Magnesium in Infectious Diseases in Older People
Abstract
:1. Introduction
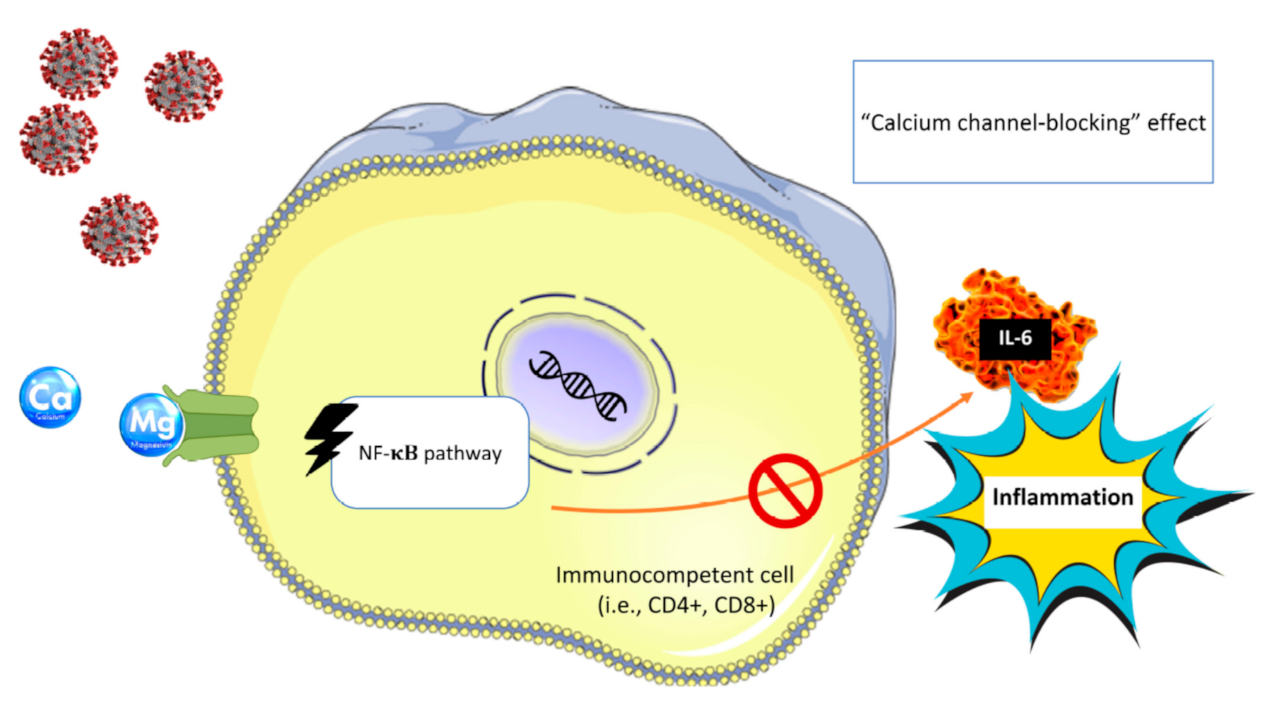
2. Mg and the Immune Responses
3. Magnesium, Inflammation, and Oxidative Stress
3.1. Inflammation
3.2. Oxidative Stress
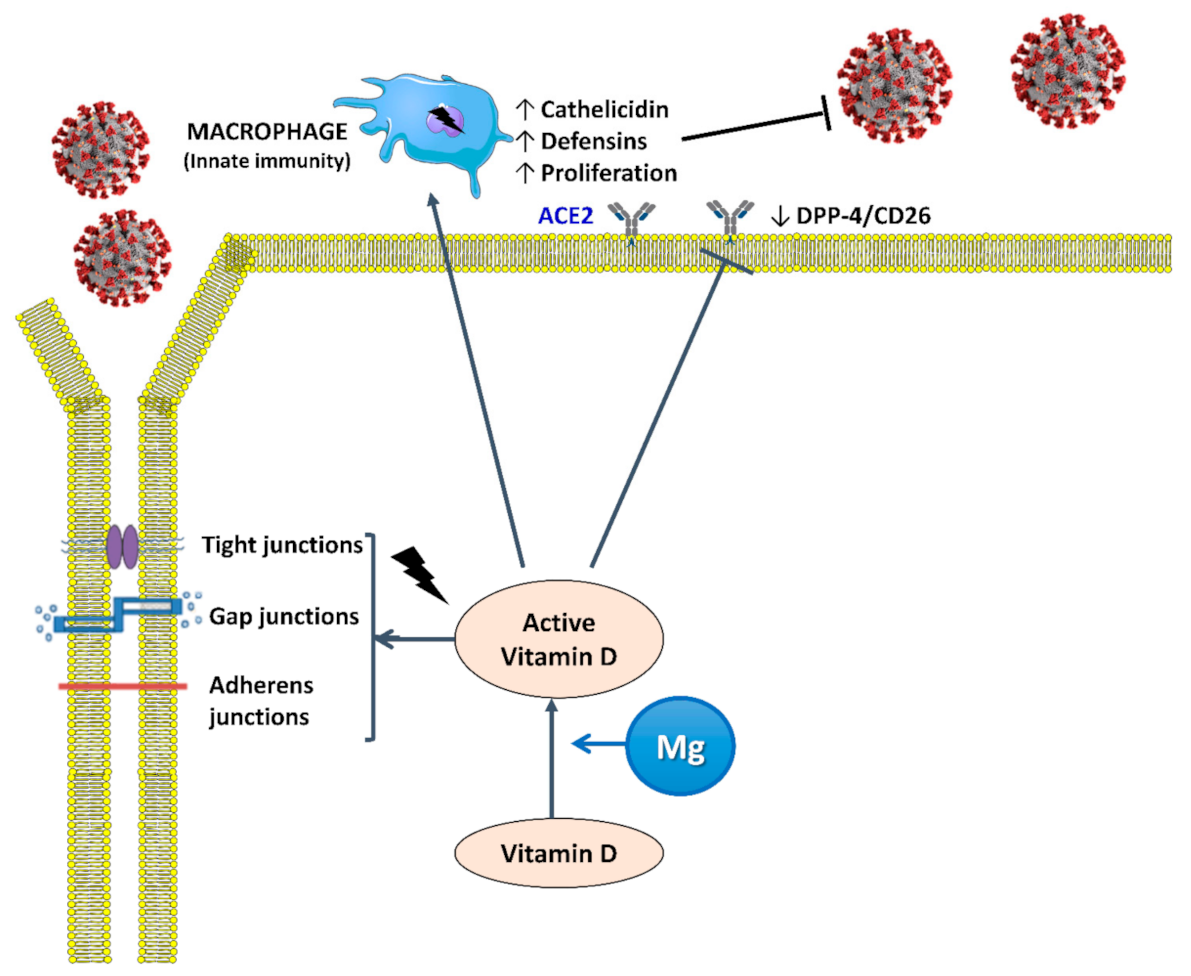
4. Mg and Vitamin D in Infectious Diseases
4.1. Interaction between Mg and Vitamin D
4.2. Vitamin D and Infections
4.3. Mg and Infectious Diseases
5. Infectious Diseases in Old Age
6. Magnesium and COVID-19 Pandemic
6.1. Cytokine Storm in COVID-19
6.2. COVID-19 and Endothelial Dysfunction
6.3. COVID-19 and Vitamin D
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wolfe, N.D.; Dunavan, C.P.; Diamond, J. Origins of major human infectious diseases. Nat. Cell Biol. 2007, 447, 279–283. [Google Scholar] [CrossRef]
- Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222.
