Diet Modifications in Primary Prevention of Asthma. Where Do We Stand?
Abstract
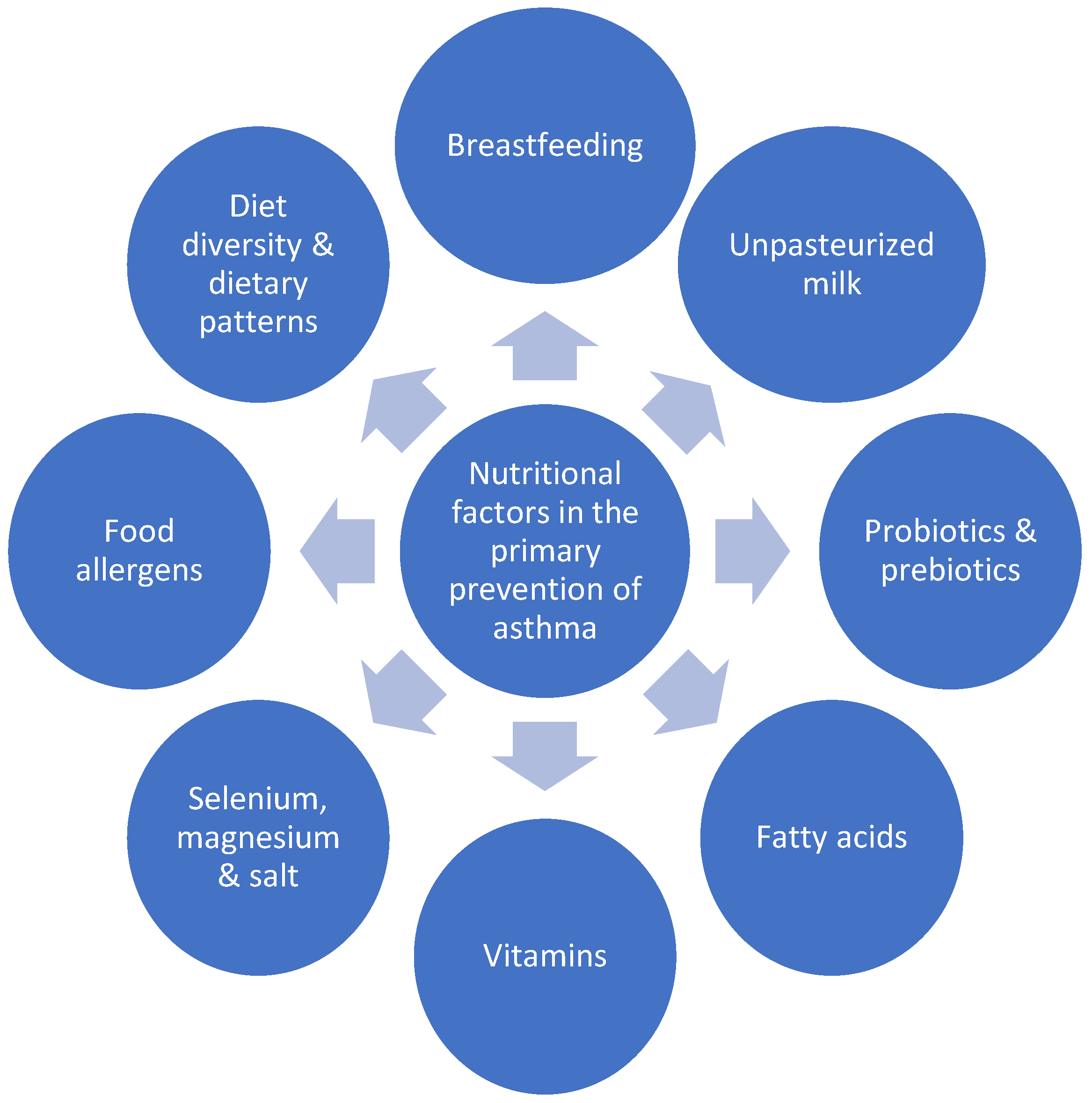
:1. Introduction
2. Breastfeeding and Infant Milk Formulas
3. Unpasteurized Milk
4. Probiotics and Prebiotics
5. Fatty Acids
6. Vitamins
6.1. Vitamin D
6.2. Vitamin A
6.3. Vitamin C
6.4. Vitamin E
7. Selenium, Magnesium and Salt
8. Food Allergens
9. Diet Diversity and Dietary Patterns
10. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- von Mutius, E.; Smits, H.H. Primary prevention of asthma: From risk and protecive factors to targeted strategies for prevention. Lancet 2020, 396, 854–866. [Google Scholar] [CrossRef]
- Brick, T.; Hettinga, K.; Kirchner, B.; Pfaffl, M.W.; Ege, M.J. The Beneficial Effect of Farm Milk Consumption on Asthma, Allergies, and Infections: From Meta-Analysis of Evidence to Clinical Trial. J. Allergy Clin. Immunol. Pract. 2020, 8, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Litonjua, A.A.; Carey, V.J.; Laranjo, N.; Harshfield, B.J.; McElrath, T.F.; O’Connor, G.T.; Sandel, M.; Iverson, R.E., Jr.; Lee-Paritz, A.; Strunk, R.C.; et al. Effect of prenatal supplementation with vitamin D on asthma or recurrent wheezing in offspring by age 3 years: The VDAART randomized clinical trial. JAMA 2016, 315, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Bisgaard, H.; Stokholm, J.; Chawes, B.L.; Vissing, N.H.; Bjarnadóttir, E.; Schoos, A.M.; Wolsk, H.M.; Pedersen, T.M.; Vinding, R.K.; Thorsteinsdóttir, S.; et al. Fish Oil-Derived Fatty Acids in Pregnancy and Wheeze and Asthma in Offspring. N. Engl. J. Med. 2016, 375, 2530–2539. [Google Scholar] [CrossRef]
- Venter, C.; Greenhawt, M.; Meyer, R.W.; Agostoni, C.; Reese, I.; du Toit, G.; Feeney, M.; Maslin, K.; Nwaru, B.I.; Roduit, C.; et al. EAACI position paper on diet diversity in pregnancy, infancy and childhood: Novel concepts and implications for studies in allergy and asthma. Allergy 2019, 75, 497–523. [Google Scholar] [CrossRef] [Green Version]
- Victora, C.G.; Bahl, R.; Barros, A.; França, G.; Horton, S.; Krasevec, J.; Murch, S.; Jeeva Sankar, M.; Walker, N.; Rollins, N.C. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef] [Green Version]
- Gdalevich, M.; Mimouni, D.; Mimouni, M. Breast-feeding and the risk of bronchial asthma in childhood: A systematic review with meta-analysis of prospective studies. J. Pediatr. 2001, 139, 261–266. [Google Scholar] [CrossRef]
- Brew, B.K.; Allen, C.W.; Toelle, B.G.; Marks, G.B. Systematic review and meta-analysis investigating breast feeding and childhood wheezing illness. Paediatr. Perinat. Epidemiol. 2011, 25, 507–518. [Google Scholar] [CrossRef]
- Dogaru, C.M.; Nyffenegger, D.; Pescatore, A.M.; Spycher, B.D.; Kuehni, C.E. Breastfeeding and childhood asthma: Systematic review and meta-analysis. Am. J. Epidemiol. 2014, 179, 1153–1167. [Google Scholar] [CrossRef] [Green Version]
- Lodge, C.J.; Tan, D.J.; Lau, M.; Dai, X.; Tham, R.; Lowe, A.J.; Bowatte, G.; Allen, K.J.; Dharmage, S.C. Breastfeeding and asthma and allergies: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 38–53. [Google Scholar] [CrossRef]
- Sansotta, N.; Piacentini, G.L.; Mazzei, F.; Minniti, F.; Boner, A.L.; Peroni, D.G. Timing of introduction of solid food and risk of allergic disease development: Understanding the evidence. Allergol. Immunopathol. 2013, 41, 337–345. [Google Scholar] [CrossRef]
- Zutavern, A.; Brockow, I.; Schaaf, B.; von Berg, A.; Diez, U.; Borte, M.; Kraemer, U.; Herbarth, O.; Behrendt, H.; Wichmann, H.E.; et al. Timing of solid food introduction in relation to eczema, asthma, allergic rhinitis, and food and inhalant sensitization at the age of 6 years: Results from the prospective birth cohort study LISA. Pediatrics 2008, 121, 44–52. [Google Scholar] [CrossRef]
- Nwaru, B.I.; Takkinen, H.M.; Niemelä, O.; Kaila, M.; Erkkola, M.; Ahonen, S.; Haapala, A.M.; Kenward, M.G.; Pekkanen, J.; Lahesmaa, R.; et al. Timing of infant feeding in relation to childhood asthma and allergic diseases. J. Allergy Clin. Immunol. 2013, 131, 78–86. [Google Scholar] [CrossRef] [PubMed]
