A Randomized Crossover Intervention Study on the Effect a Standardized Maté Extract (Ilex paraguariensis A. St.-Hil.) in Men Predisposed to Cardiovascular Risk
Abstract
:1. Introduction
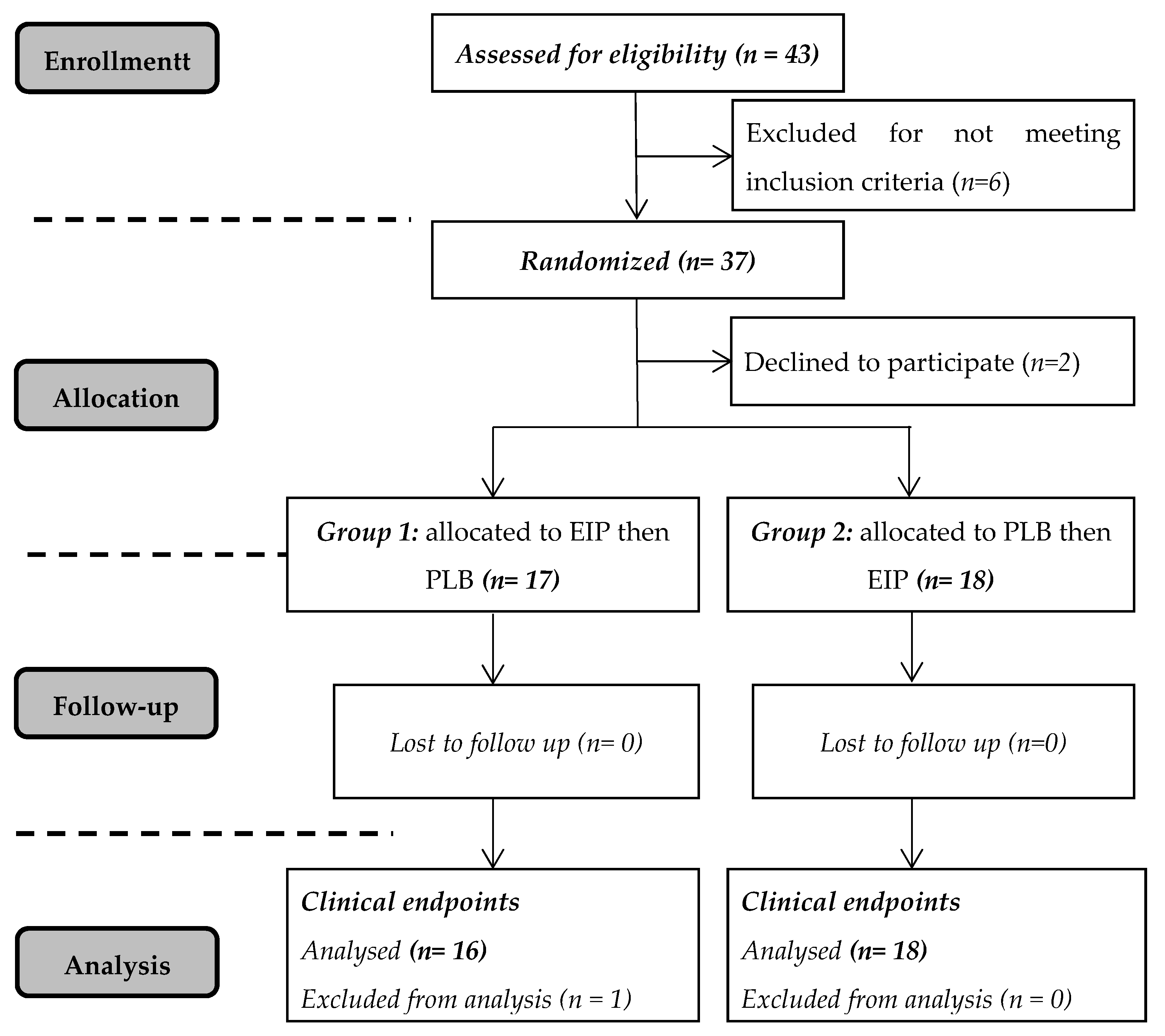
2. Materials and Methods
2.1. Subjects
2.2. Preparation and Analysis of Maté Extract
2.3. Study Intervention
2.4. Blood Pressure Measurements
2.5. Sampling and Analysis of Biological Samples
2.6. Statistical Analyses
3. Results
3.1. Baseline Characteristics of Subjects at Inclusion and Compliance
3.2. Anthropometric, Hemodynamic and Biochemical Parameters
3.3. Endothelial and Inflammatory Parameters
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Katz, D.L.; Meller, S. Can we say what diet is best for health? Annu. Rev. Public Health 2014, 35, 83–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remón, A.; Martínez-González, M.A.; de la Torre, R.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; Arós, F.; et al. Inverse association between habitual polyphenol intake and incidence of cardiovascular events in the PREDIMED study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Badimon, L. Effects of Polyphenol Intake on Metabolic Syndrome: Current Evidences from Human Trials. Oxid Med. Cell Longev. 2017, 2017, 5812401. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Polyphenols and Glycemic Control. Nutrients 2016, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, J.; Fezeu, L.; Touvier, M.; Arnault, N.; Manach, C.; Hercberg, S.; Galan, P.; Scalbert, A. Dietary intake of 337 polyphenols in French adults. Am. J. Clin. Nutr. 2011, 93, 1220–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Su, C.; Chen, X.; Wang, Q.; Jiao, W.; Luo, H.; Tang, J.; Wang, W.; Li, S.; Guo, S. Chlorogenic Acids in Cardiovascular Disease: A Review of Dietary Consumption, Pharmacology, and Pharmacokinetics. J. Agric. Food Chem. 2020, 68, 6464–6484. [Google Scholar] [CrossRef]
- Clifford, M.N.; Kerimi, A.; Williamson, G. Bioavailability and metabolism of chlorogenic acids (acyl-quinic acids) in humans. Compr. Rev. Food Sci. Food Saf. 2020. [Google Scholar] [CrossRef]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef]
- Van Dijk, A.E.; Olthof, M.R.; Meeuse, J.C.; Seebus, E.; Heine, R.J.; van Dam, R.M. Acute effects of decaffeinated coffee and the major coffee components chlorogenic acid and trigonelline on glucose tolerance. Diabetes Care 2009, 32, 1023–1025. [Google Scholar] [CrossRef] [Green Version]
