Association of Maternal Plasma Total Cysteine and Growth among Infants in Nepal: A Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Original Study
2.2. Laboratory Assessment and Anthropometric Measurements
2.3. Data Management and Analysis
3. Results
3.1. Population Characteristics
3.2. Maternal Cysteine and Infant Growth
4. Discussion
4.1. Maternal Cysteine and Birth Weight
4.2. Maternal Cysteine and Linear Growth
4.3. Maternal Cysteine and Ponderal Growth
4.4. Limitations and Strength
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Victora, C.G.; Adair, L.; Fall, C.; Hallal, P.C.; Martorell, R.; Richter, L.; Sachdev, H.S. Maternal and child undernutrition: Consequences for adult health and human capital. Lancet 2008, 371, 340–357. [Google Scholar] [CrossRef]
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- de Onis, M.; Branca, F. Childhood Stunting: A Global Perspective. Matern. Child Nutr. 2016, 12, 12–26. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.; Olofin, I.; Flaxman, S.; Fawzi, W.; Spiegelman, D.; Caulfield, L.; Black, R.; Ezzati, M.; Danaei, G. The effect of multiple anthropometric deficits on child mortality: Meta-analysis of individual data in 10 prospective studies from developing countries. Am. J. Clin. Nutr. 2013, 97, 896. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Children: Reducing mortality. Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/children-reducing-mortality (accessed on 12 December 2019).
- Ruel, M.T.; Menon, P.; Habicht, J.-P.; Loechl, C.; Bergeron, G.; Pelto, G.; Arimond, M.; Maluccio, J.; Michaud, L.; Hankebo, B. Age-based preventive targeting of food assistance and behaviour change and communication for reduction of childhood undernutrition in Haiti: A cluster randomised trial. Lancet 2008, 371, 588–595. [Google Scholar] [CrossRef]
- Emergency Nutrition Network (ENN). Child Wasting and Stunting: Time to Overcome the Separation. A Breifing Note for Policy Makers and Programme Implementers. Available online: https://www.ennonline.net/resources/timetoovercometheseparation (accessed on 12 December 2019).
- Stewart, C.P.; Iannotti, L.; Dewey, K.G.; Michaelsen, K.F.; Onyango, A.W. Contextualising complementary feeding in a broader framework for stunting prevention. Matern. Child Nutr. 2013, 9, 27–45. [Google Scholar] [CrossRef]
- World Health Organization (WHO); United Nations International Children’s Emergency Fund (UNICEF); World Food Programme (WFP). Global Nutrition Targets 2025: Wasting Policy Brief. Available online: https://www.who.int/nutrition/publications/globaltargets2025_policybrief_wasting/en/ (accessed on 10 October 2019).
- World Health Organization (WHO). Global Nutrition Targets 2025: Stunting Policy Brief. Available online: https://www.who.int/nutrition/publications/globaltargets2025_policybrief_stunting/en/ (accessed on 10 October 2019).
- Ritchie, H.; Roser, M. Micronutrient Deficiency. Available online: https://ourworldindata.org/micronutrient-deficiency (accessed on 12 December 2019).