- El-Heneidy, A.; Abdel-Rahman, M.E.; Mihala, G.; Ross, L.J.; Comans, T.A. Milk Other Than Breast Milk and the Development of Asthma in Children 3 Years of Age. A Birth Cohort Study (2006–2011). Nutrients 2018, 10, 1798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greer, F.R.; Sicherer, S.H.; Burks, A.W.; AAP Committee on Nutrition; AAP Section on Allergy and Immunology. The Effects of Early Nutritional Interventions on the Development of Atopic Disease in Infants and Children: The Role of Maternal Dietary Restriction, Breastfeeding, Hydrolyzed Formulas, and Timing of Introduction of Allergenic Complementary Foods. Pediatrics 2019, 143, e20190281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Berg, A.; Koletzko, S.; Filipiak-Pittroff, B.; Laubereau, B.; Grübl, A.; Wichmann, H.E.; Bauer, C.P.; Reinhardt, D.; Berdel, D.; German Infant Nutritional Intervention Study Group. Certain hydrolyzed formulas reduce the incidence of atopic dermatitis but not that of asthma: Three-year results of the German Infant Nutritional Intervention Study. J. Allergy Clin. Immunol. 2007, 119, 718–725. [Google Scholar] [CrossRef]
- Sozańska, B. Raw Cow’s Milk and Its Protective Effect on Allergies and Asthma. Nutrients 2019, 11, 469. [Google Scholar] [CrossRef] [Green Version]
- Braun-Fahrländer, C.; von Mutius, E. Can farm milk consumption prevent allergic diseases? Clin. Exp. Allergy 2011, 41, 29–35. [Google Scholar] [CrossRef]
- Sozańska, B.; Pearce, N.; Dudek, K.; Cullinan, P. Consumption of unpasteurized milk and its effects on atopy and asthma in children and adult inhabitants in rural Poland. Allergy 2013, 68, 644–650. [Google Scholar] [CrossRef]
- Loss, G.; Apprich, S.; Waser, M.; Kneifel, W.; von Mutius, E.; Genuneit, J.; Buchele, G.; Weber, J.; Sozańska, B.; Danielewicz, H.; et al. The protective effect of farm milk consumption on childhood asthma and atopy: The GABRIELA study. J. Allergy Clin. Immunol. 2011, 128, 766–773. [Google Scholar] [CrossRef]
- Brick, T.; Schober, Y.; Böcking, C.; Pekkanen, J.; Genuneit, J.; Loss, G.; Dalphin, J.C.; Riedler, J.; Lauener, R.; Nockher, W.A.; et al. ω-3 fatty acids contribute to the asthma-protective effect of unprocessed cow’s milk. J. Allergy Clin. Immunol. 2016, 137, 1699–1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbring, S.; Verheijden, K.A.T.; Diks, M.A.P.; Leusink-Muis, A.; Hols, G.; Baars, T.; Garssen, J.; van Esch, B.C.A.M. Raw Cow’s Milk Prevents the Development of Airway Inflammation in a Murine House Dust Mite-Induced Asthma Model. Front. Immunol. 2017, 8, 1045. [Google Scholar] [CrossRef] [Green Version]
- van Neerven, R.J.J.; Knol, E.F.; Heck, J.M.L.; Savelkoul, H.F.J. Which factors in raw cow’s milk contribute to protection against allergies? J. Allergy Clin. Immunol. 2012, 130, 853–858. [Google Scholar] [CrossRef] [PubMed]
- van Esch, B.C.; Porbahaie, M.; Abbring, S.; Garssen, J.; Potaczek, D.P.; Savelkoul, H.F.; van Neerven, R.J.J. The Impact of Milk and Its Components on Epigenetic Programming of Immune Function in Early Life and Beyond: Implications for Allergy and Asthma. Front. Immunol. 2020, 11, 2141. [Google Scholar] [CrossRef]
- Baskara-Yhuellou, I.; Tost, J. The impact of microRNAs on alterations of gene regulatory networks in allergic diseases. Adv. Protein. Chem. Struct. Biol. 2020, 120, 237–312. [Google Scholar] [PubMed]
- Malmhall, C.; Johansson, K.; Winkler, C.; Alawieh, S.; Ekerljung, L.; Radinger, M. Altered miR-155 Expression in Allergic Asthmatic Airways. Scand. J. Immunol. 2017, 85, 300–307. [Google Scholar] [CrossRef] [Green Version]
- Kirchner, B.; Pfaffl, M.W.; Dumpler, J.; von Mutius, E.; Ege, M.J. microRNA in native and processed cow’s milk and its implication for the farm milk effect on asthma. J. Allergy Clin. Immunol. 2016, 137, 1893–1895. [Google Scholar] [CrossRef] [Green Version]
- Bieli, C.; Eder, W.; Frei, R.; Klimecki, W.; Waser, M.; Riedler, J.; von Mutius, E.; Scheynius, A.; Pershagen, G.; Doekes, G.; et al. A polymorphism in CD14 modifies the effect of farm milk consumption on allergic diseases and CD14 gene expression. J. Allergy Clin. Immunol. 2007, 120, 1308–1315. [Google Scholar] [CrossRef]
- Gosalbes, M.J.; Llop, S.; Vallès, Y.; Moya, A.; Ballester, F.; Francino, M.P. Meconium microbiota types dominated by lactic acid or enteric bacteria are differentially associated with maternal eczema and respiratory problems in infants. Clin. Exp. Allergy 2013, 43, 198–211. [Google Scholar] [CrossRef]
- Tan, J.Y.; Tang, Y.C.; Huang, J. Gut Microbiota and Lung Injury. Adv. Exp. Med. Biol. 2020, 1238, 55–72. [Google Scholar]