- Johnston, K.L.; Clifford, M.N.; Morgan, L.M. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: Glycemic effects of chlorogenic acid and caffeine. Am. J. Clin. Nutr. 2003, 78, 728–733. [Google Scholar] [CrossRef]
- Zuñiga, L.Y.; Aceves-de la Mora, M.C.A.; González-Ortiz, M.; Ramos-Núñez, J.L.; Martínez-Abundis, E. Effect of Chlorogenic Acid Administration on Glycemic Control, Insulin Secretion, and Insulin Sensitivity in Patients with Impaired Glucose Tolerance. J. Med. Food 2018, 21, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Kozuma, K.; Tsuchiya, S.; Kohori, J.; Hase, T.; Tokimitsu, I. Antihypertensive effect of green coffee bean extract on mildly hypertensive subjects. Hypertens. Res. 2005, 28, 711–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, T.; Arai, Y.; Mitsui, Y.; Kusaura, T.; Okawa, W.; Kajihara, Y.; Saito, I. The blood pressure-lowering effect and safety of chlorogenic acid from green coffee bean extract in essential hypertension. Clin. Exp. Hypertens. 2006, 28, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.E.; Flury, A.; Marmet, C.; Poquet, L.; Rimoldi, S.F.; Sartori, C.; Rexhaj, E.; Brenner, R.; Allemann, Y.; Zimmermann, D.; et al. Mediation of coffee-induced improvements in human vascular function by chlorogenic acids and its metabolites: Two randomized, controlled, crossover intervention trials. Clin. Nutr. 2017, 36, 1520–1529. [Google Scholar] [CrossRef] [Green Version]
- Ochiai, R.; Sugiura, Y.; Shioya, Y.; Otsuka, K.; Katsuragi, Y.; Hashiguchi, T. Coffee polyphenols improve peripheral endothelial function after glucose loading in healthy male adults. Nutr. Res. 2014, 34, 155–159. [Google Scholar] [CrossRef]
- Cardozo, E.L., Jr.; Morand, C. Interest of mate (Ilex paraguariensis A. St.-Hil.) as a new natural functional food to preserve human cardiovascular health—A review. J. Funct. Foods 2016, 21, 440–454. [Google Scholar] [CrossRef]
- Da Silveira, T.F.F.; Meinhart, A.D.; de Souza, T.C.L.; Teixeira Filho, J.; Godoy, H.T. Phenolic compounds from yerba mate based beverages—A multivariate optimisation. Food Chem. 2016, 190, 1159–1167. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Liu, Z.; Wan, W.; Qu, X.; Chen, M. Aqueous extract of Yerba Mate Tea lowers Atherosclerotic Risk Factors in a Rat Hyperlipidemia Model. Phytother. Res. 2013, 27, 1225–1231. [Google Scholar] [CrossRef]
