Consumption of a High-Protein Meal Replacement Leads to Higher Fat Oxidation, Suppression of Hunger, and Improved Metabolic Profile After an Exercise Session
Abstract
1. Introduction
2. Materials and Methods
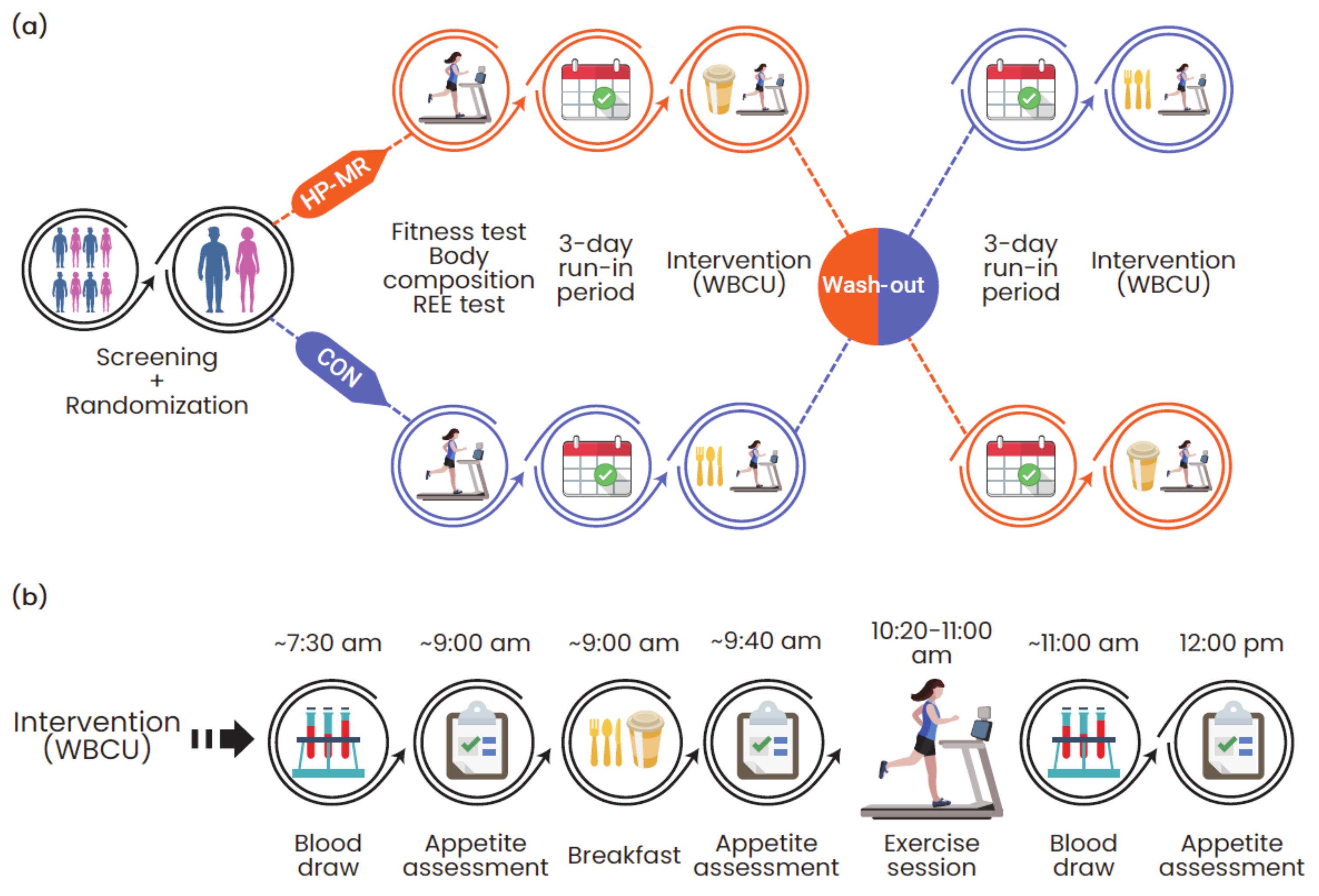
2.1. Study Design and Participant Details
2.2. Experimental Protocol
2.3. Resting Energy Expenditure
2.4. Standardized Fitness Test
2.5. Run-in Period
2.6. Energy Metabolism
2.7. Interventions
2.8. Appetite Sensations
2.9. Metabolic Blood Markers
2.10. Statistical Analysis
3. Results
3.1. Participants
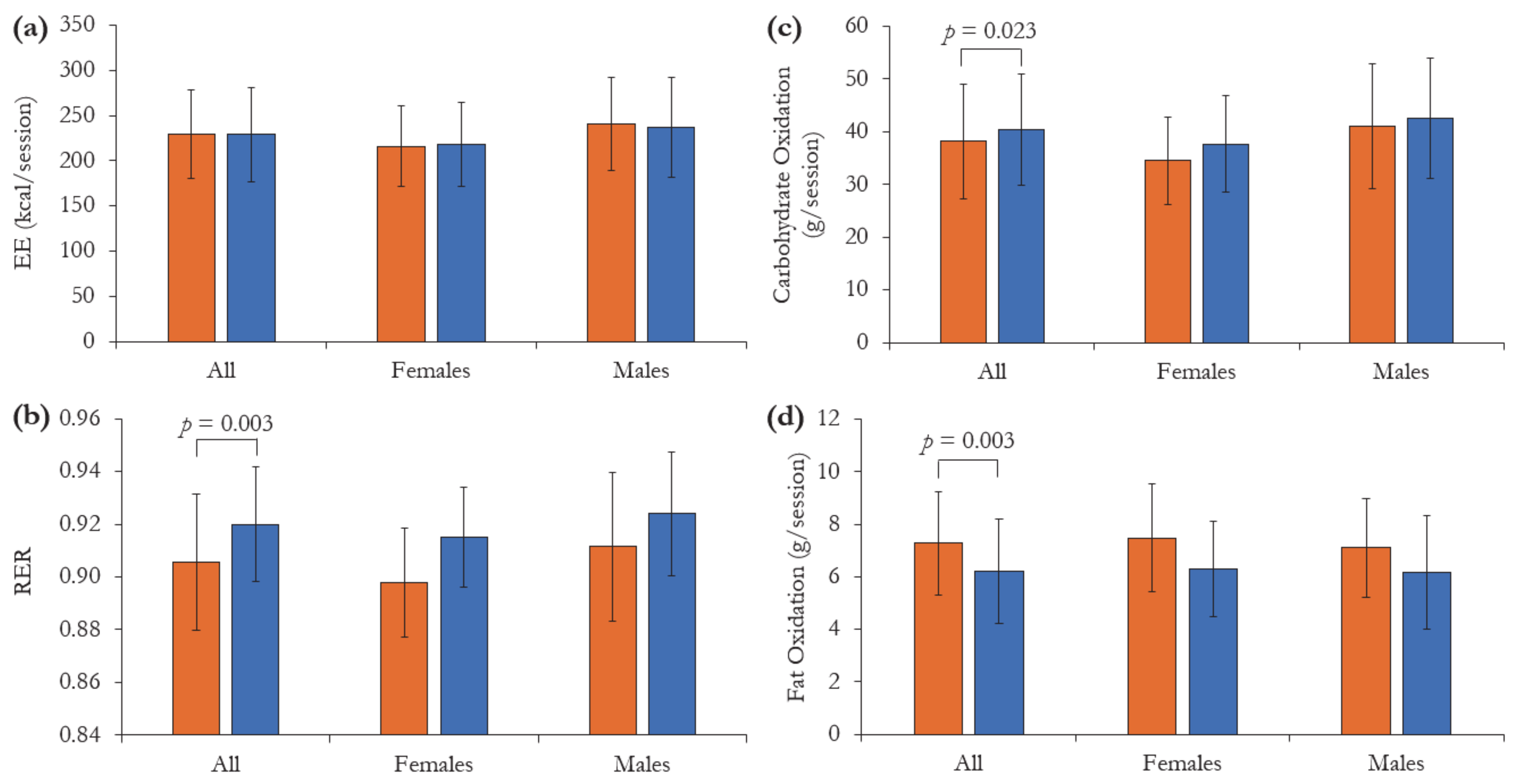
3.2. Energy Metabolism
3.3. Appetite Sensations
3.4. Metabolic Blood Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Angelantonio, E.; Bhupathiraju, S.N.; Wormser, D.; Gao, P.; Kaptoge, S.; de Gonzalez, A.B.; Cairns, B.J.; Huxley, R.; Jackson, C.L.; Joshy, G.; et al. Body-mass index and all-cause mortality: Individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016, 388, 776–786. [Google Scholar] [CrossRef]
- Abdelaal, M.; le Roux, C.W.; Docherty, N.G. Morbidity and mortality associated with obesity. Ann. Transl. Med. 2017, 5, 161. [Google Scholar] [CrossRef] [PubMed]
- Apovian, C.M. Obesity: Definition, comorbidities, causes, and burden. Am. J. Manag. Care 2016, 22, s176–s185. [Google Scholar] [PubMed]
- Garvey, W.T.; Mechanick, J.I.; Brett, E.M.; Garber, A.J.; Hurley, D.L.; Jastreboff, A.M.; Nadolsky, K.; Pessah-Pollack, R.; Plodkowski, R. American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines for Medical Care of Patients with Obesity. Endocr. Pract. 2016, 22, 1–203. [Google Scholar] [CrossRef]
- Ravussin, E.; Ryan, D.H. Three New Perspectives on the Perfect Storm: What’s Behind the Obesity Epidemic? Obesity 2018, 26, 9–10. [Google Scholar] [CrossRef]
- Bray, G.A.; Frühbeck, G.; Ryan, D.H.; Wilding, J.P. Management of obesity. Lancet 2016, 387, 1947–1956. [Google Scholar] [CrossRef]
- Hall, K.D.; Heymsfield, S.B.; Kemnitz, J.W.; Klein, S.; Schoeller, D.A.; Speakman, J.R. Energy balance and its components: Implications for body weight regulation. Am. J. Clin. Nutr. 2012, 95, 989–994. [Google Scholar] [CrossRef]
- Manore, M.M.; Larson-Meyer, D.E.; Lindsay, A.R.; Hongu, N.; Houtkooper, L. Dynamic Energy Balance: An Integrated Framework for Discussing Diet and Physical Activity in Obesity Prevention-Is it More than Eating Less and Exercising More? Nutrients 2017, 9, 905. [Google Scholar] [CrossRef]
- Keys, A.; Taylor, H.L.; Grande, F. Basal metabolism and age of adult man. Metabolism 1973, 22, 579–587. [Google Scholar] [CrossRef]
- Johns, D.J.; Hartmann-Boyce, J.; Jebb, S.A.; Aveyard, P. Diet or exercise interventions vs combined behavioral weight management programs: A systematic review and meta-analysis of direct comparisons. J. Acad. Nutr. Diet. 2014, 114, 1557–1568. [Google Scholar] [CrossRef]
