Healthy Eating Index and Nutrition Biomarkers among Army Soldiers and Civilian Control Group Indicate an Intervention Is Necessary to Raise Omega-3 Index and Vitamin D and Improve Diet Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participant Recruitment
2.2. Dietary Intake
2.3. Control Group of Normative NHANES Match
2.4. Biochemical Markers
2.5. Statistical Analysis
3. Results
3.1. Participants
3.2. Healthy Eating Index (HEI)
3.3. Biochemical Markers
4. Discussion
4.1. Healthy Eating Index
4.2. Biochemical Markers
4.3. DoD Programs to Consider
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
Appendix A
| Biochemical Marker (Units) | Reference Value/Range | Participants (n) | Army Soldiers Median (25–75% Range) | NHANES Match Median (25–75% Range) | Effect Size |
|---|---|---|---|---|---|
| Total Cholesterol (mg/dL) | <200 | 420 | 177 (163–181) | 170 (161–189) | −0.047 |
| LDL (mg/dL) | <100 | 420 | 92.7 (79.6–106.3) | 98 (90–118) | 0.160 * |
| HDL (mg/dL) | |||||
| Males | 40–50 | 319 | 53 (47–60) | 47 (45–49) | −0.360 * |
| Females | 50–59 | 101 | 59 (54–69) | 58 (55–60) | −0.250 * |
| Triglycerides (mg/dL) | 40–200 | 471 | 89 (64–128) | 87 (66–101) | −0.107 * |
| Folate (ng/mL) | 2–20 | 421 | 13.2 (12.9–13.6) | 14.3 (13.1–15.0) | −0.144 * |
| Glucose (mg/dL) | <100 | 420 | 93.2 (93–95.7) | 97 (96–100) | −0.213 * |
| Hemoglobin (g/dL) | |||||
| Males | 14–18 | 318 | 15.6 (15.0–16.6) | 15.4 (15.2–15.4) | −0.278 * |
| Females | 12–16 | 87 | 13.9 (12.8–14.6) | 13.4 (13.2–13.4) | −0.262 * |
| Hematocrit (%) | |||||
| Males | 38–54 | 332 | 47 (44–49) | 45.5 (45.2–45.8) | −0.246 * |
| Females | 36–47 | 90 | 41 (39–43) | 40 (39.8–40.2) | −0.224 * |
| Ferritin (ng/mL) | |||||
| Males | 12–300 | 101 | 97.5 (71.2–154.3) | NA | |
| Females | 12–150 | 45 | 40.7 (27.0–74.3) | 40 (32–43) | −0.141 |
| Iron (mcg/dL) | 60–160 | 199 | 109 (77–134) | 90 (82–93) | −0.2677 * |
| hsCRP (mg/L) | 0–3 | 440 | 0.8 (0.4–1.8) | 1.2 (0.8–1.6) | −0.043 |
| Vitamin D (ng/mL) | 20–40 | 421 | 25.9 (25.7–28.2) | NA | |
| Omega-3 Index (%) | 0–12 | 384 | 3.8 | NA |
References
- Todhunter, E.N. School feeding from a nutritionist’s point of view. Am. J. Public Health Nations Health 1970, 60, 2302–2306. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Guzman, C.M.; Bray, R.M.; Forman-Hoffman, V.L.; Williams, J. Overweight and obesity trends among active duty military personnel: A 13-year perspective. Am. J. Prev Med. 2015, 48, 145–153. [Google Scholar] [CrossRef]
- Gwin, J.A.; Karl, J.P.; Lutz, L.J.; Gaffney-Stomberg, E.; McClung, J.P.; Pasiakos, S.M. Higher Protein Density Diets Are Associated With Greater Diet Quality and Micronutrient Intake in Healthy Young Adults. Front. Nutr. 2019, 6, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United States Department of Health and Human Services. 2015–2020. Dietary Guidelines for Americans. Available online: https://health.gov/our-work/food-and-nutrition/2015-2020-dietary-guidelines/ (accessed on 10 February 2020).