- WHO. World Report on Ageing and Health; World Health Organisation: Geneva, Switzerland, 2015; Available online: https://www.who.int/ageing/events/world-report-2015-launch/en/ (accessed on 1 December 2020).
- Caspi, R.; Altman, T.; Dreher, K.; Fulcher, C.A.; Subhraveti, P.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Latendresse, M.; Mueller, L.A.; et al. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res. 2011, 40, D742–D753. [Google Scholar] [CrossRef] [Green Version]
- Saris, N.-E.L.; Mervaala, E.; Karppanen, H.; Khawaja, J.A.; Lewenstam, A. Magnesium: An update on physiological, clinical and analytical aspects. Clin. Chim. Acta 2000, 294, 1–26. [Google Scholar]
- Barbagallo, M.; Gupta, R.K.; Dominguez, L.J.; Resnick, L.M. Cellular Ionic Alterations with Age: Relation to Hypertension and Diabetes. J. Am. Geriatr. Soc. 2000, 48, 1111–1116. [Google Scholar] [CrossRef]
- Barbagallo, M.; Dominguez, L.J. Magnesium Metabolism in Type 2 Diabetes Mellitus. Encycl. Metalloproteins 2013, 458, 1277–1281. [Google Scholar] [CrossRef]
- Tam, M.; Gómez, S.; GonzalezGross, M.; Marcos, A. Possible roles of magnesium on the immune system. Eur. J. Clin. Nutr. 2003, 57, 1193–1197. [Google Scholar] [CrossRef] [Green Version]
- Mazur, A.; Maier, J.A.; Rock, E.; Gueux, E.; Nowacki, W.; Rayssiguier, Y. Magnesium and the inflammatory response: Potential physiopathological implications. Arch. Biochem. Biophys. 2007, 458, 48–56. [Google Scholar] [CrossRef]
- Wolf, F.I.; Trapani, V.; Simonacci, M.; Ferrè, S.; Maier, J.A.M. Magnesium deficiency and endothelial dysfunction: Is oxidative stress involved? Magnes. Res. 2008, 21, 58–64. [Google Scholar]
- Barbagallo, M.; Belvedere, M.; Dominguez, L.J. Magnesium homeostasis and aging. Magnes. Res. 2009, 22, 235–246. [Google Scholar]
- Li, F.-Y.; Chaigne-Delalande, B.; Kanellopoulou, C.; Davis, J.C.; Matthews, H.F.; Douek, D.C.; Cohen, J.I.; Uzel, G.; Su, H.C.; Lenardo, M.J. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nat. Cell Biol. 2011, 475, 471–476. [Google Scholar] [CrossRef]
- Chaigne-Delalande, B.; Li, F.-Y.; O’Connor, G.M.; Lukacs, M.J.; Jiang, P.; Zheng, L.; Shatzer, A.; Biancalana, M.; Pittaluga, S.; Matthews, H.F.; et al. Mg2+ Regulates Cytotoxic Functions of NK and CD8 T Cells in Chronic EBV Infection Through NKG2D. Science 2013, 341, 186–191. [Google Scholar] [CrossRef] [Green Version]
- Li, F.-Y.; Chaigne-Delalande, B.; Su, H.; Uzel, G.; Matthews, H.; Lenardo, M.J. XMEN disease: A new primary immunodeficiency affecting Mg2+ regulation of immunity against Epstein-Barr virus. Blood 2014, 123, 2148–2152. [Google Scholar] [CrossRef] [Green Version]
- Barbagallo, M.; Dominguez, L. Magnesium and Aging. Curr. Pharm. Des. 2010, 16, 832–839. [Google Scholar] [CrossRef]
- Galland, L. Magnesium and immune function: An overview. Magnesium 1988, 7, 290–299. [Google Scholar]
- Sugimoto, J.; Romani, A.M.; Valentin-Torres, A.M.; Luciano, A.A.; Kitchen, C.M.R.; Funderburg, N.; Mesiano, S.; Bernstein, H.B. Magnesium Decreases Inflammatory Cytokine Production: A Novel Innate Immunomodulatory Mechanism. J. Immunol. 2012, 188, 6338–6346. [Google Scholar] [CrossRef] [Green Version]
- Feske, S.; Skolnik, E.Y.; Prakriya, M. Ion channels and transporters in lymphocyte function and immunity. Nat. Rev. Immunol. 2012, 12, 532–547. [Google Scholar] [CrossRef] [Green Version]
- Bussière, F.; Tridon, A.; Zimowska, W.; Mazur, A.; Rayssiguier, Y. Increase in complement component C3 is an early response to experimental magnesium deficiency in rats. Life Sci. 2003, 73, 499–507. [Google Scholar] [CrossRef]
- Kraeuter, S.L.; Schwartz, R. Blood and Mast Cell Histamine Levels in Magnesium-Deficient Rats. J. Nutr. 1980, 110, 851–858. [Google Scholar] [CrossRef]
- Takemoto, S.; Yamamoto, A.; Tomonaga, S.; Funaba, M.; Matsui, T. Magnesium Deficiency Induces the Emergence of Mast Cells in the Liver of Rats. J. Nutr. Sci. Vitaminol. 2013, 59, 560–563. [Google Scholar] [CrossRef] [Green Version]
- Chien, M.M.; Zahradka, K.E.; Newell, M.K.; Freed, J.H. Fas-induced B cell apoptosis requires an increase in free cytosolic magnesium as an early event. J. Biol. Chem. 1999, 274, 7059–7066. [Google Scholar] [CrossRef] [Green Version]
- Bussière, F.I.; Gueux, E.; Rock, E.; Mazur, A.; Rayssiguier, Y. Protective effect of calcium deficiency on the inflammatory response in magnesium-deficient rats. Eur. J. Nutr. 2002, 41, 197–202. [Google Scholar] [CrossRef]
- Malpuech-Brugère, C.; Nowacki, W.; Daveau, M.; Gueux, E.; Linard, C.; Rock, E.; Lebreton, J.-P.; Mazur, A.; Rayssiguier, Y. Inflammatory response following acute magnesium deficiency in the rat. Biochim. Biophys. Acta Mol. Basis Dis. 2000, 1501, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Petrault, I.; Zimowska, W.; Mathieu, J.; Bayle, D.; Rock, E.; Favier, A.; Rayssiguier, Y.; Mazur, A. Changes in gene expression in rat thymocytes identified by cDNA array support the occurrence of oxidative stress in early magnesium deficiency1Presented in part at the 9th International Magnesium Symposium, 10–15 September 2000, Vichy, France.1. Biochim. Biophys. Acta Mol. Basis Dis. 2002, 1586, 92–98. [Google Scholar] [CrossRef] [Green Version]
- Zimowska, W.; Girardeau, J.P.; Kuryszko, J.; Bayle, D.; Rayssiguier, Y.; Mazur, A. Morphological and immune response alterations in the intestinal mucosa of the mouse after short periods on a low-magnesium diet. Br. J. Nutr. 2002, 88, 515–522. [Google Scholar] [CrossRef]
- Malpuech-Brugère, C.; Nowacki, W.; Gueux, E.; Kuryszko, J.; Rock, E.; Rayssiguier, Y.; Mazur, A. Accelerated thymus involution in magnesium-deficient rats is related to enhanced apoptosis and sensitivity to oxidative stress. Br. J. Nutr. 1999, 81, 405–411. [Google Scholar] [CrossRef] [Green Version]