- Hall, K.D.; Kahan, S. Maintenance of Lost Weight and Long-Term Management of Obesity. Med. Clin. N. Am. 2018, 102, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Doucet, É.; McInis, K.; Mahmoodianfard, S. Compensation in response to energy deficits induced by exercise or diet. Obes. Rev. 2018, 19, 36–46. [Google Scholar] [CrossRef]
- Dorling, J.; Broom, D.R.; Burns, S.F.; Clayton, D.J.; Deighton, K.; James, L.J.; King, J.A.; Miyashita, M.; Thackray, A.E.; Batterham, R.L.; et al. Acute and Chronic Effects of Exercise on Appetite, Energy Intake, and Appetite-Related Hormones: The Modulating Effect of Adiposity, Sex, and Habitual Physical Activity. Nutrients 2018, 10, 1140. [Google Scholar] [CrossRef]
- Thackray, A.E.; Deighton, K.; King, J.A.; Stensel, D.J. Exercise, Appetite and Weight Control: Are There Differences between Men and Women? Nutrients 2016, 8, 583. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.E.; Hill, J.O.; Jacobsen, D.J.; Potteiger, J.; Sullivan, D.K.; Johnson, S.L.; Heelan, K.; Hise, M.; Fennessey, P.V.; Sonko, B.; et al. Effects of a 16-month randomized controlled exercise trial on body weight and composition in young, overweight men and women: The Midwest Exercise Trial. Arch. Intern. Med. 2003, 163, 1343–1350. [Google Scholar] [CrossRef]
- Hagobian, T.A.; Sharoff, C.G.; Stephens, B.R.; Wade, G.N.; Silva, J.E.; Chipkin, S.R.; Braun, B. Effects of exercise on energy-regulating hormones and appetite in men and women. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R233–R242. [Google Scholar] [CrossRef] [PubMed]
- Johnston, B.C.; Kanters, S.; Bandayrel, K.; Wu, P.; Naji, F.; Siemieniuk, R.A.; Ball, G.D.C.; Busse, J.W.; Thorlund, K.; Guyatt, G.; et al. Comparison of Weight Loss Among Named Diet Programs in Overweight and Obese Adults: A Meta-analysis. JAMA 2014, 312, 923–933. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; van Mierlo, C.A.; van der Knaap, H.C.; Heo, M.; Frier, H.I. Weight management using a meal replacement strategy: Meta and pooling analysis from six studies. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 537–549. [Google Scholar] [CrossRef]
- Astbury, N.M.; Piernas, C.; Hartmann-Boyce, J.; Lapworth, S.; Aveyard, P.; Jebb, S.A. A systematic review and meta-analysis of the effectiveness of meal replacements for weight loss. Obes. Rev. 2019, 20, 569–587. [Google Scholar] [CrossRef]
- Kruschitz, R.; Wallner-Liebmann, S.; Lothaller, H.; Luger, M.; Ludvik, B. Long-Term Weight-Loss Maintenance by a Meal Replacement Based Weight Management Program in Primary Care. Obes. Facts 2017, 10, 76–84. [Google Scholar] [CrossRef]
- Feinman, R.D.; Fine, E.J. Thermodynamics and metabolic advantage of weight loss diets. Metab. Syndr. Relat. Disord. 2003, 1. [Google Scholar] [CrossRef]
- Paddon-Jones, D.; Westman, E.; Mattes, R.D.; Wolfe, R.R.; Astrup, A.; Westerterp-Plantenga, M. Protein, weight management, and satiety. Am. J. Clin. Nutr. 2008, 87, 1558s–1561s. [Google Scholar] [CrossRef]