- McGuire, S.; U.S. Department of Agriculture; U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 7th ed.; U.S. Government Printing Office: Washington, DC, USA, 2011; Volume 2, pp. 293–294. [Google Scholar]
- Montain, S.J.; Young, A.J. Diet and physical performance. Appetite 2003, 40, 255–267. [Google Scholar] [CrossRef]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and Athletic Performance. J. Acad Nutr Diet. 2016, 116, 501–528. [Google Scholar] [CrossRef] [PubMed]
- Farina, E.K.; Thompson, L.A.; Knapik, J.J.; Pasiakos, S.M.; Lieberman, H.R.; McClung, J.P. Diet Quality is Associated with Physical Performance and Special Forces Selection. Med. Sci. Sports Exerc. 2020, 52, 178–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitt, J.A. Nutrition and cognition: Meeting the challenge to obtain credible and evidence-based facts. Nutr. Rev. 2010, 68 (Suppl. 1), S2–S5. [Google Scholar] [CrossRef] [Green Version]
- Lieberman, H.R. Nutrition, brain function and cognitive performance. Appetite 2003, 40, 245–254. [Google Scholar] [CrossRef]
- Lutz, L.J.; Gaffney-Stomberg, E.; Williams, K.W.; McGraw, S.M.; Niro, P.J.; Karl, J.P.; Cable, S.J.; Cropper, T.L.; McClung, J.P. Adherence to the Dietary Guidelines for Americans Is Associated with Psychological Resilience in Young Adults: A Cross-Sectional Study. J. Acad. Nutr. Diet. 2017, 117, 396–403. [Google Scholar] [CrossRef]
- Hanlon, E.C.; Van Cauter, E. Quantification of sleep behavior and of its impact on the cross-talk between the brain and peripheral metabolism. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 3), 15609–15616. [Google Scholar] [CrossRef] [Green Version]
- Askew, E.W. Environmental and physical stress and nutrient requirements. Am. J. Clin. Nutr. 1995, 61 (Suppl. 3), 631s–637s. [Google Scholar] [CrossRef] [PubMed]
- Wrobleski, M.M.; Parker, E.A.; Hurley, K.M.; Oberlander, S.; Merry, B.C.; Black, M.M. Comparison of the HEI and HEI-2010 Diet Quality Measures in Association with Chronic Disease Risk among Low-Income, African American Urban Youth in Baltimore, Maryland. J. Am. Coll. Nutr. 2018, 37, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J. The Importance of Dietary Guidelines. Ref. Modul. Food Sci. 2019, 1–16. [Google Scholar] [CrossRef]
- Hiza, H.A.B.; Koegel, K.L.; Pannucci, T.E. Diet Quality: The Key to Healthy Eating. J. Acad. Nutr. Diet. 2018, 118, 1583–1585. [Google Scholar] [CrossRef] [PubMed]
- Chiuve, S.E.; Fung, T.T.; Rimm, E.B.; Hu, F.B.; McCullough, M.L.; Wang, M.; Stampfer, M.J.; Willett, W.C. Alternative dietary indices both strongly predict risk of chronic disease. J. Nutr. 2012, 142, 1009–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potter, J.; Brown, L.; Williams, R.L.; Byles, J.; Collins, C.E. Diet Quality and Cancer Outcomes in Adults: A Systematic Review of Epidemiological Studies. Int. J. Mol. Sci 2016, 17, 1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicklas, T.A.; O’Neil, C.E.; Fulgoni, V.L. Diet quality is inversely related to cardiovascular risk factors in adults. J. Nutr. 2012, 142, 2112–2118. [Google Scholar] [CrossRef]