- Ghosh, S.; Suri, D.; Uauy, R. Assessment of protein adequacy in developing countries: Quality matters. Br. J. Nutr. 2012, 108, S77. [Google Scholar] [CrossRef]
- Uauy, R.; Kurpad, A.; Tano-Debrah, K.; Otoo, G.; Aaron, G.; Toride, Y.; Ghosh, S. Role of Protein and Amino Acids in Infant and Young Child Nutrition: Protein and Amino Acid Needs and Relationship with Child Growth. J. Nutr. Sci. Vitaminol. 2015, 61, S192–S194. [Google Scholar] [CrossRef]
- Semba, R.D.; Trehan, I.; Gonzalez-Freire, M.; Kraemer, K.; Moaddel, R.; Ordiz, M.I.; Ferrucci, L.; Manary, M.J. Perspective: The Potential Role of Essential Amino Acids and the Mechanistic Target of Rapamycin Complex 1 (mTORC1) Pathway in the Pathogenesis of Child Stunting. Adv. Nutr. 2016, 7, 853. [Google Scholar] [CrossRef]
- Brosnan, J.T.; Brosnan, M.E. Sulfur-Containing Amino Acids: An Overview. J. Nutr. 2006, 136, 1636S–1640S. [Google Scholar] [CrossRef]
- Wu, G. Amino Acids: Biochemistry and Nutrition; CRC Press LLC: Baton Rouge, LA, USA, 2013. [Google Scholar]
- Sturman, J.A.; Gaull, G.; Neils, C.R.R. Absence of Cystathionase in Human Fetal Liver: Is Cystine Essential? Science 1970, 169, 74–76. [Google Scholar] [CrossRef]
- Gaull, G.; Sturman, J.A.; Räihä, N.C.R. Development of Mammalian Sulfur Metabolism: Absence of Cystathionase in Human Fetal Tissues. Pediatric Res. 1972, 6, 538. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.A.; Cifuentes-Zúñiga, F.; Figueroa, E.; Villanueva, C.; Hernández, C.; Alegría, R.; Arroyo-Jousse, V.; Peñaloza, E.; Farías, M.; Uauy, R.; et al. N-Acetylcysteine, a glutathione precursor, reverts vascular dysfunction and endothelial epigenetic programming in intrauterine growth restricted guinea pigs. J. Physiol. 2017, 595, 1077–1092. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.-Y.; Wang, H.; Chen, Y.-H.; Xia, M.-Z.; Zhang, C.; Xu, D.-X. N-acetylcysteine alleviates cadmium-induced placental endoplasmic reticulum stress and fetal growth restriction in mice. PLoS ONE 2018, 13, e0191667. [Google Scholar] [CrossRef]
- Luo, Z.; Xu, X.; Sho, T.; Luo, W.; Zhang, J.; Xu, W.; Yao, J.; Xu, J. Effects of n-acetyl-cysteine supplementation in late gestational diet on maternal-placental redox status, placental NLRP3 inflammasome, and fecal microbiota in sows 1. J. Anim. Sci. 2019, 97, 1757–1771. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, V.; Afsharian, P.; Shahhoseini, M.; Kalantar, S.M.; Moini, A. A review on various uses of n-acetyl cysteine. Cell J. 2017, 19, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Schwinger, C.; Chowdhury, R.; Sharma, S.; Bhandari, N.; Taneja, S.; Ueland, P.M.; Strand, T.A. Association of plasma total cysteine and anthropometric status in 6-30 months old Indian children: A cohort study. 2020, in preparation. 2020. in preparation. [Google Scholar]