- Romani, A. Magnesium Homeostasis in Mammalian Cells. Metal Ions Life Sci. 2012, 12, 69–118. [Google Scholar] [CrossRef]
- Quamme, G.A. Molecular identification of ancient and modern mammalian magnesium transporters. Am. J. Physiol. Physiol. 2010, 298, C407–C429. [Google Scholar] [CrossRef] [Green Version]
- Ryazanova, L.V.; Rondon, L.J.; Zierler, S.; Hu, Z.; Galli, J.; Yamaguchi, T.P.; Mazur, A.; Fleig, A.; Ryazanov, A.G. TRPM7 is essential for Mg2+ homeostasis in mammals. Nat. Commun. 2010, 1, 109. [Google Scholar] [CrossRef] [Green Version]
- Brandao, K.; Deason-Towne, F.; Perraud, A.-L.; Schmitz, C. The role of Mg2+ in immune cells. Immunol. Res. 2012, 55, 261–269. [Google Scholar] [CrossRef]
- Van Der Wijst, J.; Hoenderop, J.G.; Bindels, R.J. Epithelial Mg2+ channel TRPM6: Insight into the molecular regulation. Magnes. Res. 2009, 22, 127–132. [Google Scholar] [CrossRef]
- Schmitz, C.; Perraud, A.-L.; O Johnson, C.; Inabe, K.; Smith, M.K.; Penner, R.; Kurosaki, T.; Fleig, A.; Scharenberg, A.M. Regulation of Vertebrate Cellular Mg2+ Homeostasis by TRPM7. Cell 2003, 114, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.; Desai, B.N.; Navarro, B.; Donovan, A.; Andrews, N.C.; Clapham, D.E. Deletion of Trpm7 Disrupts Embryonic Development and Thymopoiesis Without Altering Mg2+ Homeostasis. Science 2008, 322, 756–760. [Google Scholar] [CrossRef] [Green Version]
- Desai, B.N.; Krapivinsky, G.; Navarro, B.; Krapivinsky, L.; Carter, B.C.; Febvay, S.; Delling, M.; Penumaka, A.; Ramsey, I.S.; Manasian, Y.; et al. Cleavage of TRPM7 Releases the Kinase Domain from the Ion Channel and Regulates Its Participation in Fas-Induced Apoptosis. Dev. Cell 2012, 22, 1149–1162. [Google Scholar] [CrossRef] [Green Version]
- Mandt, T.; Song, Y.; Scharenberg, A.M.; Sahni, J. SLC41A1 Mg2+ transport is regulated via Mg2+-dependent endosomal recycling through its N-terminal cytoplasmic domain. Biochem. J. 2011, 439, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Sahni, J.; Nelson, B.; Scharenberg, A.M. SLC41A2 encodes a plasma-membrane Mg2+ transporter. Biochem. J. 2006, 401, 505–513. [Google Scholar] [CrossRef] [Green Version]
- Rink, T.J.; Tsien, R.Y.; Pozzan, T. Cytoplasmic pH and free Mg2+ in lymphocytes. J. Cell Biol. 1982, 95, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Rijkers, G.T.; Griffioen, A.W. Changes in free cytoplasmic magnesium following activation of human lymphocytes. Biochem. J. 1993, 289, 373–377. [Google Scholar] [CrossRef]
- Goytain, A.; Quamme, G.A. Identification and characterization of a novel mammalian Mg2+ transporter with channel-like properties. BMC Genom. 2005, 6, 48. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Clapham, D.E. Mammalian MagT1 and TUSC3 are required for cellular magnesium uptake and vertebrate embryonic development. Proc. Natl. Acad. Sci. USA 2009, 106, 15750–15755. [Google Scholar] [CrossRef] [Green Version]
- Klinken, E.M.; Gray, P.E.; Pillay, B.; Worley, L.; Edwards, E.S.J.; Payne, K.; Bennetts, B.; Hung, D.; Wood, B.A.; Chan, J.J.; et al. Diversity of XMEN Disease: Description of 2 Novel Variants and Analysis of the Lymphocyte Phenotype. J. Clin. Immunol. 2019, 40, 299–309. [Google Scholar] [CrossRef]
- Matsuda-Lennikov, M.; Biancalana, M.; Zou, J.; Ravell, J.C.; Zheng, L.; Kanellopoulou, C.; Jiang, P.; Notarangelo, G.; Jing, H.; Masutani, E.; et al. Magnesium transporter 1 (MAGT1) deficiency causes selective defects in N-linked glycosylation and expression of immune-response genes. J. Biol. Chem. 2019, 294, 13638–13656. [Google Scholar] [CrossRef]
- Ravell, J.C.; Chauvin, S.D.; He, T.; Lenardo, M.J. An Update on XMEN Disease. J. Clin. Immunol. 2020, 40, 671–681. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Barbagallo, M.; Di Lorenzo, G.; Drago, A.; Scola, S.; Morici, G.; Caruso, C. Bronchial reactivity and intracellular magnesium: A possible mechanism for the bronchodilating effects of magnesium in asthma. Clin. Sci. 1998, 95, 137–142. [Google Scholar]
- A Jones, L.; Goodacre, S. Magnesium sulphate in the treatment of acute asthma: Evaluation of current practice in adult emergency departments. Emerg. Med. J. 2009, 26, 783–785. [Google Scholar] [CrossRef]
- Britton, J.; Pavord, I.; Richards, K.; Wisniewski, A.; Knox, A.; Lewis, S.; Tattersfield, A.; Weiss, S. Dietary magnesium, lung function, wheezing, and airway hyperreactivity in a random adult population sample. Lancet 1994, 344, 357–362. [Google Scholar]
- Liang, R.-Y.; Wu, W.; Huang, J.; Jiang, S.-P.; Lin, Y. Magnesium Affects the Cytokine Secretion of CD4+T Lymphocytes in Acute Asthma. J. Asthma 2012, 49, 1012–1015. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2014, 69, S4–S9. [Google Scholar] [CrossRef]
- Kramer, J.H.; Mak, I.T.; Phillips, T.M.; Weglicki, W.B. Dietary Magnesium Intake Influences Circulating Pro-Inflammatory Neuropeptide Levels and Loss of Myocardial Tolerance to Postischemic Stress. Exp. Biol. Med. 2003, 228, 665–673. [Google Scholar] [CrossRef]
- Stankovic, M.; Janjetovic, K.; Velimirović, M.; Milenković, M.; Stojković, T.; Puskas, N.; Zaletel, I.; De Luka, S.R.; Jankovic, S.; Stefanovic, S.; et al. Effects of IL-33/ST2 pathway in acute inflammation on tissue damage, antioxidative parameters, magnesium concentration and cytokines profile. Exp. Mol. Pathol. 2016, 101, 31–37. [Google Scholar] [CrossRef]
- Maier, J.A.; Malpuech-Brugere, C.; Zimowska, W.; Rayssiguier, Y.; Mazur, A. Low magnesium promotes endothelial cell dysfunction: Implications for atherosclerosis, inflammation and thrombosis. Biochim. Biophys. Acta 2004, 1689, 13–21. [Google Scholar]
- Su, N.-Y.; Peng, T.-C.; Tsai, P.-S.; Huang, C.-J. Phosphoinositide 3-kinase/Akt pathway is involved in mediating the anti-inflammation effects of magnesium sulfate. J. Surg. Res. 2013, 185, 726–732. [Google Scholar] [CrossRef]