- Lutz, L.J.; Gaffney-Stomberg, E.; Scisco, J.L.; Cable, S.J.; Karl, J.P.; Young, A.J.; McClung, J.P. Assessment of dietary intake using the healthy eating index during military training. US Army Med. Dep. J. 2013, Oct–Dec, 91–97. [Google Scholar]
- Cole, R.E.; Bukhari, A.S.; Champagne, C.M.; McGraw, S.M.; Hatch, A.M.; Montain, S.J. Performance Nutrition Dining Facility Intervention Improves Special Operations Soldiers’ Diet Quality and Meal Satisfaction. J. Nutr. Educ. Behav. 2018, 50, 993–1004. [Google Scholar] [CrossRef]
- Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Kirkpatrick, S.I.; Lerman, J.L.; Tooze, J.A.; Wilson, M.M.; Reedy, J. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 2018, 118, 1591–1602. [Google Scholar] [CrossRef] [Green Version]
- Fagnant, H.S.; Armstrong, N.J.; Lutz, L.J.; Nakayama, A.T.; Guerriere, K.I.; Ruthazer, R.; Cole, R.E.; McClung, J.P.; Gaffney-Stomberg, E.; Karl, J.P. Self-reported eating behaviors of military recruits are associated with body mass index at military accession and change during initial military training. Appetite 2019, 142, 104348. [Google Scholar] [CrossRef]
- Nindl, B.C.; Jaffin, D.P.; Dretsch, M.N.; Cheuvront, S.N.; Wesensten, N.J.; Kent, M.L.; Grunberg, N.E.; Pierce, J.R.; Barry, E.S.; Scott, J.M.; et al. Human Performance Optimization Metrics: Consensus Findings, Gaps, and Recommendations for Future Research. J. Strength Cond. Res. 2015, 29 (Suppl. 11), S221–S245. [Google Scholar] [CrossRef]
- Picó, C.; Serra, F.; Rodríguez, A.M.; Keijer, J.; Palou, A. Biomarkers of Nutrition and Health: New Tools for New Approaches. Nutrients 2019, 11, 1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Center for Disease Control and Prevention. National Health And Nutrition Exam Survey. Available online: https://www.cdc.gov/nchs/nhanes/index.html (accessed on 17 June 2020).
- National Institute of Health. HEI Overview of the Methods and Calculations. Available online: https://epi.grants.cancer.gov/hei/hei-methods-and-calculations.html (accessed on 17 June 2020).
- Coulter, I.D. The response of an expert panel to Nutritional armor for the warfighter: Can omega-3 fatty acids enhance stress resilience, wellness, and military performance? Mil. Med. 2014, 179, 192–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dretsch, M.N.; Johnston, D.; Bradley, R.S.; MacRae, H.; Deuster, P.A.; Harris, W.S. Effects of omega-3 fatty acid supplementation on neurocognitive functioning and mood in deployed U.S. soldiers: A pilot study. Mil. Med. 2014, 179, 396–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farina, E.K.; Taylor, J.C.; Means, G.E.; Murphy, N.E.; Pasiakos, S.M.; Lieberman, H.R.; McClung, J.P. Effects of deployment on diet quality and nutritional status markers of elite U.S. Army special operations forces soldiers. Nutr. J. 2017, 16, 41. [Google Scholar] [CrossRef] [PubMed]