- Balzan, S.; Hernandes, A.; Reichert, C.L.; Donaduzzi, C.M.; Pires, V.A.; Gasparotto, A., Jr.; Cardozo, E.L., Jr. Lipid-lowering effects of standardized extracts of Ilex paraguariensis in high-fat-diet rats. Fitoterapia 2013, 86, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Hussein, G.M.; Matsuda, H.; Nakamura, S.; Akiyama, T.; Tamura, K.; Yoshikawa, M. Protective and ameliorative effects of maté (Ilex paraguariensis) on metabolic syndrome in TSOD mice. Phytomedicine 2011, 19, 88–97. [Google Scholar] [CrossRef]
- Mosimann, A.L.; Wilhelm-Filho, D.; da Silva, E.L. Aqueous extract of Ilex paraguariensis attenuates the progression of atherosclerosis in cholesterol-fed rabbits. Biofactors 2006, 26, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Lima, N.d.S.; de Oliveira, E.; da Silva, A.P.S.; Maia, L.d.A.; de Moura, E.G.; Lisboa, P.C. Effects of Ilex paraguariensis (yerba mate) treatment on leptin resistance and inflammatory parameters in obese rats primed by early weaning. Life Sci. 2014, 115, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Oh, M.R.; Kim, M.G.; Chae, H.J.; Chae, S.W. Anti-obesity effects of Yerba Mate (Ilex paraguariensis): A randomized, double-blind, placebo-controlled clinical trial. BMC Complement. Altern. Med. 2015, 15, 338. [Google Scholar] [CrossRef] [Green Version]
- Arçari, D.P.; Porto, V.B.; Rodrigues, E.R.V.; Martins, F.; de Lima, R.J.; Sawaya, A.C.H.F.; Ribeiro, M.L.; Carvalho, P.O. Effect of mate tea (Ilex paraguariensis) supplementation on oxidative stress biomarkers and LDL oxidisability in normo- and hyperlipidaemic humans. J. Funct. Foods 2011, 3, 190–197. [Google Scholar] [CrossRef]
- Klein, G.A.; Stefanuto, A.; Boaventura, B.C.; de Morais, E.C.; Cavalcante, L.a.S.; de Andrade, F.; Wazlawik, E.; Di Pietro, P.F.; Maraschin, M.; da Silva, E.L. Mate tea (Ilex paraguariensis) improves glycemic and lipid profiles of type 2 diabetes and pre-diabetes individuals: A pilot study. J. Am. Coll. Nutr. 2011, 30, 320–332. [Google Scholar] [CrossRef] [PubMed]
- De Morais, E.C.; Stefanuto, A.; Klein, G.A.; Boaventura, B.C.; de Andrade, F.; Wazlawik, E.; Di Pietro, P.F.; Maraschin, M.; da Silva, E.L. Consumption of yerba mate (Ilex paraguariensis) improves serum lipid parameters in healthy dyslipidemic subjects and provides an additional LDL-cholesterol reduction in individuals on statin therapy. J. Agric. Food Chem. 2009, 57, 8316–8324. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.I.; Duncan, B.B.; Azevedo e Silva, G.; Menezes, A.M.; Monteiro, C.A.; Barreto, S.M.; Chor, D.; Menezes, P.R. Chronic non-communicable diseases in Brazil: Burden and current challenges. Lancet 2011, 377, 1949–1961. [Google Scholar] [CrossRef]