- Lin, C.Y.; Tsai, P.-S.; Hung, Y.C.; Huang, C.-J. L-type calcium channels are involved in mediating the anti-inflammatory effects of magnesium sulphate. Br. J. Anaesth. 2010, 104, 44–51. [Google Scholar] [CrossRef] [Green Version]
- King, D.E.; Mainous, I.I.I.A.G.; Geesey, M.E.; Woolson, R.F. Dietary magnesium and C-reactive protein levels. J. Am. Coll. Nutr. 2005, 24, 166–171. [Google Scholar]
- Guerrero-Romero, F.; Bermudez-Peña, C.; Rodríguez-Morán, M. Severe hypomagnesemia and low-grade inflammation in metabolic syndrome. Magnes. Res. 2011, 24, 45–53. [Google Scholar] [CrossRef]
- Song, Y.; Li, T.Y.; Van Dam, R.M.; E Manson, J.; Hu, F.B. Magnesium intake and plasma concentrations of markers of systemic inflammation and endothelial dysfunction in women. Am. J. Clin. Nutr. 2007, 85, 1068–1074. [Google Scholar] [CrossRef]
- Song, Y.; Ridker, P.M.; Manson, J.E.; Cook, N.R.; Buring, J.E.; Liu, S.-M. Magnesium Intake, C-Reactive Protein, and the Prevalence of Metabolic Syndrome in Middle-Aged and Older U.S. Women. Diabetes Care 2005, 28, 1438–1444. [Google Scholar] [CrossRef] [Green Version]
- Mazidi, M.; Kengne, A.P.; Mikhailidis, D.P.; Cicero, A.F.; Banach, M. Effects of selected dietary constituents on high-sensitivity C-reactive protein levels in U.S. adults. Ann. Med. 2017, 50, 1–6. [Google Scholar] [CrossRef]
- Konstari, S.; Sares-Jäske, L.; Heliövaara, M.; Rissanen, H.; Knekt, P.; Arokoski, J.; Sundvall, J.; Karppinen, J. Dietary magnesium intake, serum high sensitivity C-reactive protein and the risk of incident knee osteoarthritis leading to hospitalization—A cohort study of 4,953 Finns. PLoS ONE 2019, 14, e0214064. [Google Scholar] [CrossRef]
- Mazidi, M.; Rezaie, P.; Banach, M. Effect of magnesium supplements on serum C-reactive protein: A systematic review and meta-analysis. Arch. Med. Sci. 2018, 14, 707–716. [Google Scholar] [CrossRef]
- Bussière, F.I.; Mazur, A.; Fauquert, J.L.; Labbé, A.; Rayssiguier, Y.; Tridon, A. High magnesium concentration in vitro decreases human leukocyte activation. Magnes. Res. 2002, 15, 43–48. [Google Scholar]
- Kuzniar, A.; Mitura, P.; Kurys, P.; Szymonik-Lesiuk, S.; Florianczyk, B.; Stryjecka-Zimmer, M. The influence of hypomagnesemia on erythrocyte antioxidant enzyme defence system in mice. BioMetals 2003, 16, 349–357. [Google Scholar] [CrossRef]
- Weglicki, W.B.; Mak, I.T.; Kramer, J.H.; Dickens, B.F.; Cassidy, M.M.; Stafford, R.E.; Phillips, T.M. Role of free radicals and substance P in magnesium deficiency. Cardiovasc. Res. 1996, 31, 677–682. [Google Scholar]
- Calviello, G.; Ricci, P.; Lauro, L.; Palozza, P.; Cittadini, A. Mg deficiency induces mineral content changes and oxidative stress in rats. Biochem. Mol. Boil. Int. 1994, 32, 903–911. [Google Scholar]
- Kolisek, M.; Launay, P.; Beck, A.; Sponder, G.; Serafini, N.; Brenkus, M.; Froschauer-Neuhauser, E.; Martens, H.; Fleig, A.; Schweigel, M. SLC41A1 Is a Novel Mammalian Mg2+Carrier. J. Biol. Chem. 2008, 283, 16235–16247. [Google Scholar] [CrossRef] [Green Version]
- Yamanaka, R.; Tabata, S.; Shindo, Y.; Hotta, K.; Suzuki, K.; Soga, T.; Oka, K. Mitochondrial Mg2+ homeostasis decides cellular energy metabolism and vulnerability to stress. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Mastrototaro, L.; Smorodchenko, A.; Aschenbach, J.R.; Kolisek, M.; Sponder, G. Solute carrier 41A3 encodes for a mitochondrial Mg(2+) efflux system. Sci. Rep. 2016, 6, 27999. [Google Scholar]
- Liu, M.; Jeong, E.-M.; Liu, H.; Xie, A.; So, E.Y.; Shi, G.; Jeong, G.E.; Zhou, A.; Dudley, S.C. Magnesium supplementation improves diabetic mitochondrial and cardiac diastolic function. JCI Insight 2019, 4. [Google Scholar] [CrossRef]
- Liu, M.; Liu, H.; Xie, A.; Kang, G.J.; Feng, F.; Zhou, X.; Zhao, Y.; Dudley, S.C. Magnesium deficiency causes reversible diastolic and systolic cardiomyopathy. Biophys. J. 2020, 118, 245a. [Google Scholar]
- Gout, E.; Rébeillé, F.; Douce, R.; Bligny, R. Interplay of Mg2+, ADP, and ATP in the cytosol and mitochondria: Unravelling the role of Mg2+ in cell respiration. Proc. Natl. Acad. Sci. USA 2014, 111, E4560–E4567. [Google Scholar] [CrossRef] [Green Version]
- Panov, A.; Scarpa, A. Mg2+Control of Respiration in Isolated Rat Liver Mitochondria†. Biochemistry 1996, 35, 12849–12856. [Google Scholar] [CrossRef]
- Rodríguez-Zavala, J.; Moreno-Sánchez, R.; Rodriguez-Zavala, J.S. Modulation of Oxidative Phosphorylation by Mg2+in Rat Heart Mitochondria. J. Biol. Chem. 1998, 273, 7850–7855. [Google Scholar] [CrossRef] [Green Version]
- Kramer, J.H.; Mišík, V.; Weglicki, W.B. Magnesium-deficiency potentiates free radical production associated with postischemic injury to rat hearts: Vitamin E affords protection. Free Radic. Biol. Med. 1994, 16, 713–723. [Google Scholar] [CrossRef]
- Morais, J.B.; Severo, J.S.; Santos, L.R.; de Sousa Melo, S.R.; de Oliveira Santos, R.; de Oliveira, A.R.S.; Cruz, K.J.C.; do Nascimento Marreiro, D. Role of Magnesium in Oxidative Stress in Individuals with Obesity. Biol. Trace Elem. Res. 2017, 176, 20–26. [Google Scholar]
- Shah, N.C.; Liu, J.-P.; Iqbal, J.; Hussain, M.; Jiang, X.-C.; Li, Z.; Li, Y.; Zheng, T.; Li, W.; Sica, A.C.; et al. Mg deficiency results in modulation of serum lipids, glutathione, and NO synthase isozyme activation in cardiovascular tissues: Relevance to de novo synthesis of ceramide, serum Mg2+ and atherogenesis. Int. J. Clin. Exp. Med. 2011, 4, 103–118. [Google Scholar]
- Kumar, B.P.; Shivakumar, K. Depressed antioxidant defense in rat heart in experimental magnesium deficiency implications for the pathogenesis of myocardial lesions. Biol. Trace Element Res. 1997, 60, 139–144. [Google Scholar] [CrossRef]
