Purified Gymnemic Acids from Gymnema inodorum Tea Inhibit 3T3-L1 Cell Differentiation into Adipocytes
Abstract
:1. Introduction
2. Materials and Methods
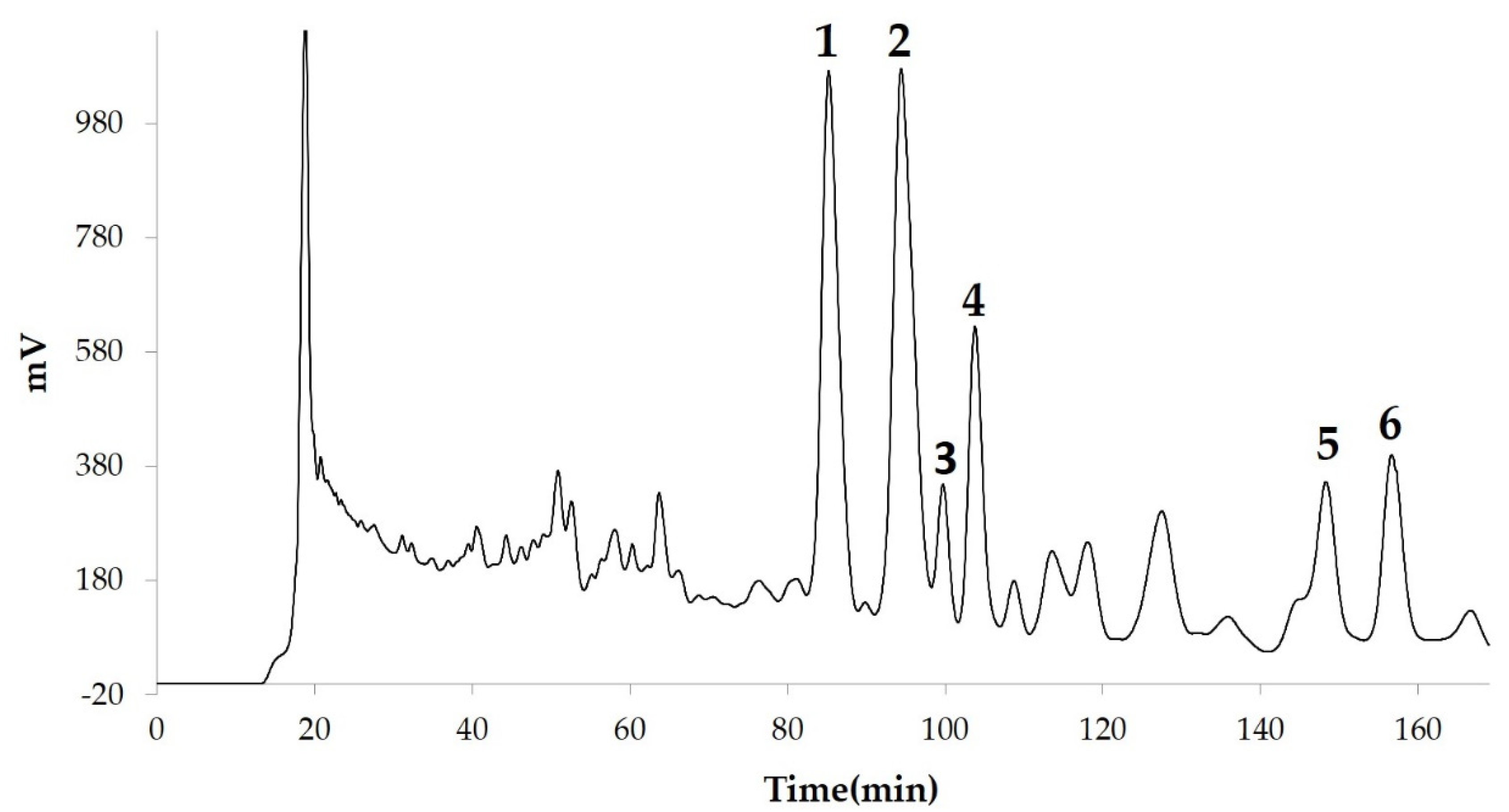
2.1. Extraction, Isolation and Purification
2.2. Mass Spectrometry
2.3. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.4. Cell Culture
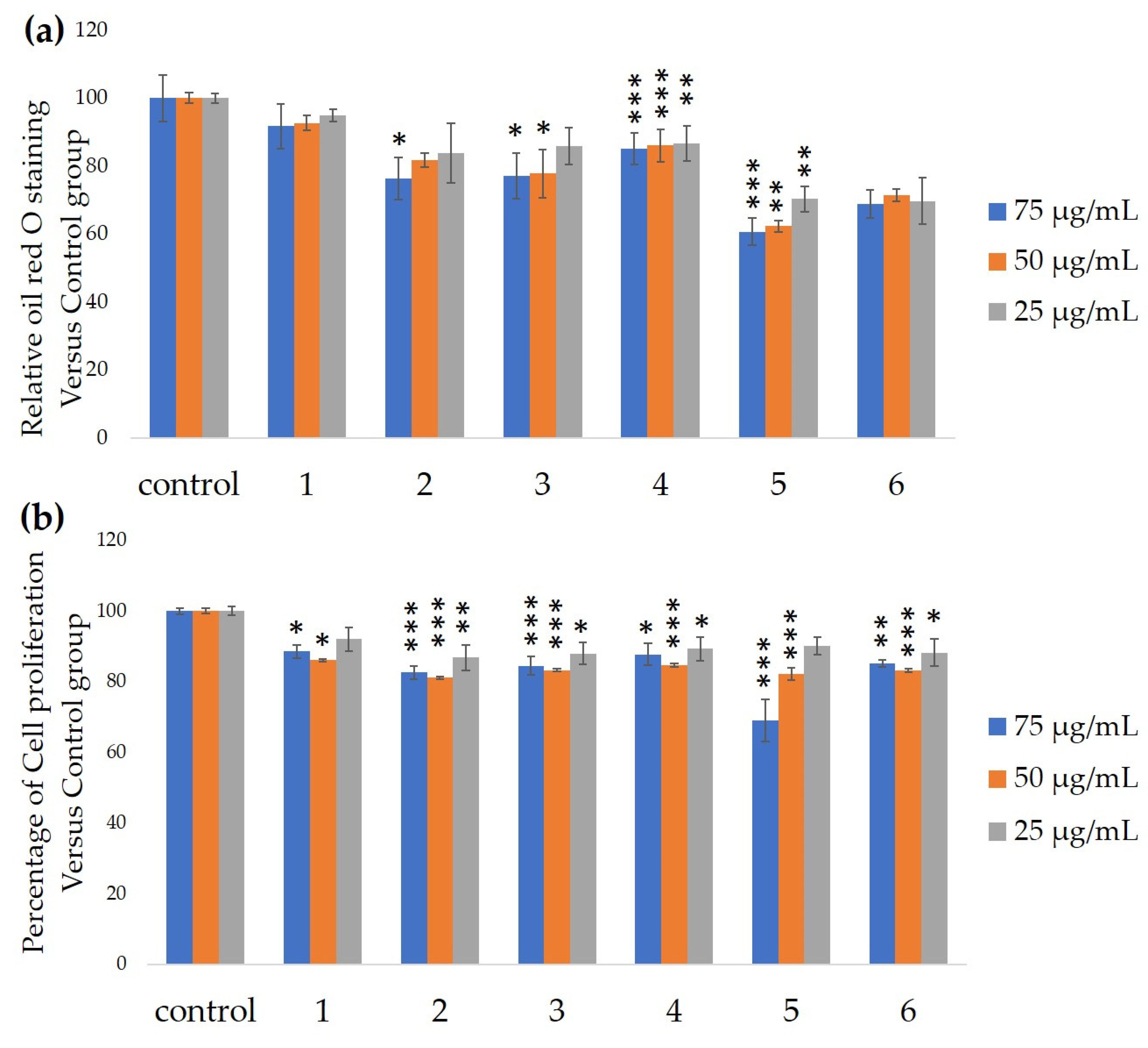
2.5. The Antiadipocyte Differentiation Activity
2.6. Quantitative Real-Time PCR
2.7. Statistical Analysis
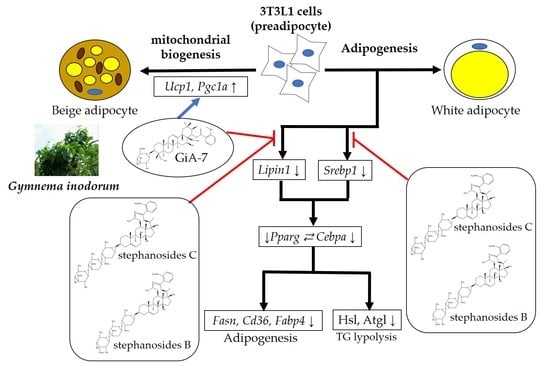
3. Results and Discussion
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shanmugasundaram, E.; Rajeswari, G.; Baskaran, K.; Kumar, B.R.; Shanmugasundaram, K.R.; Ahmath, B.K. Use of Gymnema sylvestre leaf extract in the control of blood glucose in insulin-dependent diabetes mellitus. J. Ethnopharmacol. 1990, 30, 281–294. [Google Scholar] [CrossRef]
- Shimizu, K.; Iino, A.; Nakajima, J.; Tanaka, K.; Nakajyo, S.; Urakawa, N.; Atsuchi, M.; Wada, T.; Yamashita, C. Suppression of glucose absorption by some fractions extracted from Gymnema sylvestre leaves. J. Vet. Med. Sci. 1997, 59, 245–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Romaiyan, A.; King, A.; Persaud, S.; Jones, P. A novel extract of Gymnema sylvestre improves glucose tolerance in vivo and stimulates insulin secretion and synthesis in vitro. Phytother. Res. 2013, 27, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, R.; Kathori, S.; Upadhyay, B. Effect of Gurmar (Gymnema sylvestre) powder intervention on the blood glucose levels among diabetics. Stud. Ethno-Med. 2009, 3, 133–135. [Google Scholar] [CrossRef]
- Kanetkar, P.; Singhal, R.; Kamat, M. Recent advances in indian herbal drug research guest editor: Thomas Paul Asir Devasagayam Gymnema sylvestre: A memoir. J. Clin. Biochem. Nutr. 2007, 41, 77–81. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, K.; Ozeki, M.; Iino, A.; Nakajyo, S.; Urakawa, N.; Atsuchi, M. Structure-activity relationships of triterpenoid derivatives extracted from Gymnema inodorum leaves on glucose absorption. Jpn. J. Pharmacol. 2001, 86, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Chiabchalard, A.; Tencomnao, T.; Santiyanont, R. Effect of Gymnema inodorum on postprandial peak plasma glucose levels in healthy human. Afr. J. Biotechnol. 2010, 9, 1079–1085. [Google Scholar]