- Küster, A.; Tea, I.; Ferchaud-Roucher, V.; Le Borgne, S.; Plouzennec, C.; Winer, N.; Rozé, J.-C.; Robins, R.J.; Darmaun, D.; Althabe, F. Cord Blood Glutathione Depletion in Preterm Infants: Correlation with Maternal Cysteine Depletion. PLoS ONE 2011, 6. [Google Scholar] [CrossRef]
- Chandyo, R.K.; Ulak, M.; Kvestad, I.; Shrestha, M.; Ranjitkar, S.; Basnet, S.; Hysing, M.; Shrestha, L.; Strand, T.A. The effects of vitamin B12 supplementation in pregnancy and postpartum on growth and neurodevelopment in early childhood: Study Protocol for a Randomized Placebo Controlled Trial. BMJ Open 2017, 7, e016434. [Google Scholar] [CrossRef]
- Windelberg, A.; Årseth, O.; Kvalheim, G.; Ueland, P.M. Automated Assay for the Determination of Methylmalonic Acid, Total Homocysteine, and Related Amino Acids in Human Serum or Plasma by Means of Methylchloroformate Derivatization and Gas Chromatography–Mass Spectrometry. Clin. Chem. 2005, 51, 2103–2109. [Google Scholar] [CrossRef]
- Kelleher, B.P.; Broin, S.D. Microbiological assay for vitamin B12 performed in 96-well microtitre plates. J. Clin. Pathol. 1991, 44, 592. [Google Scholar] [CrossRef] [PubMed]
- Molloy, A.M.; Scott, J.M. Microbiological assay for serum, plasma, and red cell folate using cryopreserved, microtiter plate method. Methods Enzymol. 1997, 281, 43–53. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHO Child Growth Standards. Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development; WHO Multicentre Growth Reference Study Group, World Health Organization: Geneva, Switzerland, 2006; p. 312. [Google Scholar]
- Psaki, S.; Seidman, J.; Miller, M.; Gottlieb, M.; Bhutta, Z.; Ahmed, T.; Ahmed, A.; Bessong, P.; John, S.; Kang, G.; et al. Measuring socioeconomic status in multicountry studies: Results from the eight-country MAL-ED study. Popul. Health Metr. 2014, 12. [Google Scholar] [CrossRef]
- Moon, P.-D.; Kim, M.-H.; Oh, H.-A.; Nam, S.-Y.; Han, N.-R.; Jeong, H.-J.; Kim, H.-M. Cysteine induces longitudinal bone growth in mice by upregulating IGF-1. Int. J. Mol. Med. 2015, 36, 571–576. [Google Scholar] [CrossRef] [PubMed]
- El-Khairy, L.; Vollset, S.E.; Refsum, H.; Ueland, P.M. Plasma total cysteine, pregnancy complications, and adverse pregnancy outcomes: The Hordaland Homocysteine Study. Am. J. Clin. Nutr. 2003, 77, 467–472. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S.; Sturdivant, R.X. Model-Building Strategies and Methods for Logistic Regression. In Applied Logistic Regression, 3rd ed.; Wiley: Hoboken, NJ, USA, 2013; pp. 89–151. [Google Scholar]