- Racay, P. Effect of magnesium on calcium-induced depolarisation of mitochondrial transmembrane potential. Cell Biol. Int. 2008, 32, 136–145. [Google Scholar] [CrossRef]
- Blomeyer, C.A.; Bazil, J.N.; Stowe, D.F.; Dash, R.K.; Camara, A.K. Mg2+ differentially regulates two modes of mitochondrial Ca2+ uptake in isolated cardiac mitochondria: Implications for mitochondrial Ca2+ sequestration. J. Bioenerg. Biomembr. 2016, 48, 175–188. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wei, X.; Yan, P.; Han, Y.; Sun, S.; Wu, K.-C.; Fan, D. Human mitochondrial Mrs2 protein promotes multidrug resistance in gastric cancer cells by regulating p27, cyclin D1 expression and cytochrome C release. Cancer Biol. Ther. 2009, 8, 607–614. [Google Scholar] [CrossRef] [Green Version]
- Salvi, M.; Bozac, A.; Toninello, A. Gliotoxin induces Mg2+ efflux from intact brain mitochondria. Neurochem. Int. 2004, 45, 759–764. [Google Scholar]
- Sponder, G.; Abdulhanan, N.; Fröhlich, N.; Mastrototaro, L.; Aschenbach, J.R.; Röntgen, M.; Pilchova, I.; Cibulka, M.; Racay, P.; Kolisek, M. Overexpression of Na+/Mg2+ exchanger SLC41A1 attenuates pro-survival signaling. Oncotarget 2017, 9, 5084–5104. [Google Scholar] [CrossRef] [Green Version]
- Bednarczyk, P.; Dołowy, K.; Szewczyk, A. Matrix Mg2+regulates mitochondrial ATP-dependent potassium channel from heart. FEBS Lett. 2005, 579, 1625–1632. [Google Scholar] [CrossRef] [Green Version]
- Beavis, A.D.; Powers, M.F. On the regulation of the mitochondrial inner membrane anion channel by magnesium and protons. J. Biol. Chem. 1989, 264, 17148–17155. [Google Scholar]
- Zoratti, M.; Szabò, I. The mitochondrial permeability transition. Biochim. Biophys. Acta Rev. Biomembr. 1995, 1241, 139–176. [Google Scholar] [CrossRef]
- Gorgoglione, V.; Laraspata, D.; La Piana, G.; Marzulli, D.; Lofrumento, N.E. Protective effect of magnesium and potassium ions on the permeability of the external mitochondrial membrane. Arch. Biochem. Biophys. 2007, 461, 13–23. [Google Scholar] [CrossRef]
- La Piana, G.; Gorgoglione, V.; Laraspata, D.; Marzulli, D.; Lofrumento, N.E. Effect of magnesium ions on the activity of the cytosolic NADH/cytochrome c electron transport system. FEBS J. 2008, 275, 6168–6179. [Google Scholar]
- Seo, Y.-W.; Na Shin, J.; Ko, K.H.; Cha, J.H.; Park, J.Y.; Lee, B.R.; Yun, C.-W.; Kim, Y.M.; Seol, D.-W.; Kim, D.-W.; et al. The Molecular Mechanism of Noxa-induced Mitochondrial Dysfunction in p53-Mediated Cell Death. J. Biol. Chem. 2003, 278, 48292–48299. [Google Scholar] [CrossRef] [Green Version]
- Sharikabad, M.N.; Ostbye, K.M.; Brors, O. Increased [Mg2+]o reduces Ca2+ influx and disruption of mitochondrial membrane potential during reoxygenation. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H2113–H2123. [Google Scholar]
- Huang, C.-Y.; Hsieh, Y.-L.; Ju, D.-T.; Lin, C.-C.; Kuo, C.-H.; Liou, Y.-F.; Ho, T.-J.; Tsai, C.-H.; Tsai, F.-J.; Lin, J.-Y. Attenuation of Magnesium Sulfate on CoCl(2)-Induced Cell Death by Activating ERK1/2/MAPK and Inhibiting HIF-1alpha via Mitochondrial Apoptotic Signaling Suppression in a Neuronal Cell Line. Chin. J. Physiol. 2015, 58, 244–253. [Google Scholar]
- Ferrari, R.; Albertini, A.; Curello, S.; Ceconi, C.; Di Lisa, F.; Raddino, R.; Visioli, O. Myocardial recovery during post-ischaemic reperfusion: Effects of nifedipine, calcium and magnesium. J. Mol. Cell. Cardiol. 1986, 18, 487–498. [Google Scholar] [CrossRef]
- Boelens, A.D.; Pradhan, R.K.; Blomeyer, C.A.; Camara, A.K.S.; Dash, R.K.; Stowe, D.F. Extra-matrix Mg2+ limits Ca2+ uptake and modulates Ca2+ uptake–independent respiration and redox state in cardiac isolated mitochondria. J. Bioenerg. Biomembr. 2013, 45, 203–218. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, J.; Yue, J.; Wang, Y.; Yang, C.; Cui, Q. High magnesium prevents matrix vesicle-mediated mineralization in human bone marrow-derived mesenchymal stem cells via mitochondrial pathway and autophagy. Cell Biol. Int. 2018, 42, 205–215. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Vitale, G.; Capri, M.; Salvioli, S. Inflammaging and ‘Garb-aging’. Trends Endocrinol. Metab. 2017, 28, 199–212. [Google Scholar]
- Pike, J.W.; Christakos, S. Biology and Mechanisms of Action of the Vitamin D Hormone. Endocrinol. Metab. Clin. N. Am. 2017, 46, 815–843. [Google Scholar] [CrossRef]
- Holick, M. Vitamin D Deficiency. New Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Charoenngam, N.; Holick, M. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Rude, R.K.; Adams, J.S.; Ryzen, E.; Endres, D.B.; Niimi, H.; Horst, R.L.; Haddad, J.G.; Singer, F.R. Low Serum Concentrations of 1,25-Dihydroxyvitamin D in Human Magnesium Deficiency. J. Clin. Endocrinol. Metab. 1985, 61, 933–940. [Google Scholar] [CrossRef]
- Reddy, V.; Sivakumar, B. MAGNESIUM-DEPENDENT VITAMIN-D-RESISTANT RICKETS. Lancet 1974, 303, 963–965. [Google Scholar] [CrossRef]
- Uwitonze, A.M.; Razzaque, M.S. Role of Magnesium in Vitamin D Activation and Function. J. Am. Osteopat. Assoc. 2018, 118, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Zittermann, A. Magnesium deficit—overlooked cause of low vitamin D status? BMC Med. 2013, 11. [Google Scholar] [CrossRef] [Green Version]
- Risco, F.; Traba, M.L. Influence of magnesium on the in vitro synthesis of 24,25-dihydroxyvitamin D3 and 1 alpha, 25-dihydroxyvitamin D3. Magnes. Res. 1992, 5, 5–14. [Google Scholar]
- Anast, C.S.; Mohs, J.M.; Kaplan, S.L.; Burns, T.W. Evidence for Parathyroid Failure in Magnesium Deficiency. Science 1972, 177, 606–608. [Google Scholar] [CrossRef]
- Medalle, R.; Waterhouse, C.; Hahn, T.J. Vitamin D resistance in magnesium deficiency. Am. J. Clin. Nutr. 1976, 29, 854–858. [Google Scholar] [CrossRef]