- Pesta, D.H.; Samuel, V.T. A high-protein diet for reducing body fat: Mechanisms and possible caveats. Nutr. Metab. 2014, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.L.P.; Boulé, N.G.; Sharma, A.M.; Elliott, S.; Siervo, M.; Ghosh, S.; Berg, A.; Prado, C.M. Examining the effects of a high-protein total diet replacement on energy metabolism, metabolic blood markers, and appetite sensations in healthy adults: Protocol for two complementary, randomized, controlled, crossover trials. Trials 2019, 20, 787. [Google Scholar] [CrossRef]
- Oliveira, C.L.P.; Boulé, N.G.; Sharma, A.M.; Elliott, S.; Siervo, M.; Ghosh, S.; Berg, A.; Prado, C.M. A high-protein total diet replacement increases energy expenditure and leads to negative fat balance in healthy, normal-weight adults. Am. J. Clin. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Godin, G. The Godin-Shephard Leisure-Time Physical Activity Questionnaire. Health Fit. J. Can. 2011, 4, 18–22. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients); The National Academies Press: Washington, DC, USA, 2005; p. 1357. [Google Scholar] [CrossRef]
- Smith, S.R.; de Jonge, L.; Zachwieja, J.J.; Roy, H.; Nguyen, T.; Rood, J.C.; Windhauser, M.M.; Bray, G.A. Fat and carbohydrate balances during adaptation to a high-fat diet. Am. J. Clin. Nutr. 2000, 71, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Austin, G.L.; Ogden, L.G.; Hill, J.O. Trends in carbohydrate, fat, and protein intakes and association with energy intake in normal-weight, overweight, and obese individuals: 1971–2006. Am. J. Clin. Nutr. 2011, 93, 836–843. [Google Scholar] [CrossRef]
- Brouwer, E. On simple formulae for calculating the heat expenditure and the quantities of carbohydrate and fat oxidized in metabolism of men and animals, from gaseous exchange (Oxygen intake and carbonic acid output) and urine-N. Acta Physiol. Pharmacol. Neerl. 1957, 6, 795–802. [Google Scholar]
- Koohkan, S.; McCarthy, D.H.; Berg, A. The effect of a soy-yoghurt-honey product on excess weight and related health risk factors—A review. J. Nutr. Health Food Sci. 2017, 5, 1–10. [Google Scholar] [CrossRef][Green Version]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. Relat. Metab. Disord. 2000, 24. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Gasteyger, C.; Raben, A.; Meier, D.H.; Astrup, A.; Sjodin, A. The effect of tesofensine on appetite sensations. Obesity 2012, 20, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Coyle, E.F.; Jeukendrup, A.E.; Wagenmakers, A.J.; Saris, W.H. Fatty acid oxidation is directly regulated by carbohydrate metabolism during exercise. Am. J. Physiol. 1997, 273, E268–E275. [Google Scholar] [CrossRef]
- Prentice, A.M. Manipulation of dietary fat and energy density and subsequent effects on substrate flux and food intake. Am. J. Clin. Nutr. 1998, 67, 535s–541s. [Google Scholar] [CrossRef] [PubMed]
- Spriet, L.L. New insights into the interaction of carbohydrate and fat metabolism during exercise. Sports Med. 2014, 44, S87–S96. [Google Scholar] [CrossRef]