- Shimizu, K.; Ozeki, M.; Tanaka, K.; Itoh, K.; Nakajyo, S.; Urakawa, N.; Atsuchi, M. Suppression of glucose absorption by extracts from the leaves of Gymnema inodorum. J. Vet. Med. Sci. 1997, 59, 753–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klungsupya, P.; Muangman, T.; Theangtrong, N.; Khayungarnnawee, A.; Phatvej, W.; Thisayakorn, K.; Rerk-Am, U.; Sematong, T.; Trangvacharakul, S.; Arunpairojana, V. Antioxidant and antihyperglycemic activities of Gymnema inodorum Dence. In Proceedings of the 8th NRCT-JSPS Joint Seminar Innovative Research in Natural Products for Sustainable Development, Bangkok, Thailand, 3–5 February 2009; pp. 207–209. [Google Scholar]
- Rohilla, A.; Ali, S. Alloxan induced diabetes: Mechanisms and effects. Int. J. Res. Pharm. Biomed. Sci. 2012, 3, 819–823. [Google Scholar]
- Risérus, U.; Willett, W.C.; Hu, F.B. Dietary fats and prevention of type 2 diabetes. Prog. Lipid Res. 2009, 48, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Zeyda, M.; Stulnig, T.M. Obesity, inflammation, and insulin resistance—A mini-review. Gerontology 2009, 55, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Farmer, S.R. Molecular determinants of brown adipocyte formation and function. Genes Dev. 2008, 22, 1269–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, E.D.; MacDougald, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Green, H.; Meuth, M. An established pre-adipose cell line and its differentiation in culture. Cell 1974, 3, 127–133. [Google Scholar] [CrossRef]
- Rubin, C.S.; Hirsch, A.; Fung, C.; Rosen, O.M. Development of hormone receptors and hormonal responsiveness in vitro. Insulin receptors and insulin sensitivity in the preadipocyte and adipocyte forms of 3T3-L1 cells. J. Biol. Chem. 1978, 253, 7570–7578. [Google Scholar]