- Gebara, K.S.; Gasparotto-Junior, A.; Santiago, P.G.; Cardoso, C.A.L.; de Souza, L.M.; Morand, C.; Costa, T.A.; Cardozo-Junior, E.L. Daily Intake of Chlorogenic Acids from Consumption of Maté (Ilex paraguariensis A.St.-Hil.) Traditional Beverages. J. Agric. Food Chem. 2017, 65, 10093–10100. [Google Scholar] [CrossRef] [PubMed]
- Regnault, T.R.; Oddy, H.V.; Nancarrow, C.; Sriskandarajah, N.; Scaramuzzi, R.J. Glucose-stimulated insulin response in pregnant sheep following acute suppression of plasma non-esterified fatty acid concentrations. Reprod. Biol. Endocrinol. 2004, 2, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narita, Y.; Inouye, K. Kinetic analysis and mechanism on the inhibition of chlorogenic acid and its components against porcine pancreas alpha-amylase isozymes I and II. J. Agric. Food Chem. 2009, 57, 9218–9225. [Google Scholar] [CrossRef] [PubMed]
- Vinson, J.A.; Burnham, B.R.; Nagendran, M.V. Randomized, double-blind, placebo-controlled, linear dose, crossover study to evaluate the efficacy and safety of a green coffee bean extract in overweight subjects. Diabetes Metab. Syndr. Obes. 2012, 5, 21–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akash, M.S.; Rehman, K.; Chen, S. Effects of coffee on type 2 diabetes mellitus. Nutrition 2014, 30, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Song, S.J.; Choi, S.; Park, T. Decaffeinated green coffee bean extract attenuates diet-induced obesity and insulin resistance in mice. Evid. Based Complement. Altern. Med. 2014, 2014, 718379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; He, X.; Ma, Y.; Zhao, X.; Hou, X.; Hao, E.; Deng, J.; Bai, G. Chlorogenic Acid Targeting of the AKT PH Domain Activates AKT/GSK3β/FOXO1 Signaling and Improves Glucose Metabolism. Nutrients 2018, 10, 1366. [Google Scholar] [CrossRef] [Green Version]
- Tschritter, O.; Fritsche, A.; Shirkavand, F.; Machicao, F.; Häring, H.; Stumvoll, M. Assessing the shape of the glucose curve during an oral glucose tolerance test. Diabetes Care 2003, 26, 1026–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, T.; Kobayashi, S.; Yamaguchi, T.; Hibi, M.; Fukuhara, I.; Osaki, N. Coffee Abundant in Chlorogenic Acids Reduces Abdominal Fat in Overweight Adults: A Randomized, Double-Blind, Controlled Trial. Nutrients 2019, 11, 1617. [Google Scholar] [CrossRef] [Green Version]