- Snyderman, S.E. The Protein and Animo Acid Requirements of the Premature Infant. In Metabolic Processes in the Foetus and Newborn Infant; Jonxis, J.H.P., Visser, H.K.A., Troelstra, J.A., Eds.; Springer: Dordrecht, The Netherlands, 1971; Volume 3. [Google Scholar]
- Pohlandt, F. Cystine: A semi-essential amino acid in the newborn infant. Acta Paediatr. 1974, 63, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Zlotkin, S.H.; Bryan, M.H.; Anderson, G.H. Cysteine supplementation to cysteine-free intravenous feeding regimens in newborn infants. Am. J. Clin. Nutr. 1981, 34, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, M.A.; Svardal, A.M.; Ueland, P.M. Determination of the in vivo redox status of cysteine, cysteinylglycine, homocysteine, and glutathione in human plasma. Anal. Biochem. 1992, 200, 218–229. [Google Scholar] [CrossRef]
- Andersson, A.; Isaksson, A.; Brattström, L.; Hultberg, B. Homocysteine and other thiols determined in plasma by HPLC and thiol-specific postcolumn derivatization. Clin. Chem. 1993, 39, 1590. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.; Lindgren, A.; Hultberg, B. Effect of thiol oxidation and thiol export from erythrocytes on determination of redox status of homocysteine and other thiols in plasma from healthy subjects and patients with cerebral infarction. Clin. Chem. 1995, 41, 361. [Google Scholar] [CrossRef] [PubMed]
- Ueland, P.M. Homocysteine species as components of plasma redox thiol status. Clin. Chem. 1995, 41, 340–342. [Google Scholar] [CrossRef] [PubMed]
- El-Khairy, L.; Ueland, P.M.; Nygård, O.; Refsum, H.; Vollset, S.E. Lifestyle and cardiovascular disease risk factors as determinants of total cysteine in plasma: The Hordaland Homocysteine Study. Am. J. Clin. Nutr. 1999, 70, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Dasarathy, J.; Gruca, L.L.; Bennett, C.; Parimi, P.S.; Duenas, C.; Marczewski, S.; Fierro, J.L.; Kalhan, S.C. Methionine metabolism in human pregnancy. Am. J. Clin. Nutr. 2010, 91, 357–365. [Google Scholar] [CrossRef]
- Hytten, F. Blood volume changes in normal pregnancy. Clin. Haematol. 1985, 14, 601–612. [Google Scholar] [CrossRef]
- Bhandari, S.; Sayami, J.T.; Thapa, P.; Sayami, M.; Kandel, B.P.; Banjara, M.R. Dietary intake patterns and nutritional status of women of reproductive age in Nepal: Findings from a health survey. Arch. Public Health 2016, 74, s13690-s016. [Google Scholar]