- Rude, R.K.; Oldham, S.B.; Sharp, C.F.; Singer, F.R. Parathyroid Hormone Secretion in Magnesium Deficiency*. J. Clin. Endocrinol. Metab. 1978, 47, 800–806. [Google Scholar] [CrossRef]
- Mutnuri, S.; Fernández, I.; Kochar, T. Suppression of Parathyroid Hormone in a Patient with Severe Magnesium Depletion. Case Rep. Nephrol. 2016, 2016, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Rude, R.K.; Oldham, S.B.; Singer, F.R. FUNCTIONAL HYPOPARATHYROIDISM AND PARATHYROID HORMONE END-ORGAN RESISTANCE IN HUMAN MAGNESIUM DEFICIENCY. Clin. Endocrinol. 1976, 5, 209–224. [Google Scholar] [CrossRef]
- Rösler, A.; Rabinowitz, D. Magnesium-induced reversal of vitamin-D resistance in hypoparathyroidism. Lancet 1973, 1, 803–804. [Google Scholar]
- Fuss, M.; Bergmann, P.; Bergans, A.; Bagon, J.; Cogan, E.; Pepersack, T.; Van Gossum, M.; Corvilain, J. CORRECTION OF LOW CIRCULATING LEVELS OF 1,25-DIHYDROXYVITAMIN D BY 25-HYDROXYVITAMIN D DURING REVERSAL OF HYPOMAGNESAEMIA. Clin. Endocrinol. 1989, 31, 31–38. [Google Scholar] [CrossRef]
- Hardwick, L.L.; Jones, M.R.; Brautbar, N.; Lee, D.B.N. Magnesium Absorption: Mechanisms and the Influence of Vitamin D, Calcium and Phosphate. J. Nutr. 1991, 121, 13–23. [Google Scholar] [CrossRef]
- Veronese, N.; Stubbs, B.; Solmi, M.; Noale, M.; Vaona, A.; Demurtas, J.; Maggi, S. Dietary magnesium intake and fracture risk: Data from a large prospective study. Br. J. Nutr. 2017, 117, 1570–1576. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.; Song, Y.; Manson, J.E.; Signorello, L.B.; Zhang, S.M.; Shrubsole, M.J.; Ness, R.M.; Seidner, D.L.; Dai, Q. Magnesium, vitamin D status and mortality: Results from US National Health and Nutrition Examination Survey (NHANES) 2001 to 2006 and NHANES III. BMC Med. 2013, 11. [Google Scholar] [CrossRef] [Green Version]
- Dai, Q.; Zhu, X.; E Manson, J.; Song, Y.; Li, X.; A Franke, A.; Costello, R.B.; Rosanoff, A.; Nian, H.; Fan, L.; et al. Magnesium status and supplementation influence vitamin D status and metabolism: Results from a randomized trial. Am. J. Clin. Nutr. 2018, 108, 1249–1258. [Google Scholar] [CrossRef]
- Chocano-Bedoya, P.; Ronnenberg, A.G. Vitamin D and tuberculosis. Nutr. Rev. 2009, 67, 289–293. [Google Scholar] [CrossRef] [Green Version]
- Beard, J.A.; Bearden, A.; Striker, R. Vitamin D and the anti-viral state. J. Clin. Virol. 2011, 50, 194–200. [Google Scholar] [CrossRef]
- Brighenti, S.; Bergman, P.; Martineau, A.R. Vitamin D and tuberculosis: Where next? J. Intern. Med. 2018. [Google Scholar] [CrossRef] [Green Version]
- Dini, C.; Bianchi, A. The potential role of vitamin D for prevention and treatment of tuberculosis and infectious diseases. Annali dell’Istituto Superiore Sanità 2012, 48, 319–327. [Google Scholar] [CrossRef]
- Reid, D.; Toole, B.J.; Knox, S.; Talwar, D.; Harten, J.; O’Reilly, D.S.J.; Blackwell, S.; Kinsella, J.; McMillan, D.C.; Wallace, A.M.; et al. The relation between acute changes in the systemic inflammatory response and plasma 25-hydroxyvitamin D concentrations after elective knee arthroplasty. Am. J. Clin. Nutr. 2011, 93, 1006–1011. [Google Scholar] [CrossRef] [Green Version]
- Borella, E.; Nesher, G.; Israeli, E.; Shoenfeld, Y. Vitamin D: A new anti-infective agent? Ann. N. Y. Acad. Sci. 2014, 1317, 76–83. [Google Scholar] [CrossRef]
- Arboleda, J.F.; Urcuqui-Inchima, S. Vitamin D Supplementation: A Potential Approach for Coronavirus/COVID-19 Therapeutics? Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [Green Version]
- Rejnmark, L.; Bislev, L.S.; Cashman, K.D.; Eiríksdottir, G.; Gaksch, M.; Gruebler, M.; Grimnes, G.; Gudnason, V.; Lips, P.; Pilz, S.; et al. Non-skeletal health effects of vitamin D supplementation: A systematic review on findings from meta-analyses summarizing trial data. PLoS ONE 2017, 12, e0180512. [Google Scholar] [CrossRef] [Green Version]
- Martineau, A.R.; A Jolliffe, D.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Yang, H.; Mao, Y. Magnesium and liver disease. Ann. Transl. Med. 2019, 7, 578. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Y.; Ma, H.; Wang, B.; Xu, L.; Zhang, H.; Song, X.; Gao, L.; Liang, X.; Ma, C. Hepatitis B virus X protein amplifies TGF-beta promotion on HCC motility through down-regulating PPM1a. Oncotarget 2016, 7, 33125–33135. [Google Scholar]
- Nasser, R.; Naffaa, M.E.; Mashiach, T.; Azzam, Z.S.; Braun, E. The association between serum magnesium levels and community-acquired pneumonia 30-day mortality. BMC Infect. Dis. 2018, 18, 698. [Google Scholar]
- Bhatt, S.P.; Khandelwal, P.; Nanda, S.; Stoltzfus, J.C.; Fioravanti, G.T. Serum magnesium is an independent predictor of frequent readmissions due to acute exacerbation of chronic obstructive pulmonary disease. Respir. Med. 2008, 102, 999–1003. [Google Scholar] [CrossRef] [Green Version]
- Tannou, T.; Koeberle, S.; Manckoundia, P.; Aubry, R. Multifactorial immunodeficiency in frail elderly patients: Contributing factors and management. Méd. Mal. Infect. 2019, 49, 167–172. [Google Scholar] [CrossRef]
- Sadighi Akha, A.A. Aging and the immune system: An overview. J. Immunol. Methods 2018, 463, 21–26. [Google Scholar]
- Herzig, C.T.A.; Dick, A.W.; Sorbero, M.; Pogorzelska-Maziarz, M.; Cohen, C.C.; Larson, E.L.; Stone, P.W. Infection Trends in US Nursing Homes, 2006-2013. J. Am. Med. Dir. Assoc. 2017, 18, 635.e9–635.e20. [Google Scholar] [CrossRef]
- Yorita, K.L.; Holman, R.C.; Steiner, C.A.; Sejvar, J.J.; Stoll, B.J.; Schonberger, L.B. Infectious Disease Hospitalizations in the United States. Clin. Infect. Dis. 2009, 49, 1025–1035. [Google Scholar] [CrossRef]
- WHO. Global Health Estimates 2016: Estimated Deaths by Age, Sex and Cause; WHO: Geneva, Switzerland, 2016; Available online: https://www.who.int/healthinfo/global_burden_disease/estimates/en/ (accessed on 1 December 2020).