- Lynch, S.; Pedersen, O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjorksten, B.; Naaber, P.; Sepp, E.; Mikelsaar, M. The intestinal microflora in allergic Estonian and Swedish 2-year-old children. Clin. Exp. Allergy. 1999, 29, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Stokholm, J.; Thorsen, J.; Blaser, M.J.; Rasmussen, M.A.; Hjelmsø, M.; Shah, S.; Christensen, E.D.; Chawes, B.L.; Bønnelykke, K.; Brix, S.; et al. Delivery mode and gut microbial changes correlate with an increased risk of childhood asthma. Sci. Transl. Med. 2020, 12, eaax9929. [Google Scholar] [CrossRef] [PubMed]
- Stokholm, J.; Blaser, M.J.; Thorsen, J.; Rasmussen, M.A.; Waage, J.; Vinding, R.K.; Schoos, A.M.; Kunøe, A.; Fink, N.R.; Chawes, B.L.; et al. Maturation of the gut microbiome and risk of asthma in childhood. Nat. Commun. 2018, 9, 141. [Google Scholar] [CrossRef]
- Azad, M.B.; Coneys, J.G.; Kozyrskyj, A.L.; Field, C.J.; Ramsey, C.D.; Becker, A.B.; Friesen, C.; Abou-Setta, A.M.; Zarychanski, R. Probiotic supplementation during pregnancy or infancy for the prevention of asthma and wheeze: Systematic review and meta-analysis. BMJ 2013, 347, 6471. [Google Scholar] [CrossRef] [Green Version]
- Cuello-Garcia, C.A.; Brozek, J.L.; Fiocchi, A.; Pawankar, R.; Yepes-Nuñez, J.J.; Terracciano, L.; Gandhi, S.; Agarwal, A.; Zhang, Y.; Schünemann, H.J. Probiotics for the prevention of allergy: A systematic review and meta-analysis of randomized controlled trials. J. Allergy Clin. Immunol. 2015, 136, 952–961. [Google Scholar] [CrossRef]
- Cabana, M.D.; McKean, M.; Caughey, A.B.; Fong, L.; Lynch, S.; Wong, A.; Leong, R.; Boushey, H.A.; Hilton, J.F. Early Probiotic Supplementation for Eczema and Asthma Prevention: A Randomized Controlled Trial. Pediatrics 2017, 140, e20163000. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Jiang, P.; Liu, J.; Sun, R.; Zhu, L. Association between probiotic supplementation and asthma incidence in infants: A meta-analysis of randomized controlled trials. J. Asthma 2020, 57, 167–178. [Google Scholar] [CrossRef]
- Rastall, R.A.; Gibson, G.R. Recent developments in prebiotics to selectively impact beneficial microbes and promote intestinal health. Curr. Opin. Biotechnol. 2015, 32, 42–46. [Google Scholar] [CrossRef]
- Cuello-Garcia, C.; Fiocchi, A.; Pawankar, R.; Yepes-Nuñez, J.J.; Morgano, G.P.; Zhang, Y.; Agarwal, A.; Gandhi, S.; Terracciano, L.; Schünemann, H.J.; et al. Prebiotics for the prevention of allergies: A systematic review and meta-analysis of randomized controlled trials. Clin. Exp. Allergy 2017, 47, 1468–1477. [Google Scholar] [CrossRef]
- Osborn, D.A.; Sinn, J.K. Prebiotics in infants for prevention of allergy. Cochrane Database Syst. Rev. 2013, 3, Cd006474. [Google Scholar] [CrossRef] [PubMed]
- West, C.E.; Jenmalm, M.C.; Kozyrskyj, A.L.; Prescott, S.L. Probiotics for treatment and primary prevention of allergic diseases and asthma: Looking back and moving forward. Expert Rev. Clin. Immunol. 2016, 12, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.; Meyer, R.W.; Nwaru, B.I.; Roduit, C.; Untersmayr, E.; Adel-Patient, K.; Agache, I.; Agostoni, C.; Akdis, C.A.; Bischoff, S.C.; et al. EAACI position paper: Influence of dietary fatty acids on asthma, food allergy, and atopic dermatitis. Allergy 2019, 74, 1429–1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogenkamp, A.; Ehlers, A.; Garssen, J.; Willemsen, L.E.M. Allergy Modulation by N-3 Long Chain Polyunsaturated Fatty Acids and Fat Soluble Nutrients of the Mediterranean Diet. Front. Pharmacol. 2020, 11, 1244. [Google Scholar] [CrossRef] [PubMed]
- Radzikowska, U.; Rinaldi, A.O.; Sözener, Z.C.; Karaguzel, D.; Wojcik, M.; Cypryk, K.; Akdis, M.; Akdis, C.A.; Sokolowska, M. The Influence of Dietary Fatty Acids on Immune Responses. Nutrients 2019, 11, 2990. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, A.; Hamazaki, T.; Ohshita, A.; Kohno, N.; Sakai, K.; Zhao, G.D.; Katayama, H.; Hiwada, K. Effect of aerosolized docosahexaenoic acid in a mouse model of atopic asthma. Int. Arch. Allergy Immunol. 2000, 123, 327–332. [Google Scholar] [CrossRef]
- Miyata, J.; Arita, M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol. Int. 2015, 64, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Losol, P.; Rezwan, F.I.; Patil, V.P.; Venter, C.; Ewart, S.; Zhang, H.; Arshad, S.H.; Karmaus, W.; Holloway, J.W. Effect of gestational oily fish intake on the risk of allergy in children may be influenced by FADS1/2, ELOVL5 expression and DNA methylation. Genes Nutr. 2019, 14, 20. [Google Scholar] [CrossRef]
- Acevedo, N.; Frumento, P.; Harb, H.; Alhamwe, B.A.; Johansson, C.; Eick, L.; Alm, J.; Renz, H.; Scheynius, A.; Potaczek, D.P. Histone Acetylation of Immune Regulatory Genes in Human Placenta in Association with Maternal Intake of Olive Oil and Fish Consumption. Int. J. Mol. Sci. 2019, 20, 1060. [Google Scholar] [CrossRef] [Green Version]