- Soga, S.; Ota, N.; Shimotoyodome, A. Stimulation of postprandial fat utilization in healthy humans by daily consumption of chlorogenic acids. Biosci. Biotechnol. Biochem. 2013, 77, 1633–1636. [Google Scholar] [CrossRef]
- Kotyczka, C.; Boettler, U.; Lang, R.; Stiebitz, H.; Bytof, G.; Lantz, I.; Hofmann, T.; Marko, D.; Somoza, V. Dark roast coffee is more effective than light roast coffee in reducing body weight, and in restoring red blood cell vitamin E and glutathione concentrations in healthy volunteers. Mol. Nutr. Food Res. 2011, 55, 1582–1586. [Google Scholar] [CrossRef] [PubMed]
- Agudelo-Ochoa, G.M.; Pulgarín-Zapata, I.C.; Velásquez-Rodriguez, C.M.; Duque-Ramírez, M.; Naranjo-Cano, M.; Quintero-Ortiz, M.M.; Lara-Guzmán, O.J.; Muñoz-Durango, K. Coffee Consumption Increases the Antioxidant Capacity of Plasma and Has No Effect on the Lipid Profile or Vascular Function in Healthy Adults in a Randomized Controlled Trial. J. Nutr. 2016, 146, 524–531. [Google Scholar] [CrossRef] [Green Version]
- Cho, A.S.; Jeon, S.M.; Kim, M.J.; Yeo, J.; Seo, K.I.; Choi, M.S.; Lee, M.K. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem. Toxicol. 2010, 48, 937–943. [Google Scholar] [CrossRef]
- Huang, K.; Liang, X.C.; Zhong, Y.L.; He, W.Y.; Wang, Z. 5-Caffeoylquinic acid decreases diet-induced obesity in rats by modulating PPARα and LXRα transcription. J. Sci. Food Agric. 2015, 95, 1903–1910. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.W.; Wong, C.N.; Pin, W.K.; Wong, M.H.; Kwok, C.Y.; Chan, R.Y.; Yu, P.H.; Chan, S.W. Chlorogenic acid exhibits cholesterol lowering and fatty liver attenuating properties by up-regulating the gene expression of PPAR-α in hypercholesterolemic rats induced with a high-cholesterol diet. Phytother. Res. 2013, 27, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Title, L.M.; Cummings, P.M.; Giddens, K.; Nassar, B.A. Oral glucose loading acutely attenuates endothelium-dependent vasodilation in healthy adults without diabetes: An effect prevented by vitamins C and E. J. Am. Coll. Cardiol. 2000, 36, 2185–2191. [Google Scholar] [CrossRef] [Green Version]
- Major-Pedersen, A.; Ihlemann, N.; Hermann, T.S.; Christiansen, B.; Dominguez, H.; Kveiborg, B.; Nielsen, D.B.; Svendsen, O.L.; Køber, L.; Torp-Pedersen, C. Effects of oral glucose load on endothelial function and on insulin and glucose fluctuations in healthy individuals. Exp. Diabetes Res. 2008, 2008, 672021. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Kitts, D.D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef] [Green Version]