- Patterson, R.; Potteiger, J. A comparison of normal versus low dietary carbohydrate intake on substrate oxidation during and after moderate intensity exercise in women. Eur. J. Appl. Physiol. 2011, 111, 3143–3150. [Google Scholar] [CrossRef] [PubMed]
- San-Cristobal, R.; Navas-Carretero, S.; Martínez-González, M.Á.; Ordovas, J.M.; Martínez, J.A. Contribution of macronutrients to obesity: Implications for precision nutrition. Nat. Rev. Endocrinol. 2020, 16, 305–320. [Google Scholar] [CrossRef]
- Barwell, N.D.; Malkova, D.; Leggate, M.; Gill, J.M.R. Individual responsiveness to exercise-induced fat loss is associated with change in resting substrate utilization. Metabolism 2009, 58, 1320–1328. [Google Scholar] [CrossRef]
- Zurlo, F.; Lillioja, S.; Esposito-Del Puente, A.; Nyomba, B.L.; Raz, I.; Saad, M.F.; Swinburn, B.A.; Knowler, W.C.; Bogardus, C.; Ravussin, E. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: Study of 24-h RQ. Am. J. Physiol. 1990, 259, E650–E657. [Google Scholar] [CrossRef]
- Galgani, J.; Ravussin, E. Energy metabolism, fuel selection and body weight regulation. Int. J. Obes. 2008, 32, S109–S119. [Google Scholar] [CrossRef]
- Mayer, J.; Marshall, N.B.; Vitale, J.J.; Christensen, J.H.; Mashayekhi, M.B.; Stare, F.J. Exercise, food intake and body weight in normal rats and genetically obese adult mice. Am. J. Physiol. 1954, 177, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Edholm, O.G.; Fletcher, J.G.; Widdowson, E.M.; McCance, R.A. The energy expenditure and food intake of individual men. Br. J. Nutr. 1955, 9, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, M.; King, N.A.; Blundell, J.E. Acute and long-term effects of exercise on appetite control: Is there any benefit for weight control? Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.E.; Gibbons, C.; Caudwell, P.; Finlayson, G.; Hopkins, M. Appetite control and energy balance: Impact of exercise. Obes. Rev. 2015, 16, 67–76. [Google Scholar] [CrossRef]
- Drummen, M.; Tischmann, L.; Gatta-Cherifi, B.; Adam, T.; Westerterp-Plantenga, M. Dietary Protein and Energy Balance in Relation to Obesity and Co-morbidities. Front. Endocrinol. 2018, 9. [Google Scholar] [CrossRef]
- Dougkas, A.; Östman, E. Protein-Enriched Liquid Preloads Varying in Macronutrient Content Modulate Appetite and Appetite-Regulating Hormones in Healthy Adults. J. Nutr. 2016, 146, 637–645. [Google Scholar] [CrossRef]
- Mattes, R. Fluid calories and energy balance: The good, the bad, and the uncertain. Physiol. Behav. 2006, 89, 66–70. [Google Scholar] [CrossRef]
- Martens, M.J.; Lemmens, S.G.; Born, J.M.; Westerterp-Plantenga, M.S. A solid high-protein meal evokes stronger hunger suppression than a liquefied high-protein meal. Obesity 2011, 19, 522–527. [Google Scholar] [CrossRef]
- Zanchi, D.; Depoorter, A.; Egloff, L.; Haller, S.; Mählmann, L.; Lang, U.E.; Drewe, J.; Beglinger, C.; Schmidt, A.; Borgwardt, S. The impact of gut hormones on the neural circuit of appetite and satiety: A systematic review. Neurosci. Biobehav. Rev. 2017, 80, 457–475. [Google Scholar] [CrossRef]