- Taira, J.; Ogi, T. Induction of Antioxidant Protein HO-1 Through Nrf2-ARE Signaling Due to Pteryxin in Peucedanum Japonicum Thunb in RAW264. 7 Macrophage Cells. Antioxidants 2019, 8, 621. [Google Scholar] [CrossRef] [Green Version]
- Oishi, K.; Ohyama, S.; Higo-Yamamoto, S. Chronic sleep disorder induced by psychophysiological stress induces glucose intolerance without adipose inflammation in mice. Biochem. Biophys. Res. Commun. 2018, 495, 2616–2621. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshikawa, K.; Okada, N.; Kann, Y.; Arihara, S. Steroidal glycosides from the fresh stem of Stephanotis lutchuensis var. japonica (Asclepiadaceae). Chemical structures of stephanosides AJ. Chem. Pharm. Bull. 1996, 44, 1790–1796. [Google Scholar] [CrossRef] [Green Version]
- Drolet, R.; Richard, C.; Sniderman, A.D.; Mailloux, J.; Fortier, M.; Huot, C.; Rheaume, C.; Tchernof, A. Hypertrophy and hyperplasia of abdominal adipose tissues in women. Int. J. Obes. (Lond.) 2008, 32, 283–291. [Google Scholar] [CrossRef] [Green Version]
- Xie, M.; Roy, R. AMP-Activated Kinase Regulates Lipid Droplet Localization and Stability of Adipose Triglyceride Lipase in C. elegans Dauer Larvae. PLoS ONE 2015, 10, e0130480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, D.; Wang, D.; Zhuang, X.; Wang, Z.; Ni, Y.; Chen, S.; Sun, F. Berberine increases adipose triglyceride lipase in 3T3-L1 adipocytes through the AMPK pathway. Lipids Health Dis. 2016, 15, 214. [Google Scholar] [CrossRef] [Green Version]
- Deng, T.; Shan, S.; Li, P.P.; Shen, Z.F.; Lu, X.P.; Cheng, J.; Ning, Z.Q. Peroxisome proliferator-activated receptor-gamma transcriptionally up-regulates hormone-sensitive lipase via the involvement of specificity protein-1. Endocrinology 2006, 147, 875–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kershaw, E.E.; Schupp, M.; Guan, H.-P.; Gardner, N.P.; Lazar, M.A.; Flier, J.S. PPARγ regulates adipose triglyceride lipase in adipocytes in vitro and in vivo. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1736–E1745. [Google Scholar] [CrossRef] [Green Version]