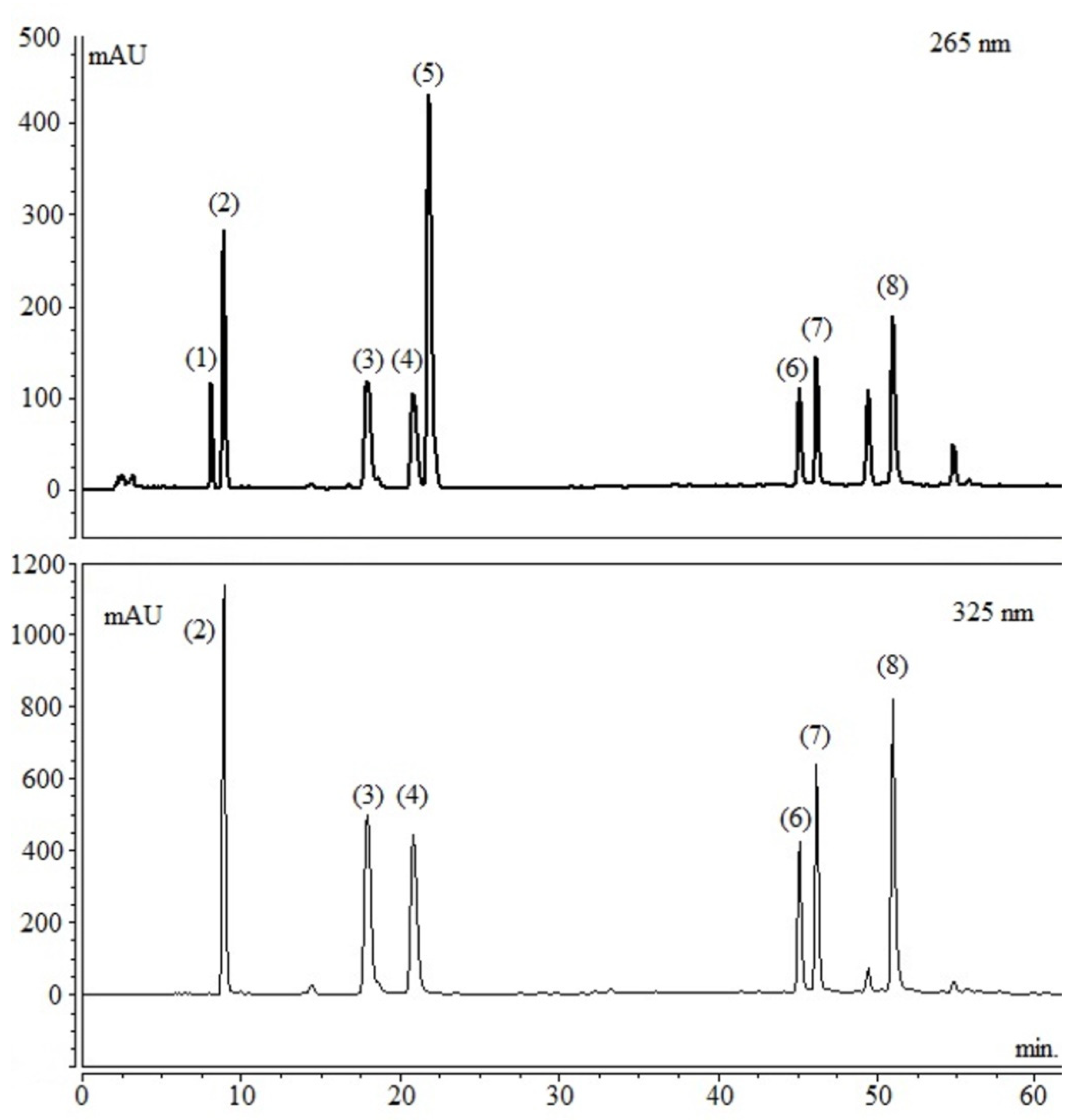


| Compounds | % in Dry Extract (w/w) | Amount per Capsule (mg) * | Daily Intake (mg) |
|---|---|---|---|
| 3-O-caffeoyl quinic acid | 4.91 | 12.28 | 110.49 |
| 5-O-caffeoyl quinic acid | 3.19 | 7.98 | 71.82 |
| 4-O-caffeoylquinic acid | 3.87 | 9.67 | 87.07 |
| ∑-Mono-O-CQAs | 11.97 | 29.93 | 269.38 |
| 3,4-Dicaffeoylquinic acid | 2.97 | 7.44 | 66.93 |
| 3,5-Dicaffeoylquinic acid | 4.40 | 10.99 | 98.95 |
| 4,5-Dicaffeoylquinic acid | 6.49 | 16.23 | 146.14 |
| ∑-Di-O-CQAs | 13.87 | 34.67 | 312.01 |
| ∑-Mono and Di-O-CQAs | 25.84 | 64.60 | 581.39 |
| Caffeine | 3.32 | 8.32 | 74.87 |
| Theobromine | 0.35 | 0.88 | 7.93 |
| ∑ Methylxanthines | 3.67 | 9.2 | 82.8 |
| Characteristics | Mean ± SD | Range | Normal Values |
|---|---|---|---|
| Age, y | 50.11 ± 4.59 | 45–63 | - |
| BW, kg | 82.08 ± 12.13 | 57.6–106.4 | - |
| WC, cm | 93.94 ± 9.40 | 75–113 | <94 (b) |
| BMI, kg/m2 | 27.28 ± 3.28 | 20.0–33.5 | 20 to 25 (b) |
| PUL, b.p.m | 69.20 ±15.63 | 40–136 | 60–100 (c) |
| SBP, mm Hg | 122 ± 12.40 | 98–160 | 120 (b) |
| DBP, mm Hg | 80 ± 11.81 | 60–116 | 80 (b) |
| Triglycerides, mmol/L | 1.61 ± 0.93 | 0.66–4.55 | 1.7 (b) |
| Total cholesterol, mmol/L | 5.57 ± 0.58 | 3.72–8.04 | <4.0 (a) |
| HDL cholesterol, mmol/L | 1.38 ± 0.34 | 0.96–2.28 | ≥1.5 (a) |
| LDL cholesterol, mmol/L | 3.39 ± 0.89 | 1.75–5.51 | <2.6 (b) |
| GLU, mmol/L | 5.22 ± 0.55 | 4.06–6.42 | 4.9–5.3 (d) |
| Creatinine, µmol/L | 84.76 ± 19.08 | - | - |
| Urea, mg/dL | 34.03 ± 7.79 | - | - |
| Alanine Transaminase, U/L | 24.20 ± 7.83 | - | - |
| Aspartate. Transaminase, U/L | 21.97 ± 7.66 | - | - |
| Framingham risk score, % | 11.04 ± 5.73 | 3.30–25.30 | <10% (e) |
| Baseline | EIP | PLB | EIP-PLB | p Value 2 | |
|---|---|---|---|---|---|
| SBP, mm Hg | 121.56 ± 10.97 | 119.57 ± 10.21 | 121.08 ± 8.92 | −1.516 | 0.224 |
| DBP, mm Hg | 76.78 ± 12.32 | 76.06 ± 12.12 | 77.52 ± 12.12 | −1.463 | 0.195 |
| PUL, bpm | 65.38 ± 7.85 | 66.74 ± 8.08 | 67.32 ± 9.19 | −0.588 | 0.693 |
| WC, cm | 93.46 ± 9.14 | 93.73 ± 9.40 | 93.51 ± 8.48 | 0.214 | 0.702 |
| BW, kg | 81.99 ± 12.24 | 81.76 ± 12.08 | 81.96 ± 11.93 | −0.196 | 0.364 |
| BMI, kg/m2 | 27.34 ± 3.51 | 27.17 ± 3.37 | 27.24 ± 3.43 | −0.065 | 0.360 |
| TC, mmol/L | 5.16 ± 0.90 | 4.97 ± 1.25 | 4.94 ± 1.15 | 0.032 | 0.881 |