- Perry, B.; Wang, Y. Appetite regulation and weight control: The role of gut hormones. Nutr. Diabetes 2012, 2, e26. [Google Scholar] [CrossRef]
- Freire, R.H.; Alvarez-Leite, J.I. Appetite control: Hormones or diet strategies? Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, M.P.; Westerterp, K.R.; Adam, T.C.; Luscombe-Marsh, N.D.; Westerterp-Plantenga, M.S. Ghrelin and glucagon-like peptide 1 concentrations, 24-h satiety, and energy and substrate metabolism during a high-protein diet and measured in a respiration chamber. Am. J. Clin. Nutr. 2006, 83, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Batterham, R.L.; Heffron, H.; Kapoor, S.; Chivers, J.E.; Chandarana, K.; Herzog, H.; Le Roux, C.W.; Thomas, E.L.; Bell, J.D.; Withers, D.J. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006, 4, 223–233. [Google Scholar] [CrossRef]
- Kavanagh, K.; Jones, K.L.; Zhang, L.; Flynn, D.M.; Shadoan, M.K.; Wagner, J.D. High isoflavone soy diet increases insulin secretion without decreasing insulin sensitivity in premenopausal nonhuman primates. Nutr. Res. 2008, 28, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Lang, V.; Bellisle, F.; Alamowitch, C.; Craplet, C.; Bornet, F.R.J.; Slama, G.; Guy-Grand, B. Varying the protein source in mixed meal modifies glucose, insulin and glucagon kinetics in healthy men, has weak effects on subjective satiety and fails to affect food intake. Eur. J. Clin. Nutr. 1999, 53, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, F.Q.; Gannon, M.C. Metabolic response of people with type 2 diabetes to a high protein diet. Nutr. Metab. 2004, 1, 6. [Google Scholar] [CrossRef]
- Liu, D.; Zhen, W.; Yang, Z.; Carter, J.D.; Si, H.; Reynolds, K.A. Genistein Acutely Stimulates Insulin Secretion in Pancreatic β-Cells Through a cAMP-Dependent Protein Kinase Pathway. Diabetes 2006, 55, 1043–1050. [Google Scholar] [CrossRef]
- Parks, E.J. Effect of dietary carbohydrate on triglyceride metabolism in humans. J. Nutr. 2001, 131, 2772s–2774s. [Google Scholar] [CrossRef]
- Wolfe, B.M.; Piche, L.A. Replacement of carbohydrate by protein in a conventional-fat diet reduces cholesterol and triglyceride concentrations in healthy normolipidemic subjects. Clin. Invest. Med. 1999, 22, 140–148. [Google Scholar] [PubMed]
- Brown, L.; Rosner, B.; Willett, W.W.; Sacks, F.M. Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am. J. Clin. Nutr. 1999, 69, 30–42. [Google Scholar] [CrossRef]
- Sánchez-Muniz, F.J. Dietary fibre and cardiovascular health. Nutr. Hosp. 2012, 27, 31–45. [Google Scholar] [PubMed]
- Reshef, L.; Olswang, Y.; Cassuto, H.; Blum, B.; Croniger, C.M.; Kalhan, S.C.; Tilghman, S.M.; Hanson, R.W. Glyceroneogenesis and the triglyceride/fatty acid cycle. J. Biol. Chem. 2003, 278. [Google Scholar] [CrossRef]
- Boulé, N.; Prud’homme, D. Physical Activity in Obesity Management. Available online: https://obesitycanada.ca/guidelines/physicalactivity/ (accessed on 29 October 2020).