- Harb, H.; Irvine, J.; Amarasekera, M.; Hii, C.S.; Kesper, D.A.; Ma, Y.; D’Vaz, N.; Renz, H.; Potaczek, D.P.; Prescott, S.L.; et al. The role of PKCζ in cord blood T-cell maturation towards Th1 cytokine profile and its epigenetic regulation by fish oil. Biosci. Rep. 2017, 37, 85. [Google Scholar] [CrossRef] [Green Version]
- Rucci, E.; den Dekker, H.T.; de Jongste, J.C.; Steenweg-de-Graaff, J.; Gaillard, R.; Pasmans, S.G.; Hofman, A.; Tiemeier, H.; Jaddoe, V.W.V.; Duijts, L. Maternal fatty acid levels during pregnancy, childhood lung function and atopic diseases. The Generation R Study. Clin. Exp. Allergy 2016, 46, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Blümer, N.; Renz, H. Consumption of omega3-fatty acids during perinatal life: Role in immuno-modulation and allergy prevention. J. Perinat Med. 2007, 1, S12–S18. [Google Scholar] [CrossRef] [PubMed]
- Rago, D.; Rasmussen, M.A.; Lee-Sarwar, K.A.; Weiss, S.T.; Lasky-Su, J.; Stokholm, J.; Bønnelykke, K.; Chawes, B.L.; Bisgaard, H. Fish-oil supplementation in pregnancy, child metabolomics and asthma risk. EBioMedicine 2019, 46, 399–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, S.; Strøm, M.; Maslova, E.; Dahl, R.; Hoffmann, H.J.; Rytter, D.; Bech, B.H.; Henriksen, T.B.; Granström, C.; Halldorsson, T.I.; et al. Fish oil supplementation during pregnancy and allergic respiratory disease in the adult offspring. J. Allergy Clin. Immunol. 2017, 139, 104–111. [Google Scholar] [CrossRef] [Green Version]
- Best, K.P.; Sullivan, T.R.; Palmer, D.J.; Gold, M.; Martin, J.; Kennedy, D.; Makrides, M. Prenatal omega-3 LCPUFA and symptoms of allergic disease and sensitization throughout early childhood—A longitudinal analysis of long-term follow-up of a randomized controlled trial. World Allergy Organ. J. 2018, 11, 10. [Google Scholar] [CrossRef]
- Marks, G.B.; Mihrshahi, S.; Kemp, A.S.; Tovey, E.R.; Webb, K.; Almqvist, C.; Ampon, R.D.; Crisafulli, D.; Belousova, E.G.; Mellis, C.M.; et al. Prevention of asthma during the first 5 years of life: A randomized controlled trial. J. Allergy Clin. Immunol. 2006, 118, 53–61. [Google Scholar] [CrossRef]
- Almqvist, C.; Garden, F.; Xuan, W.; Mihrshahi, S.; Leeder, S.R.; Oddy, W.; Webb, K.; Marks, G.B.; CAPS team. Omega-3 and omega-6 fatty acid exposure from early life does not affect atopy and asthma at age 5 years. J. Allergy Clin. Immunol. 2007, 119, 1438–1444. [Google Scholar] [CrossRef]
- Birch, E.E.; Khoury, J.C.; Berseth, C.L.; Castañeda, Y.S.; Couch, J.M.; Bean, J.; Tamer, R.; Harris, C.L.; Mitmesser, S.H.; Scalabrin, D.M. The impact of early nutrition on incidence of allergic manifestations and common respiratory illnesses in children. J. Pediatr. 2010, 156, 902–906. [Google Scholar] [CrossRef]
- Foiles, A.M.; Kerling, E.H.; Wick, J.A.; Scalabrin, D.M.F.; Colombo, J.; Carlson, S.E. Formula with long-chain polyunsaturated fatty acids reduces incidence of allergy in early childhood. Pediatr. Allergy Immunol. 2016, 27, 156–161. [Google Scholar] [CrossRef] [Green Version]
- D’Vaz, N.; Meldrum, S.J.; Dunstan, J.A.; Martino, D.; McCarthy, S.; Metcalfe, J.; Tulic, M.K.; Mori, T.A.; Prescott, S.L. Postnatal fish oil supplementation in high-risk infants to prevent allergy: Randomized controlled trial. Pediatrics 2012, 130, 674–682. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, S.; Miyake, Y.; Sasaki, S.; Tanaka, K.; Ohya, Y.; Matsunaga, I.; Yoshida, T.; Oda, H.; Ishiko, O.; Hirota, Y.; et al. Fat and fish intake and asthma in Japanese women: Baseline data from the Osaka Maternal and Child Health Study. Int. J. Tuberc. Lung Dis. 2007, 11, 103–109. [Google Scholar]
- McKeever, T.M.; Lewis, S.A.; Cassano, P.A.; Ocké, M.; Burney, P.; Britton, J.; Smit, H.A. The relation between dietary intake of individual fatty acids, FEV1 and respiratory disease in Dutch adults. Thorax 2008, 63, 208–214. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Shi, L.; Pang, W.; Liu, W.; Li, J.; Wang, H.; Shi, G. Dietary Fiber Intake Regulates Intestinal Microflora and Inhibits Ovalbumin-Induced Allergic Airway Inflammation in a Mouse Model. PLoS ONE 2016, 11, e0147778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Roduit, C.; Frei, R.; Ferstl, R.; Loeliger, S.; Westermann, P.; Rhyner, C.; Schiavi, E.; Barcik, W.; Rodriguez-Perez, N.; Wawrzyniak, M.; et al. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy 2019, 74, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Muehleisen, B.; Gallo, R.L. Vitamin D in allergic disease: Shedding light on a complex problem. J. Allergy Clin. Immunol. 2013, 131, 324–329. [Google Scholar] [CrossRef]