| HDL-c, mmol/L | 1.35 ± 0.33 | 1.41 ± 0.40 | 1.34 ± 0.31 | 0.066 | 0.20 |
| LDL-c, mmol/L | 3.02 ± 0.81 | 2.95 ± 1.07 | 2.89 ± 0.93 | 0.0567 | 0.748 |
| TGY, mmol/L | 1.70 ±1.01 | 1.51 ± 0.86 | 1.63 ± 0.98 | −0.117 | 0.348 |
| GLU, mmol/L | 4.95 ± 0.44 | 4.42 ± 0.63 | 4.60 ± 0.52 | −0.172 | 0.132 |
| EIP | PLB | |||||
|---|---|---|---|---|---|---|
| D0 | D28 | D28+ | D0 | D28 | D28+ | |
| CRP (mg/dL) | 1.13 ± 0.35 a | 1.070 ± 0.25 a | 0.50 ± 0.18 b | 1.15 ± 0.34 a | 0.82 ± 0.26 a | 0.60 ± 0.25 b |
| ICAM-1 (ng/mL) | 532.6 ± 46.54 | 523.60 ± 55.07 | 474.9 ± 58.12 | 444.2 ± 56.68 | 469.20 ± 72.94 | 456.00 ± 63.97 |
| VCAM-1 (ng/mL) | 478.5 ± 43.09 | 535.3 ± 67.16 | 467.19 ± 50.36 | 478.9 ± 63.87 | 459.6 ± 60.64 | 480.4 ± 69.85 |
| IL-6 (pg/mL) | 1.71 ± 0.26 a | 1.39 ± 0.17 b | 1.73 ± 0.16 ab | 1.51 ± 0.22 | 1.20 ± 0.22 | 1.78 ± 0.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gebara, K.S.; Gasparotto Junior, A.; Palozi, R.A.C.; Morand, C.; Bonetti, C.I.; Gozzi, P.T.; de Mello, M.R.F.; Costa, T.A.; Cardozo Junior, E.L. A Randomized Crossover Intervention Study on the Effect a Standardized Maté Extract (Ilex paraguariensis A. St.-Hil.) in Men Predisposed to Cardiovascular Risk. Nutrients 2021, 13, 14. https://doi.org/10.3390/nu13010014
Gebara KS, Gasparotto Junior A, Palozi RAC, Morand C, Bonetti CI, Gozzi PT, de Mello MRF, Costa TA, Cardozo Junior EL. A Randomized Crossover Intervention Study on the Effect a Standardized Maté Extract (Ilex paraguariensis A. St.-Hil.) in Men Predisposed to Cardiovascular Risk. Nutrients. 2021; 13(1):14. https://doi.org/10.3390/nu13010014
Chicago/Turabian StyleGebara, Karimi S., Arquimedes Gasparotto Junior, Rhanany A. C. Palozi, Christine Morand, Carla I. Bonetti, Paula T. Gozzi, Martha R. F. de Mello, Telma A. Costa, and Euclides L. Cardozo Junior. 2021. "A Randomized Crossover Intervention Study on the Effect a Standardized Maté Extract (Ilex paraguariensis A. St.-Hil.) in Men Predisposed to Cardiovascular Risk" Nutrients 13, no. 1: 14. https://doi.org/10.3390/nu13010014
APA StyleGebara, K. S., Gasparotto Junior, A., Palozi, R. A. C., Morand, C., Bonetti, C. I., Gozzi, P. T., de Mello, M. R. F., Costa, T. A., & Cardozo Junior, E. L. (2021). A Randomized Crossover Intervention Study on the Effect a Standardized Maté Extract (Ilex paraguariensis A. St.-Hil.) in Men Predisposed to Cardiovascular Risk. Nutrients, 13(1), 14. https://doi.org/10.3390/nu13010014