- Goodpaster, B.H.; Katsiaras, A.; Kelley, D.E. Enhanced Fat Oxidation through Physical Activity Is Associated With Improvements in Insulin Sensitivity in Obesity. Diabetes 2003, 52, 2191. [Google Scholar] [CrossRef] [PubMed]
- Burton, F.L.; Malkova, D.; Caslake, M.J.; Gill, J.M. Energy replacement attenuates the effects of prior moderate exercise on postprandial metabolism in overweight/obese men. Int. J. Obes. 2008, 32, 481–489. [Google Scholar] [CrossRef]
- Westerterp-Plantenga, M.S.; Lejeune, M.P.; Smeets, A.J.; Luscombe-Marsh, N.D. Sex differences in energy homeostatis following a diet relatively high in protein exchanged with carbohydrate, assessed in a respiration chamber in humans. Physiol. Behav. 2009, 97, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Lori, A.; Nori, G. Modulation of Appetite by Gonadal Steroid Hormones. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1251. [Google Scholar] [CrossRef]
- Cornier, M.-A.; Salzberg, A.K.; Endly, D.C.; Bessesen, D.H.; Tregellas, J.R. Sex-based differences in the behavioral and neuronal responses to food. Physiol. Behav. 2010, 99, 538–543. [Google Scholar] [CrossRef]
- Brennan, I.M.; Feltrin, K.L.; Nair, N.S.; Hausken, T.; Little, T.J.; Gentilcore, D.; Wishart, J.M.; Jones, K.L.; Horowitz, M.; Feinle-Bisset, C. Effects of the phases of the menstrual cycle on gastric emptying, glycemia, plasma GLP-1 and insulin, and energy intake in healthy lean women. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G602–G610. [Google Scholar] [CrossRef]
- Buffenstein, R.; Poppitt, S.D.; McDevitt, R.M.; Prentice, A.M. Food intake and the menstrual cycle: A retrospective analysis, with implications for appetite research. Physiol. Behav. 1995, 58, 1067–1077. [Google Scholar] [CrossRef]
- Asarian, L.; Geary, N. Sex differences in the physiology of eating. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R1215–R1267. [Google Scholar] [CrossRef]

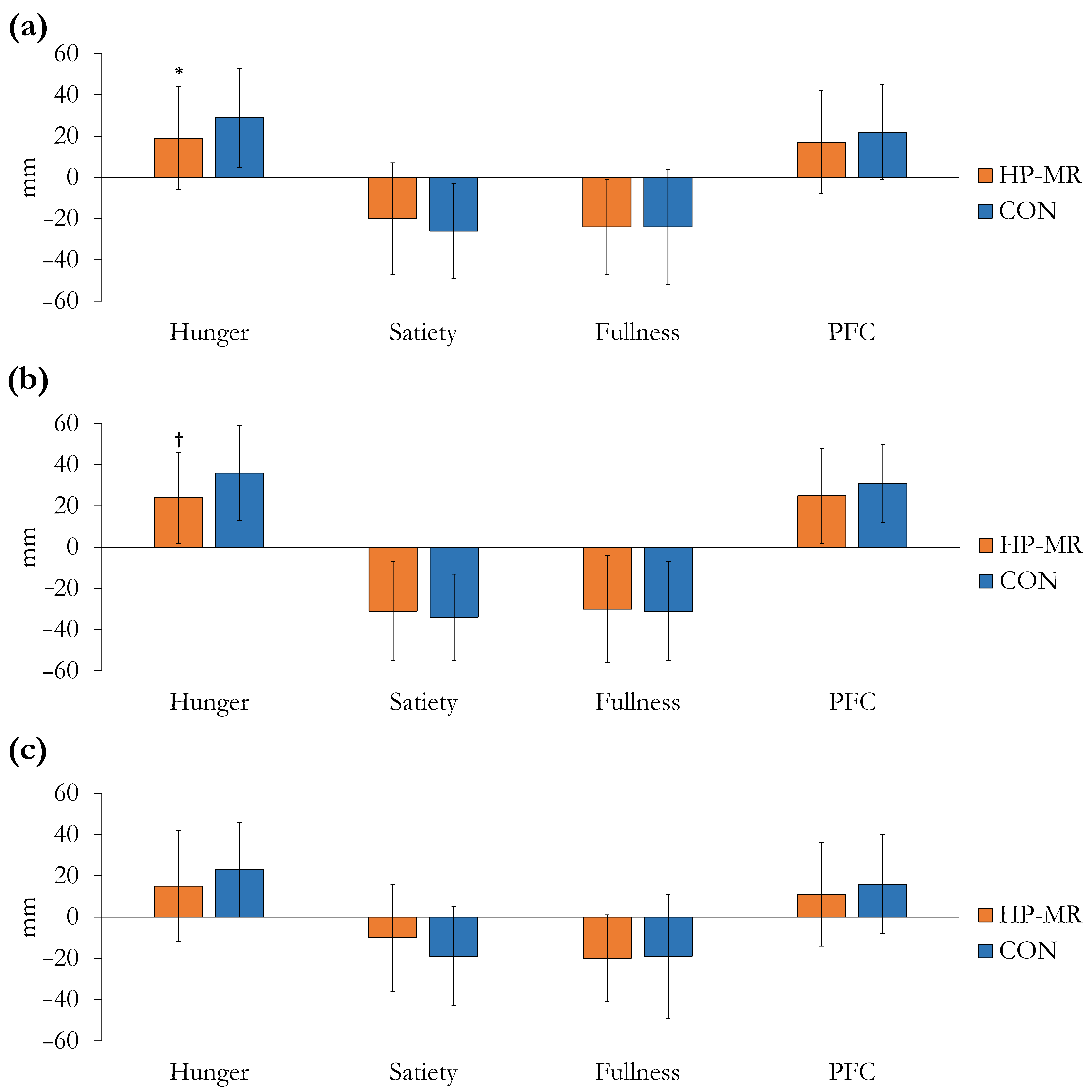

| HP-MR | CON | Diet Difference a | |||||
|---|---|---|---|---|---|---|---|
| All (n = 43) | Females (n = 19) | Males (n = 24) | All (n = 43) | Females (n = 19) | Males (n = 24) | ||
| Energy | |||||||
| Kcal/meal | 413 ± 74 | 366 ± 59 | 450 ± 63 | 409 ± 72 | 360 ± 51 | 448 ± 63 | <0.001 |
| kcal/kg body weight | 6 ± 1 | 6 ± 1 | 7 ± 1 | 6 ± 1 | 6 ± 1 | 7 ± 1 | <0.001 |
| Protein | |||||||
| % energy | 42.6 ± 0.8 | 43.0 ± 0.9 | 42.3 ± 0.4 | 14.7 ± 0.8 | 14.3 ± 1.1 | 15.1 ± 0.2 | <0.001 |
| g/meal | 44 ± 7 | 39 ± 6 | 47 ± 6 | 16 ± 3 | 14 ± 3 | 17 ± 2 | <0.001 |
| g/kg body weight | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.0 | <0.001 |