- Sembongi, H.; Miranda, M.; Han, G.S.; Fakas, S.; Grimsey, N.; Vendrell, J.; Carman, G.M.; Siniossoglou, S. Distinct roles of the phosphatidate phosphatases lipin 1 and 2 during adipogenesis and lipid droplet biogenesis in 3T3-L1 cells. J. Biol. Chem. 2013, 288, 34502–34513. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.E.; Bae, E.; Jeong, D.-Y.; Kim, M.-J.; Jin, W.-J.; Park, S.-W.; Han, G.-S.; Carman, G.M.; Koh, E.; Kim, K.-S. Lipin1 regulates PPARγ transcriptional activity. Biochem. J. 2013, 453, 49–60. [Google Scholar] [CrossRef]
- Zhang, P.; Takeuchi, K.; Csaki, L.S.; Reue, K. Lipin-1 phosphatidic phosphatase activity modulates phosphatidate levels to promote peroxisome proliferator-activated receptor gamma (PPARgamma) gene expression during adipogenesis. J. Biol. Chem. 2012, 287, 3485–3494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, J.; Peterfy, M.; Reue, K. Lipin expression preceding peroxisome proliferator-activated receptor-gamma is critical for adipogenesis in vivo and in vitro. J. Biol. Chem. 2004, 279, 29558–29564. [Google Scholar] [CrossRef] [Green Version]
- Puigserver, P.; Spiegelman, B.M. Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α): Transcriptional coactivator and metabolic regulator. Endocr. Rev. 2003, 24, 78–90. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, M.; Fujii, N.; Narita, T.; Higami, Y. SREBP-1c-Dependent Metabolic Remodeling of White Adipose Tissue by Caloric Restriction. Int. J. Mol. Sci. 2018, 19, 3335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharp, L.Z.; Shinoda, K.; Ohno, H.; Scheel, D.W.; Tomoda, E.; Ruiz, L.; Hu, H.; Wang, L.; Pavlova, Z.; Gilsanz, V. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS ONE 2012, 7, e49452. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Bostrom, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannon, B.; Nedergaard, J. Neither brown nor white. Nature 2012, 488, 286–287. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, Y.; Nojima, H.; Matsuda, H.; Murakami, T.; Yoshikawa, M.; Kimura, I. Antihyperglycemic effects of gymnemic acid IV, a compound derived from Gymnema sylvestre leaves in streptozotocin-diabetic mice. J. Asian Nat. Prod. Res. 2000, 2, 321–327. [Google Scholar] [CrossRef]