- Rogne, T.; Tielemans, M.J.; Chong, M.F.-F.; Yajnik, C.S.; Krishnaveni, G.V.; Poston, L.; Jaddoe, V.W.V.; Steegers, E.A.P.; Joshi, S.; Chong, Y.-S.; et al. Associations of Maternal Vitamin B12 Concentration in Pregnancy With the Risks of Preterm Birth and Low Birth Weight: A Systematic Review and Meta-Analysis of Individual Participant Data. Am. J. Epidemiol. 2017, 185, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Stipanuk, M.; Caudill, M. Biochemical, Physiological, and Molecular Aspects of Human Nutrition, 3rd ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Roberts, J.M.; Taylor, R.N.; Goldfien, A. Clinical and Biochemical Evidence of Endothelial Cell Dysfunction in the Pregnancy Syndrome Preeclampsia. Am. J. Hypertens. 1991, 4, 700–708. [Google Scholar] [CrossRef]
- Hultberg, B.; Andersson, A.; Arnadottir, M. Reduced, Free and Total Fractions of Homocysteine and Other Thiol Compounds in Plasma from Patients with Renal Failure. Nephron 1995, 70, 62–67. [Google Scholar] [CrossRef]
- Redman, C.W. Current topic: Pre-eclampsia and the placenta. Placenta 1991, 12, 301–308. [Google Scholar] [CrossRef]
- Eriksson, J.G.; Kajantie, E.; Osmond, C.; Thornburg, K.; Barker, D.J.P. Boys live dangerously in the womb. Am. J. Hum. Biol. 2010, 22, 330–335. [Google Scholar] [CrossRef]
- Chong, M.F.-F.; Chia, A.-R.; Colega, M.; Tint, M.-T.; Aris, I.M.; Chong, Y.-S.; Gluckman, P.; Godfrey, K.M.; Kwek, K.; Saw, S.-M.; et al. Maternal Protein Intake during Pregnancy Is Not Associated with Offspring Birth Weight in a Multiethnic Asian Population. J. Nutr. 2015, 145, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Alwasel, S.H.; Abotalib, Z.; Aljarallah, J.S.; Osmond, C.; Alkharaz, S.M.; Alhazza, I.M.; Harrath, A.; Thornburg, K.; Barker, D.J.P. Sex Differences in birth size and intergenerational effects of intrauterine exposure to Ramadan in Saudi Arabia. Am. J. Hum. Biol. 2011, 23, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Jahoor, F.; Badaloo, A.; Reid, M.; Forrester, T. Sulfur amino acid metabolism in children with severe childhood undernutrition: Cysteine kinetics. Am. J. Clin. Nutr. 2006, 84, 1393–1399. [Google Scholar] [CrossRef][Green Version]
- Badaloo, A.; Hsu, J.W.; Taylor-Bryan, C.; Green, C.; Reid, M.; Forrester, T.; Jahoor, F. Dietary cysteine is used more efficiently by children with severe acute malnutrition with edema compared with those without edema. Am. J. Clin. Nutr. 2012, 95, 84–90. [Google Scholar] [CrossRef]
- Elshorbagy, A.K.; Nurk, E.; Gjesdal, C.G.; Tell, G.S.; Ueland, P.M.; Nygard, O.; Tverdal, A.; Vollset, S.E.; Refsum, H. Homocysteine, cysteine, and body composition in the Hordaland Homocysteine Study: Does cysteine link amino acid and lipid metabolism? Am. J. Clin. Nutr. 2008, 88, 738. [Google Scholar] [CrossRef]