- Chiara, T.; Victoria, C.C.; Giulia, A.; Cristina, B.; Stefano, C.; Matilde, C.; Rita, D.M.; Viviana, G.; Beata, J.; Leopoldo, S.; et al. Health-Care-Associated Infections Management, sow the seed of good habits: A grounded theory study. Acta Biomed. 2019, 90, 26–33. [Google Scholar]
- Freedman, V.A.; Martin, L.G. Contribution of chronic conditions to aggregate changes in old-age functioning. Am. J. Public Health 2000, 90, 1755–1760. [Google Scholar] [CrossRef] [Green Version]
- Meurer, W.J.; Losman, E.D.; Smith, B.L.; Malani, P.N.; Younger, J.G. Short-term functional decline of older adults admitted for suspected sepsis. Am. J. Emerg. Med. 2011, 29, 936–942. [Google Scholar] [CrossRef]
- Johnstone, J.; Eurich, D.T.; Majumdar, S.R.; Jin, Y.; Marrie, T.J. Long-term morbidity and mortality after hospitalization with community-acquired pneumonia: A population-based cohort study. Medicine 2008, 87, 329–334. [Google Scholar]
- Curns, A.T.; Holman, R.C.; Sejvar, J.J.; Owings, M.F.; Schonberger, L.B. Infectious disease hospitalizations among older adults in the United States from 1990 through 2002. Arch. Intern. Med. 2005, 165, 2514–2520. [Google Scholar]
- Elias, R.; Hartshorn, K.; Rahma, O.; Lin, N.; Snyder-Cappione, J.E. Aging, immune senescence, and immunotherapy: A comprehensive review. Semin. Oncol. 2018, 45, 187–200. [Google Scholar]
- Agarwal, S.; Busse, P.J. Innate and adaptive immunosenescence. Ann. Allergy Asthma Immunol. 2010, 104, 183–190. [Google Scholar]
- Castle, S.C.; Uyemura, K.; Fulop, T.; Makinodan, T. Host Resistance and Immune Responses in Advanced Age. Clin. Geriatr. Med. 2007, 23, 463–479. [Google Scholar] [CrossRef]
- Shahid, Z.; Kalayanamitra, R.; McClafferty, B.; Kepko, D.; Ramgobin, D.; Nitasa Sahu, M.D.; Dhirisha Bhatt, M.D.; Kirk Jones, P.D.; Reshma Golamari, M.D.; Rohit Jain, M.D. COVID-19 and Older Adults: What We Know. J. Am. Geriatr. Soc. 2020, 68, 926–929. [Google Scholar]
- Emami, A.; Javanmardi, F.; Pirbonyeh, N.; Akbari, A. Prevalence of Underlying Diseases in Hospitalized Patients with COVID-19: A Systematic Review and Meta-Analysis. Arch. Acad. Emerg. Med. 2020, 8, e35. [Google Scholar]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nat. Cell Biol. 2020, 579, 270–273. [Google Scholar] [CrossRef] [Green Version]
- WHO. Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int/?gclid=Cj0KCQjw0rr4BRCtARIsAB0_48NF8a417ap3xz5a6rC5bv4LHq4iaWP5iTQPyvEhFlQLpGa7fyo6R0aAhVTEALw_wcB (accessed on 14 December 2020).
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Perrotta, F.; Corbi, G.; Mazzeo, G.; Boccia, M.; Aronne, L.; D’Agnano, V.; Komici, K.; Mazzarella, G.; Parrella, R.; Bianco, A. COVID-19 and the elderly: Insights into pathogenesis and clinical decision-making. Aging Clin. Exp. Res. 2020, 32, 1599–1608. [Google Scholar] [CrossRef]
- Li, P.; Chen, L.; Liu, Z.; Pan, J.; Zhou, D.; Wang, H.; Gong, H.; Fu, Z.; Song, Q.; Min, Q.; et al. Clinical features and short-term outcomes of elderly patients with COVID-19. Int. J. Infect. Dis. 2020, 97, 245–250. [Google Scholar] [CrossRef]
- Sepulveda, E.R.; Stall, N.M.; Sinha, S.K. A Comparison of COVID-19 Mortality Rates Among Long-Term Care Residents in 12 OECD Countries. J. Am. Med Dir. Assoc. 2020, 21, 1572–1574. [Google Scholar] [CrossRef]
- Abebe, E.C.; Dejenie, T.A.; Shiferaw, M.Y.; Malik, T. The newly emerged COVID-19 disease: A systemic review. Virol. J. 2020, 17, 1–8. [Google Scholar] [CrossRef]
- Nielsen, F.H. Magnesium, inflammation, and obesity in chronic disease. Nutr. Rev. 2010, 68, 333–340. [Google Scholar] [CrossRef]
- Dominguez, L.; Barbagallo, M. The biology of the metabolic syndrome and aging. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 5–11. [Google Scholar] [CrossRef]
- Dietz, W.; Santos Burgoa, C. Obesity and its Implications for COVID-19 Mortality. Obesity 2020, 28, 1005. [Google Scholar]
- Wadman, M. Why obesity worsens COVID-19. Science 2020, 369, 1280–1281. [Google Scholar] [CrossRef]
- Iotti, S.; Wolf, F.; Mazur, A.; A Maier, J. The COVID-19 pandemic: Is there a role for magnesium? Hypotheses and perspectives. Magnes. Res. 2020, 1. [Google Scholar] [CrossRef]
- Wallace, T. Combating COVID-19 and Building Immune Resilience: A Potential Role for Magnesium Nutrition? J. Am. Coll. Nutr. 2020, 39, 685–693. [Google Scholar] [CrossRef]
- Tan, C.W.; Ho, L.P.; Kalimuddin, S.; Cherng, B.P.Z.; Teh, Y.E.; Thien, S.Y.; Wong, H.M.; Tern, P.J.W.; Chandran, M.; Chay, J.W.M.; et al. Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B12 in combination on progression to severe outcomes in older patients with coronavirus (COVID-19). Nutrient 2020, 79. [Google Scholar] [CrossRef]
- Tang, C.-F.; Ding, H.; Jiao, R.-Q.; Wu, X.-X.; Kong, L.-D. Possibility of magnesium supplementation for supportive treatment in patients with COVID-19. Eur. J. Pharmacol. 2020, 886. [Google Scholar] [CrossRef]
- Ilie, P.C.; Stefanescu, S.; Smith, L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 2020, 32, 1195–1198. [Google Scholar] [CrossRef]
- Meltzer, D.O.; Best, T.J.; Zhang, H.; Vokes, T.; Arora, V.; Solway, J. Association of Vitamin D Status and Other Clinical Characteristics with COVID-19 Test Results. JAMA Netw. Open 2020, 3, e2019722. [Google Scholar] [CrossRef]
- Bernheim, A.; Mei, X.; Huang, M.; Yang, Y.; Fayad, Z.A.; Zhang, N.; Diao, K.; Lin, B.; Zhu, X.; Li, K.; et al. Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology 2020, 295. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.-Y.; Ma, Y.-T.; Zhang, J.; Xie, X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020, 17, 259–260. [Google Scholar] [CrossRef] [Green Version]
- Zimmer, H.G. Effects of magnesium orotate on rat heart function. Cardioscience 1994, 5, 55–61. [Google Scholar]