- Wang, L.; Luo, H.; Miyoshi, M.; Imoto, T.; Hiji, Y.; Sasaki, T. Inhibitory effect of gymnemic acid on intestinal absorption of oleic acid in rats. Can. J. Physiol. Pharmacol. 1998, 76, 1017–1023. [Google Scholar] [CrossRef]
- Liu, H.-M.; Kiuchi, F.; Tsuda, Y. Isolation and structure elucidation of gymnemic acids, antisweet principles of Gymnema sylvestre. Chem. Pharm. Bull. 1992, 40, 1366–1375. [Google Scholar] [CrossRef] [Green Version]
- Asare-Anane, H.; Huang, G.; Amiel, S.; Jones, P.; Persaud, S. Stimulation of insulin secretion by an aqueous extract of Gymnema sylvestre: Role of intracellular calcium. In Proceedings of the 196th Meeting of the Society for Endocrinology and Society for Endocrinology Joint Endocrinology and Diabetes Day, London, UK, 7–9 November 2005. [Google Scholar]
- Libman, A.; Zhang, H.; Ma, C.; Southavong, B.; Sydara, K.; Bouamanivong, S.; Tan, G.T.; Fong, H.H.; Soejarto, D.D. A first new antimalarial pregnane glycoside from Gongronema napalense. Asian J. Tradit. Med. 2008, 3, 203. [Google Scholar]



| Target Gene | Direction | Primer Sequence (5′–3′) |
|---|---|---|
| Pparγ | Forward | AACTCTGGGAGATTCTCCTGTTGA |
| Reverse | TGGTAATTTCTTGTGAAGTGCTCATA | |
| Fasn | Forward | GGAGGTGGTGATAGCCGGTAT |
| Reverse | TGGGTAATCCATAGAGCCCAG | |
| Cebpα | Forward | AAGAAGTCGGTGGACAAGAACAG |
| Reverse | GTTGCGTTGTTTGGCTTTATCTC | |
| Pgc1α | Forward | GTAGGCCCAGGTACGACAGC |
| Reverse | GCTCTTTGCGGTATTCATCCC | |
| Lipin-1 | Forward | CCATAGAGATGAGCTCGGAT |
| Reverse | AACTGGGATACGATGCTGACT | |
| Atgl | Forward | CTTGAGCAGCTAGAACAATG |
| Reverse | GGACACCTCAATAATGTTGGC | |
| Hsl | Forward | GCTGGAGGAGTGTTTTTTTGC |
| Reverse | AGTTGAACCAAGCAGGTCACA | |
| Srebp-1c | Forward | ATCGGCGCGGAAGCTGTCGGGGTAGCGTC |
| Reverse | ACTGTCTTGGTTGTTGATGAGCTGGAGCAT | |
| Glut4 | Forward | CTGTCGCTGGTTTCTCCAAC |
| Reverse | CAGGAGGACGGCAAATAGAA | |
| Ucp1 | Forward | GGCAACAAGAGCTGACAGTAAAT |
| Reverse | GGCCCTTGTAAACAACAAAATAC | |
| Fabp4 | Forward | CCGCAGACGACAGGA |
| Reverse | CTCATGCCCTTTCATAAACT | |