- Adams, J.S.; Hewison, M. Unexpected actions of vitamin D: New perspectives on the regulation of innate and adaptive immunity. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 80–90. [Google Scholar] [CrossRef] [Green Version]
- Matheu, V.; Back, O.; Mondoc, E.; Issazadeh-Navikas, S. Dual effects of vitamin D-induced alteration of TH1/TH2 cytokine expression: Enhancing IgE production and decreasing airway eosinophilia in murine allergic airway disease. J. Allergy Clin. Immunol. 2003, 112, 585–592. [Google Scholar] [CrossRef]
- Damera, G.; Fogle, H.W.; Lim, P.; Goncharova, E.A.; Zhao, H.; Banerjee, A.; Tliba, O.; Krymskaya, V.P.; Panettieri, R.A., Jr. Vitamin D inhibits growth of human airway smooth muscle cells through growth factor-induced phosphorylation of retinoblastoma protein and checkpoint kinase 1. Br. J. Pharmacol. 2009, 158, 1429–1441. [Google Scholar] [CrossRef]
- Sikorska-Szaflik, H.; Sozańska, B. The Role of Vitamin D in Respiratory Allergies Prevention. Why the Effect Is so Difficult to Disentangle? Nutrients 2020, 12, 1801. [Google Scholar] [CrossRef]
- Hollams, E.M.; Hart, P.H.; Holt, B.J.; Serralha, M.; Parsons, F.; de Klerk, N.H.; Zhang, G.; Sly, P.D.; Holt, P.G. Vitamin D and atopy and asthma phenotypes in children: A longitudinal cohort study. Eur. Respir. J. 2011, 38, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Xun, P.; Pike, K.; Wills, A.K.; Chawes, B.L.; Bisgaard, H.; Cai, W.; Wan, Y.; He, K. In utero exposure to 25-hydroxyvitamin D and risk of childhood asthma, wheeze, and respiratory tract infections: A meta-analysis of birth cohort studies. J. Allergy Clin. Immunol. 2017, 139, 1508–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothers, J.; Wright, A.L.; Stern, D.A.; Halonen, M.; Camargo, C.A., Jr. Cord blood 25-hydroxyvitamin D levels are associated with aeroallergen sensitization in children from Tucson, Arizona. J. Allergy Clin. Immunol. 2011, 128, 1093–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bisgaard, H.; Vissing, N.H.; Carson, C.G.; Bischoff, A.L.; Følsgaard, N.V.; Kreiner-Møller, E.; Chawes, B.L.; Stokholm, J.; Pedersen, L.; Bjarnadóttir, E.; et al. Deep phenotyping of the unselected COPSAC2010 birth cohort study. Clin. Exp. Allergy 2013, 43, 1384–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolsk, H.M.; Chawes, B.L.; Litonjua, A.A.; Hollis, B.W.; Waage, J.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H.; Weiss, S.T. Prenatal vitamin D supplementation reduces risk of asthma/recurrent wheeze in early childhood: A combined analysis of two randomized controlled trials. PLoS ONE 2017, 12, e0186657. [Google Scholar] [CrossRef]
- Litonjua, A.A.; Carey, V.J.; Laranjo, N.; Stubbs, B.J.; Mirzakhani, H.; O’Connor, G.T.; Sandel, M.; Beigelman, A.; Bacharier, L.B.; Zeiger, R.S.; et al. Six-Year Follow-up of a Trial of Antenatal Vitamin D for Asthma Reduction. N. Engl. J. Med. 2020, 382, 525–533. [Google Scholar] [CrossRef]
- von Mutius, E.; Martinez, F.D. Vitamin D Supplementation during Pregnancy and the Prevention of Childhood Asthma. N. Engl. J. Med. 2020, 382, 574–575. [Google Scholar] [CrossRef]
- Litonjua, A.A. Fat-soluble vitamins and atopic disease: What is the evidence? Proc. Nutr. Soc. 2012, 71, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.Y.; Blatter, J.; Brehm, J.M.; Forno, E.; Litonjua, A.A.; Celedón, J.C. Diet and asthma: Vitamins and methyl donors. Lancet Respir. Med. 2013, 10, 813–822. [Google Scholar] [CrossRef] [Green Version]
- Mora, J.R.; Iwata, M.; von Andrian, U.H. Vitamin effects on the immune system: Vitamins A and D take centre stage. Nat. Rev. Immunol. 2008, 8, 685–698. [Google Scholar] [CrossRef] [Green Version]
- Checkley, W.; West, K.P., Jr.; Wise, R.A.; Wu, L.; LeClerq, S.C.; Khatry, S.; Katz, J.; Christian, P.; Tielsch, J.M.; Sommer, A. Supplementation with vitamin A early in life and subsequent risk of asthma. Eur. Respir. J. 2011, 38, 1310–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Checkley, W.; West, K.P., Jr.; Wise, R.A.; Baldwin, M.R.; Wu, L.; LeClerq, S.C.; Christian, P.; Katz, J.; Tielsch, J.M.; Khatry, S.; et al. Effects of maternal vitamin A supplementation on lung function in preadolescent offspring: A follow-up study of a randomized, double-blinded, placebo-controlled trial cohort in rural Nepal. N. Engl. J. Med. 2010, 362, 1784–1794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen S, Britton JR, Leonardi-Bee JA Association between antioxidant vitamins and asthma outcome measures: Systematic review and meta-analysis. Thorax 2009, 64, 610–619. [CrossRef] [PubMed] [Green Version]