- Gao, C.; Cai, Y.; Zhang, K.; Zhou, L.; Zhang, Y.; Zhang, X.; Li, Q.; Li, W.; Yang, S.; Zhao, X.; et al. Association of hypertension and antihypertensive treatment with COVID-19 mortality: A retrospective observational study. Eur. Heart J. 2020, 41, 2058–2066. [Google Scholar] [CrossRef]
- Katulanda, P.; Dissanayake, H.A.; Ranathunga, I.; Vithiya, R.; Wijewickrama, P.S.A.; Yogendranathan, N.; Gamage, K.K.K.; De Silva, N.L.; Sumanatilleke, M.; Somasundaram, N.; et al. Prevention and management of COVID-19 among patients with diabetes: An appraisal of the literature. Diabetologia 2020, 63, 1440–1452. [Google Scholar] [CrossRef]
- O’Driscoll, M.; Ribeiro Dos Santos, G.; Wang, L.; Cummings, D.A.T.; Azman, A.S.; Paireau, J.; Fontanet, A.; Cauchemez, S.; Salje, H. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature 2020. [Google Scholar] [CrossRef]
- Barbagallo, M.; Dominguez, L.J.; Galioto, A.; Ferlisi, A.; Cani, C.; Malfa, L.; Pineo, A.; Paolisso, G. Role of magnesium in insulin action, diabetes and cardio-metabolic syndrome X. Mol. Aspects Med. 2003, 24, 39–52. [Google Scholar]
- Chernow, B.; Bamberger, S.; Stoiko, M.; Vadnais, M.; Mills, S.; Hoellerich, V.; Warshaw, A.L. Hypomagnesemia in Patients in Postoperative Intensive Care. Chest 1989, 95, 391–397. [Google Scholar] [CrossRef]
- Escuela, M.P.; Guerra, M.; Celaya, S.; Añón, J.M.; Martínez-Vizcaíno, V.; Zapatero, M.D.; García-Jalón, A. Total and ionized serum magnesium in critically ill patients. Intensiv. Care Med. 2005, 31, 151–156. [Google Scholar] [CrossRef]
- Hansen, B.-A.; Bruserud, Ø. Hypomagnesemia in critically ill patients. J. Intensiv. Care 2018, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Libby, P.; Luscher, T. COVID-19 is, in the end, an endothelial disease. Eur. Heart J. 2020, 41, 3038–3044. [Google Scholar]
- Barbagallo, M.; Dominguez, L.; Galioto, A.; Pineo, A.; Belvedere, M. Oral magnesium supplementation improves vascular function in elderly diabetic patients. Magnes. Res. 2010, 23, 131–137. [Google Scholar]
- Shechter, M.; Sharir, M.; Labrador, M.J.P.; Forrester, J.; Silver, B.; Merz, C.N.B. Oral Magnesium Therapy Improves Endothelial Function in Patients With Coronary Artery Disease. Circulation 2000, 102, 2353–2358. [Google Scholar] [CrossRef] [Green Version]
- Marques, B.C.A.A.; Klein, M.R.S.T.; Da Cunha, M.R.; Mattos, S.D.S.; Nogueira, L.D.P.; De Paula, T.; Corrêa, F.M.; Oigman, W.; Neves, M.F. Effects of Oral Magnesium Supplementation on Vascular Function: A Systematic Review and Meta-analysis of Randomized Controlled Trials. High Blood Press. Cardiovasc. Prev. 2019, 27, 19–28. [Google Scholar] [CrossRef]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020, 53, 25–32. [Google Scholar] [CrossRef]
- Altura, B.M.; Gebrewold, A.; Ising, H.; Gunther, T. Magnesium deficiency and hypertension: Correlation between magnesium-deficient diets and microcirculatory changes in situ. Science 1984, 223, 1315–1317. [Google Scholar] [CrossRef]
- Iseri, L.T.; French, J.H. Magnesium: Nature’s physiologic calcium blocker. Am. Hear. J. 1984, 108, 188–193. [Google Scholar] [CrossRef]
- Louvet, L.; Büchel, J.; Steppan, S.; Passlick-Deetjen, J.; Massy, Z. Magnesium prevents phosphate-induced calcification in human aortic vascular smooth muscle cells. Nephrol. Dial. Transplant. 2012, 28, 869–878. [Google Scholar] [CrossRef] [Green Version]
- Evans, P.C.; Rainger, G.E.; Mason, J.C.; Guzik, T.J.; Osto, E.; Stamataki, Z.; Neil, D.; E Hoefer, I.; Fragiadaki, M.; Waltenberger, J.; et al. Endothelial dysfunction in COVID-19: A position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc. Res. 2020, 116, 2177–2184. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; A Schuepbach, R.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar]
- Maier, J. Endothelial cells and magnesium: Implications in atherosclerosis. Clin. Sci. 2011, 122, 397–407. [Google Scholar] [CrossRef] [Green Version]
- Sheu, J.-R.; Hsiao, G.; Shen, M.-Y.; Fong, T.-H.; Chen, Y.-W.; Lin, C.-H.; Chou, D.-S. Mechanisms involved in the antiplatelet activity of magnesium in human platelets. Br. J. Haematol. 2002, 119, 1033–1041. [Google Scholar]
- Pereira, M.; Damascena, A.D.; Azevedo, L.M.G.; Oliveira, T.D.A.; Santana, J.D.M. Vitamin D deficiency aggravates COVID-19: Systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2020, 1–9. [Google Scholar] [CrossRef]
- De Smet, D.; De Smet, K.; Herroelen, P.; Gryspeerdt, S.; Martens, G.A. Serum 25(OH)D Level on Hospital Admission Associated With COVID-19 Stage and Mortality. Am. J. Clin. Pathol. 2020. [Google Scholar] [CrossRef]
- Jain, A.; Chaurasia, R.; Sengar, N.S.; Singh, M.; Mahor, S.; Narain, S. Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers. Sci. Rep. 2020, 10, 20191. [Google Scholar] [CrossRef]



| Decreased | Increased | |
|---|---|---|
| Innate immune system | ||
|
| |
| Adaptive immune system | ||
| T cells |
|
|
| B cells |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dominguez, L.J.; Veronese, N.; Guerrero-Romero, F.; Barbagallo, M. Magnesium in Infectious Diseases in Older People. Nutrients 2021, 13, 180. https://doi.org/10.3390/nu13010180
Dominguez LJ, Veronese N, Guerrero-Romero F, Barbagallo M. Magnesium in Infectious Diseases in Older People. Nutrients. 2021; 13(1):180. https://doi.org/10.3390/nu13010180
Chicago/Turabian StyleDominguez, Ligia J., Nicola Veronese, Fernando Guerrero-Romero, and Mario Barbagallo. 2021. "Magnesium in Infectious Diseases in Older People" Nutrients 13, no. 1: 180. https://doi.org/10.3390/nu13010180
APA StyleDominguez, L. J., Veronese, N., Guerrero-Romero, F., & Barbagallo, M. (2021). Magnesium in Infectious Diseases in Older People. Nutrients, 13(1), 180. https://doi.org/10.3390/nu13010180