| 36b4 | Forward | CTTCATTGTGGGAGCAGACA |
| Reverse | TCTCCAGAGCTGGGTTGTTC |
| C-No. | Carbon Type | GiA-7 | Compound 2 |
|---|---|---|---|
| 1 | —CH2— | 39.7 | 39.7 |
| 2 | —CH2— | 26.2 | 26.4 |
| 3 | >CH—O— | 82.3 | 82.9 |
| 4 | >C< | 43.9 | 44.0 |
| 5 | >CH— | 48.1 | 48.1 |
| 6 | —CH2— | 18.8 | 18.9 |
| 7 | —CH2— | 33.2 | 33.2 |
| 8 | >C< | 41.2 | 41.3 |
| 9 | >CH— | 48.2 | 48.2 |
| 10 | >C< | 37.5 | 37.5 |
| 11 | —CH2— | 24.8 | 24.8 |
| 12 | —CH= | 124.9 | 125.0 |
| 13 | >C= | 142.8 | 142.8 |
| 14 | >C< | 43.9 | 44.0 |
| 15 | —CH2— | 37.0 | 37.0 |
| 16 | >CH—O— | 66.8 | 66.8 |
| 17 | >C< | 46.5 | 46.5 |
| 18 | >CH— | 44.9 | 44.9 |
| 19 | —CH2— | 47.1 | 47.1 |
| 20 | >C< | 33.0 | 33.1 |
| 21 | —CH2— | 39.9 | 39.9 |
| 22 | >CH— | 74.3 | 74.3 |
| 23 | —CH2—O— | 64.8 | 64.7 |
| 24 | —CH3 | 13.4 | 13.4 |
| 25 | —CH3 | 16.7 | 16.7 |
| 26 | —CH3 | 17.5 | 17.5 |
| 27 | —CH3 | 28.0 | 28.0 |
| 28 | —CH2—O— | 61.2 | 61.1 |
| 29 | —CH3 | 33.5 | 33.5 |
| 30 | —CH3 | 25.6 | 25.6 |
| O-NMAt | |||
| N1 | >C=O | 169.6 | 169.7 |
| N2 | >C= | 112.1 | 112.1 |
| N3 | >C= | 153.0 | 153.0 |
| N4 | —CH= | 111.9 | 112.0 |
| N5 | —CH= | 135.6 | 135.6 |
| N6 | —CH= | 115.3 | 115.4 |
| N7 | —CH= | 133.0 | 133.0 |
| N8 | —CH3 | 29.7 | 29.7 |
| β-glu | |||
| 1’ | —O—CH—O— | 105.3 | 105.7 |
| 2’ | >CH—O— | 75.0 | 75.2 |
| 3’ | >CH—O— | 78.0 | 78.0 |
| 4’ | >CH—O— | 73.5 | 73.5 |
| 5’ | >CH—O— | 76.6 | 78.0 |
| 6’ | —COO— |
| C-No. | Carbon Type | Stephanoside C | Compound 5 |
|---|---|---|---|
| 1 | —CH2— | 38.9 | 38.9 |
| 2 | —CH2— | 30.0 | 30.0 |
| 3 | >CH—O— | 77.8 | 77.7 |
| 4 | —CH2— | 39.3 | 39.4 |
| 5 | >C= | 139.3 | 139.4 |
| 6 | —CH= | 119.5 | 119.5 |
| 7 | —CH2— | 35.0 | 35.0 |
| 8 | >C< | 74.4 | 74.4 |
| 9 | >CH— | 44.1 | 44.2 |
| 10 | >C< | 37.3 | 37.4 |
| 11 | —CH2— | 25.7 | 25.7 |
| 12 | >CH— | 74.7 | 74.6 |
| 13 | >C< | 57.0 | 57.0 |
| 14 | >C< | 89.0 | 89.0 |
| 15 | —CH2— | 33.8 | 33.8 |
| 16 | —CH2— | 34.0 | 34.0 |
| 17 | >C< | 87.7 | 87.7 |
| 18 | —CH3 | 11.4 | 11.4 |
| 19 | —CH3 | 18.1 | 18.1 |
| 20 | >CH—O— | 75.0 | 75.0 |
| 21 | —CH3 | 15.6 | 15.7 |
| 12-O-Acetyl moiety | |||