| Fat | |||||||
| % energy | 26.6 ± 0.6 | 26.4 ± 0.4 | 26.7 ± 0.6 | 30.2 ± 1.8 | 30.5 ± 2.8 | 29.9 ± 0.3 | <0.001 |
| g/meal | 12 ± 2 | 11 ± 2 | 13 ± 2 | 14 ± 3 | 12 ± 2 | 15 ± 2 | <0.001 |
| g/kg body weight | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | <0.001 |
| Carbohydrate | |||||||
| % energy | 30.8 ± 0.6 | 30.6 ± 0.8 | 31.0 ± 0.4 | 55.0 ± 1.9 | 55.2 ± 2.8 | 54.9 ± 0.3 | <0.001 |
| g/meal | 32 ± 6 | 28 ± 5 | 35 ± 5 | 58 ± 10 | 52 ± 8 | 63 ± 9 | <0.001 |
| g/kg body weight | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.1 | <0.001 |
| Sugars (g/meal) | 32 ± 6 | 28 ± 5 | 35 ± 5 | 27 ± 4 | 25 ± 3 | 29 ± 4 | <0.001 |
| Fiber (g/meal) | 1 ± 0 | 1 ± 0 | 1 ± 0 | 7 ± 1 | 6 ± 1 | 8 ± 1 | <0.001 |
| Saturated Fat (g/meal) | 3 ± 0 | 2 ± 0 | 3 ± 0 | 3 ± 1 | 3 ± 0 | 3 ± 0 | <0.001 |
| Monounsaturated Fat (g/meal) | 7 ± 1 | 6 ± 1 | 8 ± 1 | 6 ± 1 | 6 ± 1 | 7 ± 1 | <0.001 |
| Polyunsaturated Fat (g/meal) | 1 ± 0 | 1 ± 0 | 1 ± 0 | 4 ± 1 | 3 ± 1 | 4 ± 1 | <0.001 |
| Cholesterol (mg/meal) | 12 ± 3 | 10 ± 3 | 13 ± 2 | 4 ± 28 | 10 ± 42 | 0 ± 0 | <0.001 |
| Characteristics | All (n = 43) |
|---|---|
| Age (years) | 24 ± 4 |
| Height (cm) | 171.1 ± 7.3 |
| Weight (kg) | 64.4 ± 6.9 |
| Waist Circumference (cm) | 74.4 ±5.6 |
| BMI (kg/m2) | 22.0 ± 1.4 |
| FM (kg) (F/M) | 18.6 ± 3.3/12.7 ± 4.9 |
| LST (kg) (F/M) | 40.1 ± 4.4/51.4 ± 5.6 |
| Ethnicity | |
| White | 19 (44) |
| Asian | 14 (33) |
| Hispanic | 3 (7) |
| Black | 1 (2) |
| Other | 6 (14) |
| HP-MR | CON | ∆a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fasting | Post-Exercise | ∆ a | Time Effect b | Time × Sex b | Fasting | Post-Exercise | ∆ a | Time Effect b | Time × Sex b | Diet Effect c | Diet × Sex c | |
| Glucose (mmol/L) | 4.8 ± 0.3 | 5.1 ± 0.4 | 0.3 ± 0.4 | <0.001 | 0.371 | 4.8 ± 0.3 | 5.1 ± 0.6 | 0.2 ± 0.6 | 0.008 | 0.366 | 0.662 | 0.800 |
| Insulin (pmol/L) d | 43.1 ± 15.4 | 95.8 ± 59.7 | 52.9 ± 52.2 | <0.001 | 0.123 | 44.3 ± 18.4 | 78.5 ± 40.0 | 34.1 ± 33.2 | <0.001 | 0.057 | 0.042 | 0.744 |
| Lipid Panel d | ||||||||||||
| Total Cholesterol (mmol/L) | 4.34 ± 0.73 | 4.37 ± 0.73 | 0.02 ± 0.18 | 0.196 | 0.017 | 4.28 ± 0.68 | 4.29 ± 0.69 | 0.01 ± 0.17 | 0.643 | 0.294 | 0.528 | 0.266 |
| HDL Cholesterol (mmol/L) | 1.45 ± 0.43 | 1.46 ± 0.45 | 0.01 ± 0.06 | 0.125 | 0.056 | 1.43 ± 0.43 | 1.44 ± 0.43 | 0.01 ± 0.07 | 0.214 | 0.795 | 0.987 | 0.251 |
| Non-HDL Cholesterol (mmol/L) | 2.89 ± 0.57 | 2.90 ± 0.55 | 0.01 ± 0.13 | 0.287 | 0.020 | 2.85 ± 0.49 | 2.85 ± 0.49 | −0.01 ± 0.11 | 0.909 | 0.146 | 0.323 | 0.376 |
| LDL Cholesterol (mmol/L) | 2.40 ± 0.52 | 2.38 ± 0.48 | −0.02 ± 0.13 | 0.314 | 0.010 | 2.36 ± 0.48 | 2.26 ± 0.47 | −0.11 ± 0.14 | <0.001 | 0.014 | 0.003 | 0.978 |
