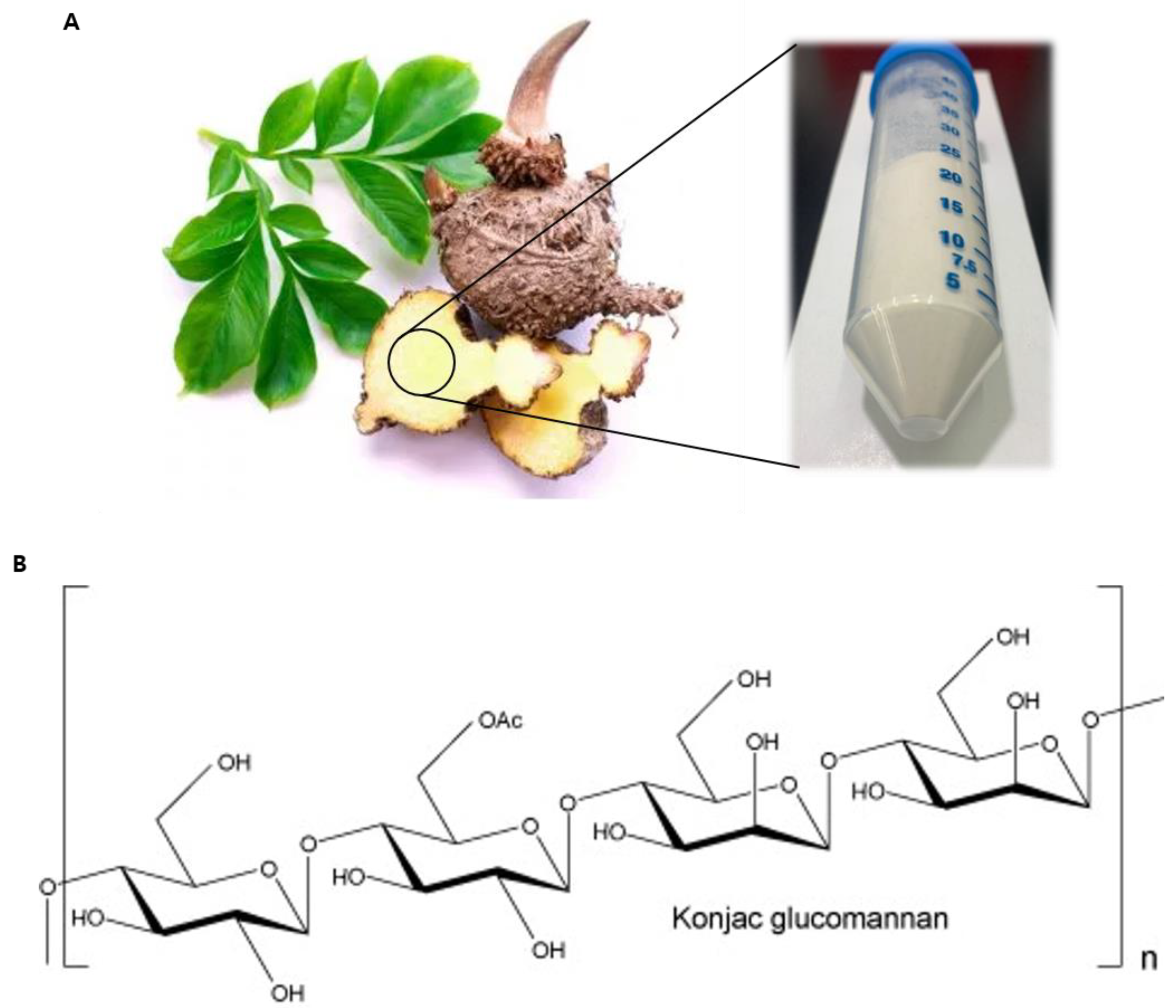

| Category | Viscosity (mPa.s) | Glucomannan (%) ≥ | pH (1% in DW) | Water (%) ≤ | Ash (%) ≤ | Lead (Pb) (mg/kg) ≤ | Sulfate (g/kg) ≤ | Arsenic (As) (mg/kg) ≤ | Aflatoxin B1 (ug/kg) ≤ |
|---|---|---|---|---|---|---|---|---|---|
| Purified KGM | 36,000 | 95 | 5.0~7.0 | 8 | 2 | 0.8 | 0.004 | 2 | 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, K.H.; Kim, S.T.; Bin, B.H.; Park, P.J. Effect of Konjac Glucomannan (KGM) on the Reconstitution of the Dermal Environment against UVB-Induced Condition. Nutrients 2020, 12, 2779. https://doi.org/10.3390/nu12092779
Choi KH, Kim ST, Bin BH, Park PJ. Effect of Konjac Glucomannan (KGM) on the Reconstitution of the Dermal Environment against UVB-Induced Condition. Nutrients. 2020; 12(9):2779. https://doi.org/10.3390/nu12092779
Chicago/Turabian StyleChoi, Kyung Ho, Sung Tae Kim, Bum Ho Bin, and Phil June Park. 2020. "Effect of Konjac Glucomannan (KGM) on the Reconstitution of the Dermal Environment against UVB-Induced Condition" Nutrients 12, no. 9: 2779. https://doi.org/10.3390/nu12092779