| A1 | —COO— | 171.5 | 171.4 |
| A2 | —CH3 | 22.1 | 22.1 |
| 20-O-N-Methylanthraniloyl moiety | |||
| N1 | —COO— | 111.0 | 111.1 |
| N2 | >C= | 152.7 | 152.7 |
| N3 | >C= | 111.6 | 111.6 |
| N4 | CH= | 135.1 | 135.2 |
| N5 | —CH= | 114.8 | 114.8 |
| N6 | —CH= | 132.7 | 132.7 |
| N7 | —CH= | 168.3 | 168.3 |
| N8 | —CH3 | 29.7 | 29.6 |
| C-No. | Carbon Type | Stephanoside C | Compound 5 |
|---|---|---|---|
| d-Cymarose | |||
| 1′ | —O—CH—O— | 96.5 | 96.5 |
| 2′ | —CH2— | 37.3 | 37.4 |
| 3′ | >CH—O— | 78.0 | 78.0 |
| 4′ | >CH—O— | 83.5 | 83.5 |
| 5′ | >CH—O— | 69.1 | 69.1 |
| 6′ | —CH3 | 18.7 | 18.7 |
| O-Me | —O—CH3 | 59.0 | 59.0 |
| d-Olenadrose | |||
| 1′′ | —O—CH—O— | 102.2 | 102.1 |
| 2′′ | —CH2— | 37.7 | 37.8 |
| 3′′ | >CH—O— | 79.3 | 79.3 |
| 4′′ | >CH—O— | 83.2 | 83.2 |
| 5′′ | >CH—O— | 72.1 | 72.2 |
| 6′′ | —CH3 | 19.0 | 19.0 |
| O-Me | —O—CH3 | 57.4 | 57.4 |
| d-Thevetose | |||
| 1′′′ | ―O—CH—O— | 104.2 | 104.3 |
| 2′′′ | >CH—O— | 75.3 | 75.4 |
| 3′′′ | >CH—O— | 88.2 | 88.3 |
| 4′′′ | >CH—O— | 76.1 | 76.1 |
| 5′′′ | >CH—O— | 72.9 | 73.0 |
| 6′′′ | —CH3 | 18.8 | 18.8 |
| O-Me | —O—CH3 | 61.1 | 61.1 |
| C-No. | Carbon Type | Stephanoside B | Compound 6 |
|---|---|---|---|
| 1 | —CH2— | 38.8 | 38.8 |
| 2 | —CH2— | 30.0 | 29.9 |
| 3 | >CH—O— | 77.7 | 77.6 |
| 4 | —CH2— | 39.3 | 39.2 |
| 5 | >C= | 139.3 | 139.2 |
| 6 | —CH= | 119.4 | 119.4 |
| 7 | —CH2— | 34.9 | 34.9 |
| 8 | >C< | 74.3 | 74.3 |
| 9 | >CH— | 44.1 | 44.0 |
| 10 | >C< | 37.3 | 37.2 |
| 11 | —CH2— | 25.6 | 25.6 |
| 12 | >CH— | 74.6 | 74.6 |
| 13 | >C< | 56.9 | 56.9 |
| 14 | >C< | 88.9 | 88.9 |
| 15 | —CH2— | 33.8 | 33.7 |
| 16 | —CH2— | 33.9 | 33.9 |
| 17 | >C< | 87.6 | 87.6 |
| 18 | —CH3 | 11.3 | 11.3 |
| 19 | —CH3 | 18.1 | 18.0 |
| 20 | >CH—O— | 74.9 | 74.9 |
| 21 | —CH3 | 15.6 | 15.6 |
| 12-O-Acetyl moiety | |||
| A1 | —COO— | 171.3 | 171.3 |
| A2 | —CH3 | 22.0 | 22.1 |
| 20-O-N-Methylanthraniloyl moiety | |||
| N1 | —COO— | 111.0 | 111.0 |
| N2 | >C= | 152.6 | 152.6 |
| N3 | >C= | 111.5 | 111.5 |
| N4 | —CH= | 135.1 | 135.1 |
| N5 | —CH= | 114.7 | 114.7 |
| N6 | —CH= | 132.6 | 132.6 |
| N7 | —CH= | 168.2 | 168.2 |