- Elshorbagy, A.; Refsum, H.; Smith, A.; Graham, I. The Association of Plasma Cysteine and γ-Glutamyltransferase With BMI and Obesity. Obesity 2009, 17, 1435–1440. [Google Scholar] [CrossRef]
- Elshorbagy, A.K.; Valdivia-Garcia, M.; Mattocks, D.A.L.; Plummer, J.D.; Smith, A.D.; Drevon, C.A.; Refsum, H.; Perrone, C.E. Cysteine supplementation reverses methionine restriction effects on rat adiposity: Significance of stearoyl-coenzyme A desaturase. J. Lipid Res. 2011, 52, 104. [Google Scholar] [CrossRef]
- Elshorbagy, A.K.; Kozich, D.V.; Smith, D.A.; Refsum, H. Cysteine and Obesity: Consistency of the Evidence Across Epidemiologic, Animal and Cellular Studies. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 49–57. [Google Scholar] [CrossRef]
- Elshorbagy, A.K.; Valdivia-Garcia, M.; Refsum, H.; Butte, N. The Association of Cysteine with Obesity, Inflammatory Cytokines and Insulin Resistance in Hispanic Children and Adolescents. PLoS ONE 2012, 7, e44166. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health—Nepal; New ERA; ICF. Nepal Demographic and Health Survey 2016; Ministry of Health: Kathmandu, Nepal, 2017.
- Jones, D.P.; Park, Y.; Gletsu-Miller, N.; Liang, Y.; Yu, T.; Accardi, C.J.; Ziegler, T.R. Dietary sulfur amino acid effects on fasting plasma cysteine/cystine redox potential in humans. Nutrition 2011, 27, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Blanco, R.A.; Ziegler, T.R.; Carlson, B.A.; Cheng, P.-Y.; Park, Y.; Cotsonis, G.A.; Accardi, C.J.; Jones, D.P. Diurnal variation in glutathione and cysteine redox states in human plasma. Am. J. Clin. Nutr. 2007, 86, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Ziegler, T.R.; Gletsu-Miller, N.; Liang, Y.; Yu, T.; Accardi, C.J.; Jones, D.P. Postprandial cysteine/cystine redox potential in human plasma varies with meal content of sulfur amino acids. J. Nutr. 2010, 140, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Elshorbagy, A.K.; Nijpels, G.; Valdivia-Garcia, M.; Stehouwer, C.D.A.; Ocke, M.; Refsum, H.; Dekker, J.M. S-Adenosylmethionine Is Associated with Fat Mass and Truncal Adiposity in Older Adults. J. Nutr. 2013, 143, 1982–1988. [Google Scholar] [CrossRef]
| Maternal Characteristics (n = 561) | Estimate |
|---|---|
| Mean age (SD), years | 27.5 (3.8) |
| Mean gestational age (SD) at enrollment by LMP 1, weeks | 10.2 (3.0) |
| Mean weight (SD), kg | 55.3 (7.7) |
| Mean height (SD), cms | 152.8 (5.3) |
| Mean BMI (SD), kg/m2 | 23.7 (3.0) |
| Nutritional status, % (n) Underweight (BMI < 18.5 kg/m2) Normal weight (BMI ≥ 18.5 and < 25 kg/m2) Overweight (BMI ≥ 25 and < 30 kg/m2) Obese (BMI ≥ 30 kg/m2) | 1.3 (7) 65.0 (365) 33.0 (185) 0.7 (4) |