- Chambial, S.; Dwivedi, S.; Shukla, K.K.; John, P.J.; Sharma, P. Vitamin C in disease prevention and cure: An overview. Indian J. Clin. Biochem. 2013, 28, 314–328. [Google Scholar] [CrossRef] [Green Version]
- Chatzi, L.; Apostolaki, G.; Bibakis, I.; Skypala, I.; Bibaki-Liakou, V.; Tzanakis, N.; Kogevinas, M.; Cullinan, P. Protective effect of fruits, vegetables and the Mediterranean diet on asthma and allergies among children in Crete. Thorax 2007, 62, 677–683. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, B.; Berthon, B.S.; Wark, P.; Wood, L.G. Effects of Fruit and Vegetable Consumption on Risk of Asthma, Wheezing and Immune Responses: A Systematic Review and Meta-Analysis. Nutrients 2017, 9, 341. [Google Scholar] [CrossRef]
- Picado, C.; Deulofeu, R.; Lleonart, R.; Agustí, M.; Mullol, J.; Quintó, L.; Torra, M. Dietary micronutrients/ antioxidants and their relationship with bronchial asthma severity. Allergy 2001, 56, 43–49. [Google Scholar] [CrossRef]
- Harik-Khan, R.I.; Muller, D.C.; Wise, R.A. Serum Vitamin Levels and the Risk of Asthma in Children. Am. J. Epidemiol. 2004, 159, 351–357. [Google Scholar] [CrossRef] [Green Version]
- Bando, N.; Yamanishi, R.; Terao, J. Inhibition of immunoglobulin E production in allergic model mice by supplementation with vitamin E and beta-carotene. Biosci. Biotechnol. Biochem. 2003, 67, 2176–2182. [Google Scholar] [CrossRef]
- Devereux, G. Early life events in asthma–diet. Pediatr. Pulmonol. 2007, 42, 663–673. [Google Scholar] [CrossRef]
- Stone, C.A., Jr.; Cook-Mills, J.; Gebretsadik, T.; Rosas-Salazar, C.; Turi, K.; Brunwasser, S.M.; Connolly, A.; Russell, P.; Liu, Z.; Costello, K.; et al. Delineation of the Individual Effects of Vitamin E Isoforms on Early Life Incident Wheezing. J. Pediatr. 2019, 206, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Nwaru, B.I.; Virtanen, S.M.; Alfthan, G.; Karvonen, A.M.; Genuneit, J.; Lauener, R.P.; Dalphin, J.C.; Hyvärinen, A.; Pfefferle, P.; Riedler, J.; et al. Serum vitamin E concentrations at 1 year and risk of atopy, atopic dermatitis, wheezing, and asthma in childhood: The PASTURE study. Allergy 2014, 69, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Cook-Mills, J.M.; Abdala-Valencia, H.; Hartert, T. Two faces of vitamin E in the lung. Am. J. Respir. Crit. Care Med. 2013, 188, 279–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCary, C.A.; Abdala-Valencia, H.; Berdnikovs, S.; Cook-Mills, J.M. Supplemental and highly elevated tocopherol doses differentially regulate allergic inflammation: Reversibility of alpha-tocopherol and gammatocopherol’s effects. J. Immunol. 2011, 186, 3674–3685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdala-Valencia, H.; Berdnikovs, S.; Cook-Mills, J.M. Vitamin E isoforms as modulators of lung inflammation. Nutrients 2013, 5, 4347–4363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Ferrie, R.P.; Balmert, L.C.; Kienzl, M.; Rifas-Shiman, S.L.; Gold, D.R.; Sordillo, J.E.; Kleinman, K.; Camargo, C.A., Jr.; Litonjua, A.A. Associations of α- and γ-tocopherol during early life with lung function in childhood. J. Allergy Clin. Immunol. 2020, 146, 1349–1357. [Google Scholar] [CrossRef]
- Shaheen, S.O.; Newson, R.B.; Rayman, M.P.; Wong, A.P.; Tumilty, M.K.; Phillips, J.M.; Potts, J.F.; Kelly, F.J.; White, P.T.; Burney, P.G. Randomised, double blind, placebo-controlled trial of selenium supplementation in adult asthma. Thorax 2007, 62, 483–490. [Google Scholar] [CrossRef] [Green Version]
- Shaheen, S.O.; Sterne, J.A.; Thompson, R.L.; Songhurst, C.E.; Margetts, B.M.; Burney, P.G. Dietary antioxidants and asthma in adults: Population-based case-control study. Am. J. Respir. Crit. Care Med. 2001, 164, 1823–1828. [Google Scholar] [CrossRef]
- Gontijo-Amaral, C.; Ribeiro, M.A.; Gontijo, L.S.; Condino-Neto, A.; Ribeiro, J.D. Oral magnesium supplementation in asthmatic children: A double-blind randomized placebo-controlled trial. Eur. J. Clin. Nutr. 2007, 61, 54–60. [Google Scholar] [CrossRef]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2020. Available online: www.ginasthma.org (accessed on 15 November 2020).