| N8 | —CH2 | 29.6 | 29.5 |
| C-No. | Carbon Type | Stephanoside B | Compound 6 |
|---|---|---|---|
| d-Cymarose | |||
| 1′ | —O—CH—O— | 96.4 | 96.4 |
| 2′ | —CH2— | 37.3 | 37.2 |
| 3′ | >CH—O— | 77.9 | 77.9 |
| 4′ | >CH—O— | 83.5 | 83.5 |
| 5′ | >CH—O— | 69.0 | 68.9 |
| 6′ | —CH3 | 18.7 | 18.7 |
| O—Me | —O—CH3 | 58.9 | 58.8 |
| d-Olenadrose | |||
| 1′′ | —O—CH—O— | 101.9 | 101.9 |
| 2′′ | —CH2— | 37.5 | 37.6 |
| 3′′ | >CH—O— | 79.3 | 79.2 |
| 4′′ | >CH—O— | 82.8 | 82.9 |
| 5′′ | >CH—O— | 72.0 | 72.0 |
| 6′′ | —CH3 | 19.0 | 18.9 |
| O-Me | —O—CH3 | 57.2 | 57.2 |
| d-Allomethylose | |||
| 1‴ | —O—CH—O— | 102.2 | 102.1 |
| 2‴ | >CH—O— | 73.2 | 73.3 |
| 3‴ | >CH—O— | 84.0 | 84.1 |
| 4‴ | >CH—O— | 74.6 | 74.5 |
| 5‴ | >CH—O— | 71.0 | 71.0 |
| 6‴ | —CH3 | 18.7 | 18.7 |
| O-Me | —O—CH3 | 62.1 | 62.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saiki, P.; Kawano, Y.; Ogi, T.; Klungsupya, P.; Muangman, T.; Phantanaprates, W.; Kongchinda, P.; Pinnak, N.; Miyazaki, K. Purified Gymnemic Acids from Gymnema inodorum Tea Inhibit 3T3-L1 Cell Differentiation into Adipocytes. Nutrients 2020, 12, 2851. https://doi.org/10.3390/nu12092851
Saiki P, Kawano Y, Ogi T, Klungsupya P, Muangman T, Phantanaprates W, Kongchinda P, Pinnak N, Miyazaki K. Purified Gymnemic Acids from Gymnema inodorum Tea Inhibit 3T3-L1 Cell Differentiation into Adipocytes. Nutrients. 2020; 12(9):2851. https://doi.org/10.3390/nu12092851
Chicago/Turabian StyleSaiki, Papawee, Yasuhiro Kawano, Takayuki Ogi, Prapaipat Klungsupya, Thanchanok Muangman, Wimonsri Phantanaprates, Papitchaya Kongchinda, Nantaporn Pinnak, and Koyomi Miyazaki. 2020. "Purified Gymnemic Acids from Gymnema inodorum Tea Inhibit 3T3-L1 Cell Differentiation into Adipocytes" Nutrients 12, no. 9: 2851. https://doi.org/10.3390/nu12092851
APA StyleSaiki, P., Kawano, Y., Ogi, T., Klungsupya, P., Muangman, T., Phantanaprates, W., Kongchinda, P., Pinnak, N., & Miyazaki, K. (2020). Purified Gymnemic Acids from Gymnema inodorum Tea Inhibit 3T3-L1 Cell Differentiation into Adipocytes. Nutrients, 12(9), 2851. https://doi.org/10.3390/nu12092851