| Parity, % (n) 0 ≥1 | 48.0 (269) 52.0 (292) |
| Mean education (SD), years | 11.0 (3.5) |
| Mean tCys concentration (SD), μmol/L | 207.3 (24.6) |
| Median plasma folate concentration (IQR), nmol/L | 57.3 (33.0–76.4) |
| Mean plasma cobalamin concentration (SD), pmol/L | 204.5 (78.5) |
| Mean WAMI-index score (SD) 2 | 0.65 (0.14) |
| Infant characteristics (n = 521) 3 | |
| Gender, % (n) Male | 53.6 (279) |
| Mean birth weight (SD) measured at hospital, g | 3009 (428) |
| Median age (IQR) for assessment at birth, days | 3 (2–5) |
| Median age (IQR) for assessment at six months 4, days | 182 (181–184) |
| Mean LAZ score (SD) at birth | −0.85 (1.07) |
| Mean LAZ score (SD) at six months 4 | −0.56 (0.90) |
| Mean WLZ score (SD) at birth 5 | −0.80 (1.11) |
| Mean WLZ score (SD) at six months 4 | 0.26 (1.03) |
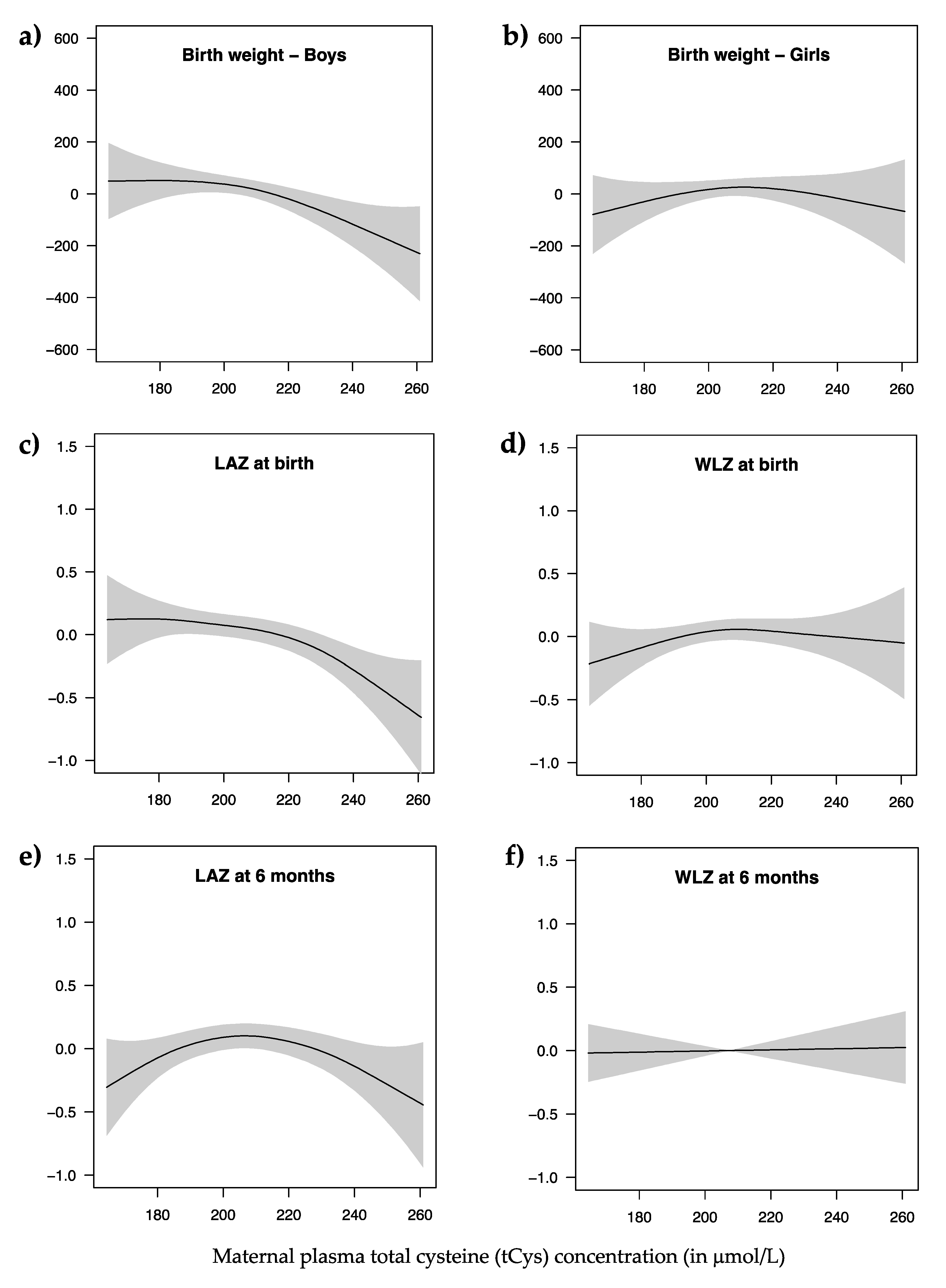
| Anthropometric Indices | n | Crude β-coefficients (95% CI) for tCys | Adjusted β-coefficients (95% CI) for tCys |
|---|---|---|---|
| Birth weight 1, g | 521 | −1.072 (−2.579, 0.434) | - |
| Boys | - | −2.611 (−4.547, −0.676) * | |
| Girls | - | 0.502 (−1.792, 2.796) | |
| LAZ score at birth | 521 | −0.005 (−0.009, −0.001) ** | −0.005 (−0.009, −0.001) ** |
| WLZ score at birth 2 | 503 | 0.003 (−0.001, 0.007) | 0.003 (−0.002, 0.007) |
| LAZ score at six months 3 | 376 | −0.002 (−0.006, 0.001) | −0.001 (−0.005, 0.003) |
| WLZ score at six months 4 | 376 | 0.002 (−0.002, 0.006) | 0.001 (−0.003, 0.005) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arora, N.; Strand, T.A.; Chandyo, R.K.; Elshorbagy, A.; Shrestha, L.; Ueland, P.M.; Ulak, M.; Schwinger, C. Association of Maternal Plasma Total Cysteine and Growth among Infants in Nepal: A Cohort Study. Nutrients 2020, 12, 2849. https://doi.org/10.3390/nu12092849
Arora N, Strand TA, Chandyo RK, Elshorbagy A, Shrestha L, Ueland PM, Ulak M, Schwinger C. Association of Maternal Plasma Total Cysteine and Growth among Infants in Nepal: A Cohort Study. Nutrients. 2020; 12(9):2849. https://doi.org/10.3390/nu12092849
Chicago/Turabian StyleArora, Nikhil, Tor A. Strand, Ram K. Chandyo, Amany Elshorbagy, Laxman Shrestha, Per M. Ueland, Manjeswori Ulak, and Catherine Schwinger. 2020. "Association of Maternal Plasma Total Cysteine and Growth among Infants in Nepal: A Cohort Study" Nutrients 12, no. 9: 2849. https://doi.org/10.3390/nu12092849
APA StyleArora, N., Strand, T. A., Chandyo, R. K., Elshorbagy, A., Shrestha, L., Ueland, P. M., Ulak, M., & Schwinger, C. (2020). Association of Maternal Plasma Total Cysteine and Growth among Infants in Nepal: A Cohort Study. Nutrients, 12(9), 2849. https://doi.org/10.3390/nu12092849