- Song, W.J.; Chang, Y.S. Magnesium sulfate for acute asthma in adults: A systematic literature review. Asia Pac. Allergy 2012, 2, 76–85. [Google Scholar] [CrossRef] [Green Version]
- Silverman, R.A.; Osborn, H.; Runge, J.; Gallagher, E.J.; Chiang, W.; Feldman, J.; Gaeta, T.; Freeman, K.; Levin, B.; Mancherje, N.; et al. IV magnesium sulfate in the treatment of acute severe asthma: A multicenter randomized controlled trial. Chest 2002, 122, 489–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mickleborough, T.D.; Fogarty, A. Dietary sodium intake and asthma: An epidemiological and clinical review. Int. J. Clin. Pract. 2006, 60, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- Gotshall, R.W.; Mickleborough, T.D.; Cordain, L. Dietary salt restriction improves pulmonary function in exercise induced asthma. Med. Sci. Sports Exerc. 2000, 32, 1815–1819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pogson, Z.; McKeever, T. Dietary sodium manipulation and asthma. Cochrane Database Syst. Rev. 2011, 3, CD000436. [Google Scholar] [CrossRef]
- Hill, D.A.; Grundmeier, R.W.; Ram, G.; Spergel, J.M. The epidemiologic characteristics of healthcare provider-diagnosed eczema, asthma, allergic rhinitis, and food allergy in children: A retrospective cohort study. BMC Pediatr. 2016, 16, 133. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, A.; Kumar, R.; Pongracic, J.A.; Sullivan, C.L.; Caruso, D.M.; Costello, J.; Meyer, K.E.; Vucic, Y.; Gupta, R.; Kim, J.S.; et al. Food allergy is associated with an increased risk of asthma. Clin. Exp. Allergy 2009, 39, 261–270. [Google Scholar] [CrossRef] [Green Version]
- Tariq, S.M.; Matthews, S.M.; Hakim, E.A.; Arshad, S.H. Egg allergy in infancy predicts respiratory allergic disease by 4 years of age. Pediatr. Allergy Immunol. 2000, 11, 162–167. [Google Scholar] [CrossRef]
- du Toit, G.; Roberts, G.; Sayre, P.H.; Bahnson, H.T.; Radulovic, S.; Santos, A.F.; Brough, H.A.; Phippard, D.; Basting, M.; Feeney, M. Randomized trial of peanut consumption in infants at risk for peanut allergy. N. Engl. J. Med. 2015, 372, 803–813. [Google Scholar] [CrossRef] [Green Version]
- du Toit, G.; Sayre, P.H.; Roberts, G.; Lawson, K.; Sever, M.L.; Bahnson, H.T.; Fisher, H.R.; Feeney, M.; Radulovic, S.; Basting, M.; et al. Allergen specificity of early peanut consumption and effect on development of allergic disease in the Learning Early About Peanut Allergy study cohort. J. Allergy Clin. Immunol. 2018, 141, 1343–1353. [Google Scholar] [CrossRef] [Green Version]
- Perkin, M.R.; Logan, K.; Tseng, A.; Raji, B.; Ayis, S.; Peacock, J.; Brough, H.; Marrs, T.; Radulovic, S.; Craven, J.; et al. Randomized Trial of Introduction of Allergenic Foods in Breast-Fed Infants. N. Engl. J. Med. 2016, 374, 1733–1743. [Google Scholar] [CrossRef] [Green Version]
- Arshad, S.H.; Matthews, S.; Gant, C.; Hide, D.W. Effect of allergen avoidance on development of allergic disorders in infancy. Lancet 1992, 339, 1493–1497. [Google Scholar] [CrossRef]
- Arshad, S.H.; Bateman, B.; Matthews, S.M. Primary prevention of asthma and atopy during childhood by allergen avoidance in infancy: A randomised controlled study. Thorax 2003, 58, 489–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan-Yeung, M.; Manfreda, J.; Dimich-Ward, H.; Ferguson, A.; Watson, W.; Becker, A. A randomized controlled study on the effectiveness of a multifaceted intervention program in the primary prevention of asthma in high-risk infants. Arch. Pediatr. Adolesc Med. 2000, 154, 657–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bisgaard, H.; Li, N.; Bonnelykke, K.; Chawes, B.L.; Skov, T.; Paludan-Müller, G.; Stokholm, J.; Smith, B.; Krogfelt, K.A. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J. Allergy Clin. Immunol. 2011, 128, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Roduit, C.; Frei, R.; Depner, M.; Schaub, B.; Loss, G.; Genuneit, J.; Pfefferle, P.; Hyvärinen, A.; Karvonen, A.M.; Riedler, J.; et al. Increased food diversity in the first year of life is inversely associated with allergic diseases. J. Allergy Clin. Immunol. 2014, 133, 1056–1064. [Google Scholar] [CrossRef]
- Nwaru, B.I.; Takkinen, H.M.; Kaila, M.; Erkkola, M.; Ahonen, S.; Pekkanen, J.; Simell, O.; Veijola, R.; Ilonen, J.; Hyöty, H.; et al. Food diversity in infancy and the risk of childhood asthma and allergies. J. Allergy Clin. Immunol. 2014, 133, 1084–1091. [Google Scholar] [CrossRef]