- Gaffney-Stomberg, E.; Lutz, L.J.; Rood, J.C.; Cable, S.J.; Pasiakos, S.M.; Young, A.J.; McClung, J.P. Calcium and vitamin D supplementation maintains parathyroid hormone and improves bone density during initial military training: A randomized, double-blind, placebo controlled trial. Bone 2014, 68, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Del Gobbo, L.; Tintle, N.L. The Omega-3 Index and relative risk for coronary heart disease mortality: Estimation from 10 cohort studies. Atherosclerosis 2017, 262, 51–54. [Google Scholar] [CrossRef] [PubMed]
- McClung, J.P.; Karl, J.P.; Cable, S.J.; Williams, K.W.; Nindl, B.C.; Young, A.J.; Lieberman, H.R. Randomized, double-blind, placebo-controlled trial of iron supplementation in female soldiers during military training: Effects on iron status, physical performance, and mood. Am. J. Clin. Nutr. 2009, 90, 124–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, S.J.; Adair, L. An alternative to dietary data exclusions. J. Am. Diet. Assoc. 2007, 107, 792–799. [Google Scholar] [CrossRef]
- Rhee, J.J.; Sampson, L.; Cho, E.; Hughes, M.D.; Hu, F.B.; Willett, W.C. Comparison of methods to account for implausible reporting of energy intake in epidemiologic studies. Am. J. Epidemiol. 2015, 181, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, S.I.; Reedy, J.; Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Wilson, M.M.; Lerman, J.L.; Tooze, J.A. Applications of the Healthy Eating Index for Surveillance, Epidemiology, and Intervention Research: Considerations and Caveats. J. Acad. Nutr. Diet. 2018, 118, 1603–1621. [Google Scholar] [CrossRef] [PubMed]
- Purvis, D.L.; Lentino, C.V.; Jackson, T.K.; Murphy, K.J.; Deuster, P.A. Nutrition as a component of the performance triad: How healthy eating behaviors contribute to soldier performance and military readiness. US Army Med. Dep. J. 2013, 66–78. [Google Scholar]
- Smith, T.J.; Dotson, L.E.; Young, A.J.; White, A.; Hadden, L.; Bathalon, G.P.; Funderburk, L.; Marriott, B.P. Eating patterns and leisure-time exercise among active duty military personnel: Comparison to the Healthy People objectives. J. Acad. Nutr. Diet. 2013, 113, 907–919. [Google Scholar] [CrossRef]
- Bray, R.M.; Pemberton, M.R.; Hourani, L.L.; Witt, M.; Olmsted, K.L.; Brown, J.M.; Scheffler, S. Department of Defense Survey of Health Related Behaviors Among Active Duty Military Personnel: A Component of the Defense Lifestyle AssessmentProgram(DLAP); Defense Technical Information Center: Fort Belvoir, VA, USA, 2009. [Google Scholar]