| Triglyceride (mmol/L) | 1.06 ± 0.42 | 1.15 ± 0.50 | 0.08 ± 0.19 | 0.008 | 0.829 | 1.07 ± 0.42 | 1.30 ± 0.51 | 0.22 ± 0.27 | <0.001 | 0.194 | 0.002 | 0.337 |
| Glycerol (µM) e | 27.5 ± 19.6 | 36.3 ± 28.8 | 7.8 ± 15.1 | <0.001 | 0.001 | 23.1 ± 14.1 | 37.3 ± 22.3 | 14.3 ± 14.8 | <0.001 | 0.001 | 0.015 | 0.975 |
| NEFA (µM) e | 201.2 ± 191.6 | 188.5 ± 222.9 | −13.9 ± 204.4 | 0.774 | 0.296 | 176.5 ± 147.9 | 183.6 ± 173.4 | 7.1 ± 114.9 | 0.607 | 0.391 | 0.514 | 0.551 |
| Leptin (pg/mL) d | 8702.86 ± 9994.55 | 7418.60 ± 8780.62 | −1241.71 ± 1903.88 | <0.001 | <0.001 | 9026.12 ± 10798.76 | 7300.37 ± 8360.41 | −1725.74 ± 3147.62 | <0.001 | <0.001 | 0.154 | 0.153 |
| PYY (pg/mL) f | 123.34 ± 61.70 | 196.76 ± 79.42 | 73.90 ± 50.70 | <0.001 | 0.265 | 124.89 ± 64.05 | 168.55 ± 79.87 | 47.65 ± 61.48 | <0.001 | 0.229 | 0.031 | 0.052 |
| GLP-1 (pM) e | 1.44 ± 3.17 | 5.03 ± 5.59 | 3.68 ± 3.25 | <0.001 | <0.001 | 1.47 ± 3.16 | 3.54 ± 4.47 | 2.07 ± 2.74 | <0.001 | 0.001 | 0.001 | 0.009 |
| Ghrelin (pg/mL) e | 442.76 ± 296.13 | 292.54 ± 190.23 | −149.38 ± 157.07 | <0.001 | 0.023 | 474.33 ± 316.86 | 288.58 ± 183.35 | −185.75 ± 199.17 | <0.001 | 0.009 | 0.128 | 0.335 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, C.L.P.; Boulé, N.G.; Berg, A.; Sharma, A.M.; Elliott, S.A.; Siervo, M.; Ghosh, S.; Prado, C.M. Consumption of a High-Protein Meal Replacement Leads to Higher Fat Oxidation, Suppression of Hunger, and Improved Metabolic Profile After an Exercise Session. Nutrients 2021, 13, 155. https://doi.org/10.3390/nu13010155
Oliveira CLP, Boulé NG, Berg A, Sharma AM, Elliott SA, Siervo M, Ghosh S, Prado CM. Consumption of a High-Protein Meal Replacement Leads to Higher Fat Oxidation, Suppression of Hunger, and Improved Metabolic Profile After an Exercise Session. Nutrients. 2021; 13(1):155. https://doi.org/10.3390/nu13010155
Chicago/Turabian StyleOliveira, Camila L. P., Normand G. Boulé, Aloys Berg, Arya M. Sharma, Sarah A. Elliott, Mario Siervo, Sunita Ghosh, and Carla M. Prado. 2021. "Consumption of a High-Protein Meal Replacement Leads to Higher Fat Oxidation, Suppression of Hunger, and Improved Metabolic Profile After an Exercise Session" Nutrients 13, no. 1: 155. https://doi.org/10.3390/nu13010155
APA StyleOliveira, C. L. P., Boulé, N. G., Berg, A., Sharma, A. M., Elliott, S. A., Siervo, M., Ghosh, S., & Prado, C. M. (2021). Consumption of a High-Protein Meal Replacement Leads to Higher Fat Oxidation, Suppression of Hunger, and Improved Metabolic Profile After an Exercise Session. Nutrients, 13(1), 155. https://doi.org/10.3390/nu13010155