- Castro-Rodriguez, J.A.; Ramirez-Hernandez, M.; Padilla, O.; Pacheco-Gonzalez, R.M.; Pere-Fernandez, V.; Garcia-Marcos, L. Effect of foods and Mediterranean diet during pregnancy and first years of life on wheezing, rhinitis and dermatitis in preschoolers. Allergol. Immunopathol. 2016, 44, 400–409. [Google Scholar] [CrossRef]
- Chatzi, L.; Garcia, R.; Roumeliotaki, T.; Basterrechea, M.; Begiristain, H.; Iñiguez, C.; Vioque, J.; Kogevinas, M.; Sunyer, J.; INMA Study Group; et al. Mediterranean diet adherence during pregnancy and risk of wheeze and eczema in the first year of life: INMA (Spain) and RHEA (Greece) mother-child cohort studies. Br. J. Nutr. 2013, 110, 2058–2068. [Google Scholar] [CrossRef]
- Chatzi, L.; Torrent, M.; Romieu, I.; Garcia-Esteban, R.; Ferrer, C.; Vioque, J.; Kogevinas, M.; Sunyer, J. Mediterranean diet in pregnancy is protective for wheeze and atopy in childhood. Thorax 2008, 63, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Marcos, L.; Canflanca, I.M.; Garrido, J.B.; Varela, A.L.; Garcia-Hernandez, G.; Guillen Grima, F.; Gonzalez-Diaz, C.; Carvajal-Urueña, I.; Arnedo-Pena, A.; Busquets-Monge, R.M.; et al. Relationship of asthma and rhinoconjunctivitis with obesity, exercise and Mediterranean diet in Spanish schoolchildren. Thorax 2007, 62, 503–508. [Google Scholar] [CrossRef] [Green Version]
- Nagel, G.; Weinmayr, G.; Kleiner, A.; Garcia-Marcos, L.; Strachan, D.P. Effect of diet on asthma and allergic sensitisation in the International Study on Allergies and Asthma in Childhood (ISAAC) Phase Two. Thorax 2010, 65, 516–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro-Rodriguez, J.A.; Garcia-Marcos, L. What are the effects of a mediterranean diet on allergies and asthma in children? Front. Pediatr. 2017, 5, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akcay, A.; Tamay, Z.; Hocaoglu, A.B.; Ergin, A.; Guler, N. Risk factors affecting asthma prevalence in adolescents living in Istanbul, Turkey. Allergol. Immunopathol. 2014, 42, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.O.; Northstone, K.; Newson, R.B.; Emmett, P.M.; Sherriff, A.; Henderson, A.J. Dietary patterns in pregnancy and respiratory and atopic outcomes in childhood. Thorax 2009, 64, 411–417. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.; Custovic, A.; Smith, J.A.; Simpson, A.; Kerry, G.; Murray, C.S. Cross-sectional association of dietary patterns with asthma and atopic sensitization in childhood—In a cohort study. Pediatr. Allergy Immunol. 2014, 25, 565–571. [Google Scholar]
- Lee, S.C.; Yang, Y.H.; Chuang, S.Y.; Liu, S.C.; Yang, H.C.; Pan, W.H. Risk of asthma associated with energy-dense but nutrient-poor dietary pattern in Taiwanese children. Asia Pac. J. Clin. Nutr. 2012, 21, 73–81. [Google Scholar]
- de Cassia Ribeiro Silva, R.; Assis, A.M.; Cruz, A.A.; Fiaccone, R.L.; Dinnocenzo, S.; Barreto, M.L.; da Silva, L.A.; Cunha, L. Dietary patterns and wheezing in the midst of nutritional transition: A study in Brazil. Pediatr. Allergy Immunol. Pulmonol. 2013, 26, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Wickens, K.; Barry, D.; Friezema, A.; Rhodius, R.; Bone, N.; Purdie, G.; Crane, J. Fast foods—Are they a risk factor for asthma? Allergy 2005, 60, 1537–1541. [Google Scholar] [CrossRef]
- Mai, X.M.; Becker, A.B.; Liem, J.J.; Kozyrskyj, A.L. Fast food consumption counters the protective effect of breastfeeding on asthma in children? Clin. Exp. Allergy 2009, 39, 556–561. [Google Scholar] [CrossRef]




| Vitamins | |
|---|---|
| D |
|
| A |
|
| C |
|
| E |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sozańska, B.; Sikorska-Szaflik, H. Diet Modifications in Primary Prevention of Asthma. Where Do We Stand? Nutrients 2021, 13, 173. https://doi.org/10.3390/nu13010173
Sozańska B, Sikorska-Szaflik H. Diet Modifications in Primary Prevention of Asthma. Where Do We Stand? Nutrients. 2021; 13(1):173. https://doi.org/10.3390/nu13010173
Chicago/Turabian StyleSozańska, Barbara, and Hanna Sikorska-Szaflik. 2021. "Diet Modifications in Primary Prevention of Asthma. Where Do We Stand?" Nutrients 13, no. 1: 173. https://doi.org/10.3390/nu13010173