- Robinson, S.M.; Jameson, K.A.; Batelaan, S.F.; Martin, H.J.; Syddall, H.E.; Dennison, E.M.; Cooper, C.; Sayer, A.A. Diet and its relationship with grip strength in community-dwelling older men and women: The Hertfordshire cohort study. J. Am. Geriatr. Soc. 2008, 56, 84–90. [Google Scholar] [CrossRef]
- Houston, D.K.; Stevens, J.; Cai, J.; Haines, P.S. Dairy, fruit, and vegetable intakes and functional limitations and disability in a biracial cohort: The Atherosclerosis Risk in Communities Study. Am. J. Clin. Nutr. 2005, 81, 515–522. [Google Scholar] [CrossRef]
- Robinson, S.M.; Westbury, L.D.; Cooper, R.; Kuh, D.; Ward, K.; Syddall, H.E.; Sayer, A.A.; Cooper, C. Adult Lifetime Diet Quality and Physical Performance in Older Age: Findings From a British Birth Cohort. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1532–1537. [Google Scholar] [CrossRef] [Green Version]
- Drenowatz, C.; Shook, R.P.; Hand, G.A.; Hebert, J.R.; Blair, S.N. The independent association between diet quality and body composition. Sci. Rep. 2014, 4, 4928. [Google Scholar] [CrossRef] [Green Version]
- United States Department of Agriculture. Healthy Eating Index. Available online: https://www.fns.usda.gov/resource/healthy-eating-index-hei (accessed on 28 January 2020).
- Walker, R.E.; Jackson, K.H.; Tintle, N.L.; Shearer, G.C.; Bernasconi, A.; Masson, S.; Latini, R.; Heydari, B.; Kwong, R.Y.; Flock, M.; et al. Predicting the effects of supplemental EPA and DHA on the omega-3 index. Am. J. Clin. Nutr. 2019, 110, 1034–1040. [Google Scholar] [CrossRef]
- Anzalone, A.; Carbuhn, A.; Jones, L.; Gallop, A.; Smith, A.; Johnson, P.; Swearingen, L.; Moore, C.; Rimer, E.; McBeth, J.; et al. The Omega-3 Index in National Collegiate Athletic Association Division I Collegiate Football Athletes. J. Athl. Train. 2019, 54, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Block, G.; Woods, M.; Potosky, A.; Clifford, C. Validation of a self-administered diet history questionnaire using multiple diet records. J. Clin. Epidemiol. 1990, 43, 1327–1335. [Google Scholar] [CrossRef]
- Cummings, S.R.; Block, G.; McHenry, K.; Baron, R.B. Evaluation of two food frequency methods of measuring dietary calcium intake. Am. J. Epidemiol. 1987, 126, 796–802. [Google Scholar] [CrossRef] [PubMed]
| HEI Components | Standard (Per 1000 Kcal) | Max Score |
|---|---|---|
| Total fruit | ≥0.8 cup equivalents | 5 |
| Whole fruit | ≥0.4 cup equivalents | 5 |
| Total vegetables | ≥1.1 cup equivalents | 5 |
| Greens and beans | ≥0.2 cup equivalents | 5 |
| Whole grains | ≥1.5 oz equivalents | 10 |
| Dairy | 1.3 cup equivalents | 10 |
| Total protein foods | ≥2.5 oz equivalents | 5 |
| Seafood and plant protein | ≥0.8 oz equivalents | 5 |
| Fatty acids | (MUFA + PUFA)/SFA * | 10 |
| HEI Components | Standard for Maximum Score (10) | Standard for Minimum Score (0) |
|---|---|---|
| Refined grains | 1.8 oz equivalents (per 1000 kcal) | ≥4.3 oz equivalents (per 1000 kcal) |
| Sodium | ≤1.1 g (per 1000 kcal) | ≥2.0 g (per 1000 kcal) |
| Added sugars | ≤6.5% of total energy | ≥26% of total energy |
| Saturated fats | ≤8% of total energy | 16% of total energy |
| Category | # of Participants (% of Category) |
|---|---|
| Sex | n = 487 |
| Female | 105 (22) |
| Male | 382 (78) |
| No response | 41 |
| Race | n = 415 |
| Asian | 16 (3.9) |
| Black or African American | 99 (23.9) |
| Native American/Alaskan Native | 4 (0.9) |
| Native Hawaiian/Pacific Islander | 6 (1.4) |
| White or Caucasian | 248 (59.8) |
| Other | 21 (5.0) |
| No response | 134 |
| Rank | n = 431 |
| Enlisted | 391 (91) |
| Officer | 40 (9) |
| No response | 97 |
| Age | n = 425 |
| 25 and under | 217 (51) |
| 26 and older | 208 (49) |
| No response | 103 |
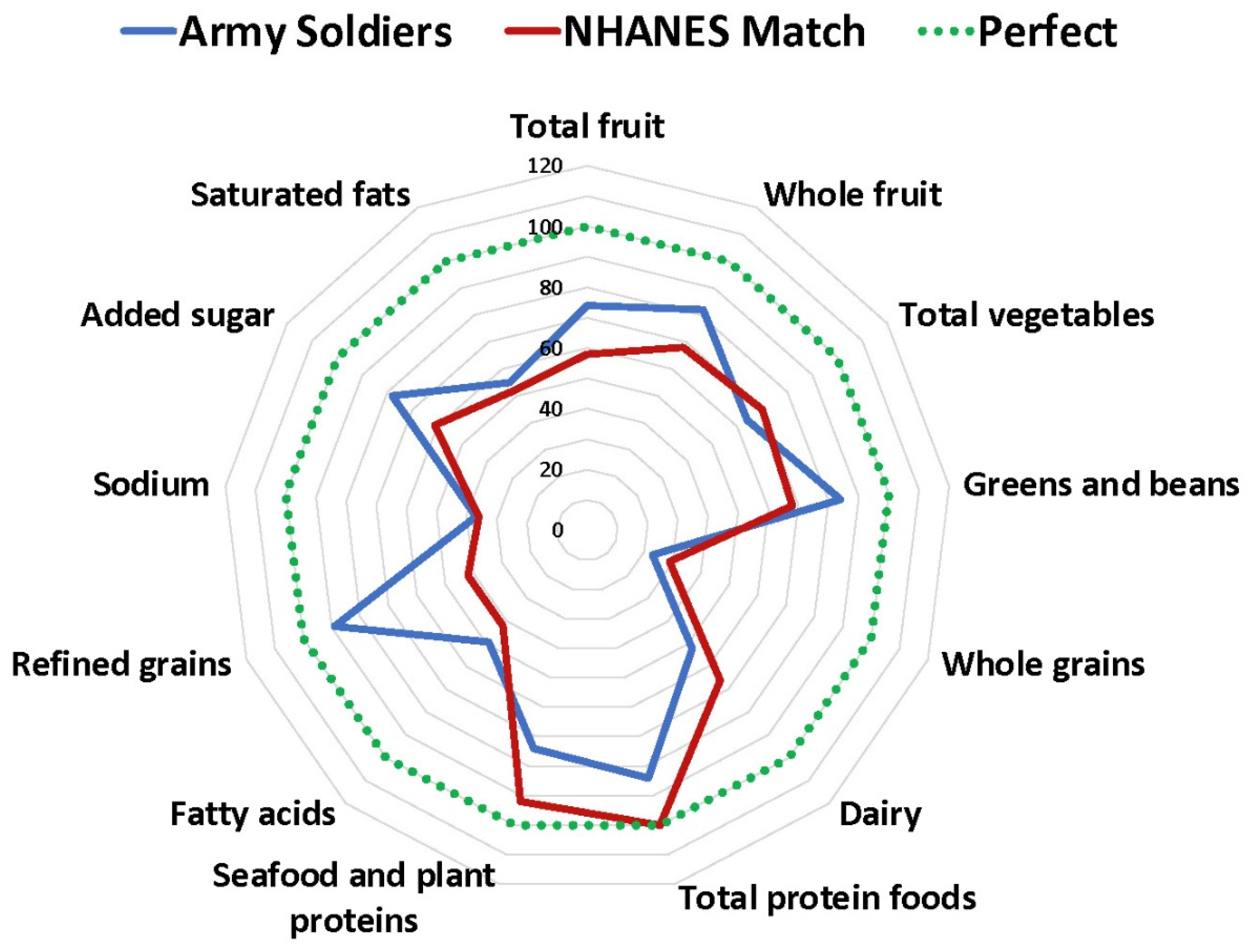
| HEI Component | Army Soldier Median (25–75% Range) | NHANES Match Median (25–75% Range) |
|---|---|---|
| Adequate components | ||
| Total fruit | 3.7 (2.4–5.0) | 2.9 (2.4–3.0) |
| Whole fruit | 4.1 (2.0–5.0) | 3.4 (2.9–3.9) |
| Total vegetables | 3.2 (2.4–4.3) | 3.5 (3.1–4.0) |
| Greens and beans | 4.2 (2.3–5.0) | 3.4 (2.3–3.9) |
| Whole grains | 2.3 (1.4–4.1) | 2.9 (2.4–3.4) |
| Dairy | 5.2 (3.7–7.4) | 6.6 (5.9–7.6) |
| Total protein foods | 4.2 (3.5–5.0) | 5 (5–5) |
| Seafood and plant proteins | 3.7 (1.8–5.0) | 4.6 (4.0–4.8) |
| Fatty acids | 4.9 (3.3–6.5) | 4.2 (4.2–4.2) |
| Moderation components | ||
| Refined grains | 8.9 (7.2–10) | 4.2 (3.1–5.2) |
| Sodium | 3.7 (2.0–5.7) | 3.6 (3.6–3.6) |
| Added sugar | 7.8 (5.6–9.3) | 6.1 (5.5–6.9) |
| Saturated fats | 5.5 (3.6–7.3) | 5.2 (5.2–5.2) |
| Total HEI | 59.9 (53–67) | 55.4 (52–58) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rittenhouse, M.; Scott, J.; Deuster, P. Healthy Eating Index and Nutrition Biomarkers among Army Soldiers and Civilian Control Group Indicate an Intervention Is Necessary to Raise Omega-3 Index and Vitamin D and Improve Diet Quality. Nutrients 2021, 13, 122. https://doi.org/10.3390/nu13010122
Rittenhouse M, Scott J, Deuster P. Healthy Eating Index and Nutrition Biomarkers among Army Soldiers and Civilian Control Group Indicate an Intervention Is Necessary to Raise Omega-3 Index and Vitamin D and Improve Diet Quality. Nutrients. 2021; 13(1):122. https://doi.org/10.3390/nu13010122
Chicago/Turabian StyleRittenhouse, Melissa, Jonathan Scott, and Patricia Deuster. 2021. "Healthy Eating Index and Nutrition Biomarkers among Army Soldiers and Civilian Control Group Indicate an Intervention Is Necessary to Raise Omega-3 Index and Vitamin D and Improve Diet Quality" Nutrients 13, no. 1: 122. https://doi.org/10.3390/nu13010122






