Nutritional Status and Diet Style Affect Cognitive Function in Alcoholic Liver Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Neuropsychological Test
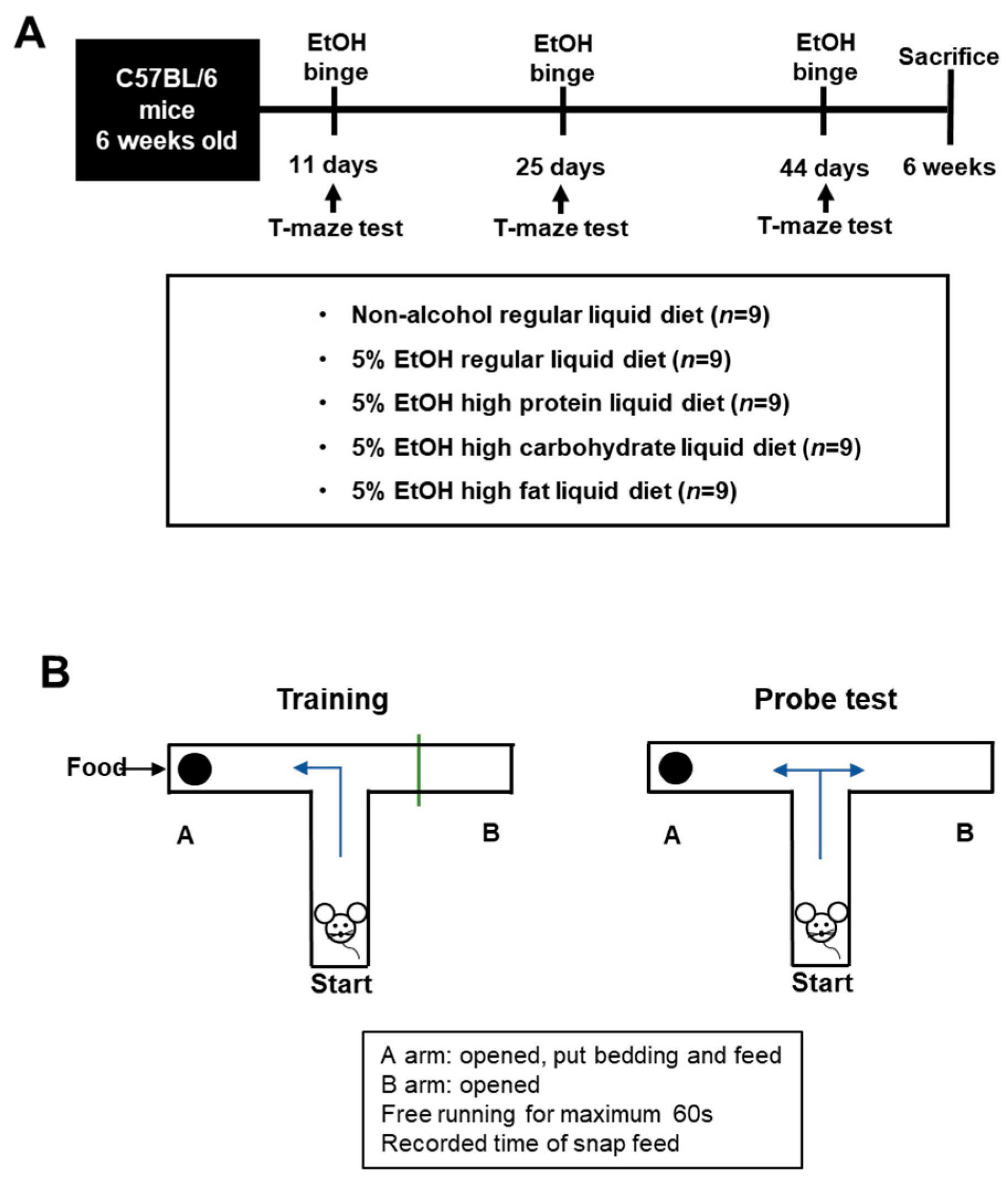
2.3. Experimental Animals
2.4. Pathology
2.5. Western Blot Analysis of the Brain
3. Results
3.1. Patient Characteristics
3.2. Body Mass Index and Cognitive Function
3.3. Changes in the Body Weight and Pathology
3.4. Cognitive Function Based on Diet Groups
3.5. Brain Markers in the High-Protein Diet Group
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rehm, J.; Samokhvalov, A.V.; Shield, K.D. Global burden of alcoholic liver diseases. J. Hepatol. 2013, 59, 160–168. [Google Scholar] [CrossRef] [Green Version]
- Room, R.; Babor, T.; Rehm, J. Alcohol and public health. Lancet 2005, 365, 519–530. [Google Scholar] [CrossRef]
- Sacks, J.J.; Roeber, J.; Bouchery, E.E.; Gonzales, K.; Chaloupka, F.J.; Brewer, R.D. State costs of excessive alcohol consumption, 2006. Am. J. Prev. Med. 2013, 45, 474–485. [Google Scholar] [CrossRef] [Green Version]
- Burke, T.R. The economic impact of alcohol abuse and alcoholism. Public Health Rep. 1988, 103, 564. [Google Scholar]
- Thomas, V.S.; Rockwood, K.J. Alcohol abuse, cognitive impairment, and mortality among older people. J. Am. Geriatr. Soc. 2001, 49, 415–420. [Google Scholar] [CrossRef]
- Suk, K.T.; Kim, M.Y.; Baik, S.K. Alcoholic liver disease: Treatment. World J. Gastroenterol. 2014, 20, 12934–12944. [Google Scholar] [CrossRef]
- Song, D.S. Medical Treatment of Alcoholic Liver Disease. Korean J. Gastroenterol. 2020, 76, 65–70. [Google Scholar] [CrossRef]
- Koretz, R.L.; Avenell, A.; Lipman, T.O. Nutritional support for liver disease. Cochrane Database Syst. Rev. 2012, CD008344. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, C.; Suk, K.T.; Choi, H.C.; Bang, C.S.; Yoon, J.H.; Baik, G.H.; Kim, D.J.; Jang, M.U.; Sohn, J.H. Differences in cognitive function between patients with viral and alcoholic compensated liver cirrhosis. Metab. Brain Dis. 2016, 31, 369–376. [Google Scholar] [CrossRef]
- Harper, C. The neuropathology of alcohol-related brain damage. Alcohol Alcohol. 2009, 44, 136–140. [Google Scholar] [CrossRef] [Green Version]
- Oscar-Berman, M.; Marinković, K. Alcohol: Effects on neurobehavioral functions and the brain. Neuropsychol. Rev. 2007, 17, 239–257. [Google Scholar] [CrossRef]
- Stranahan, A.M.; Norman, E.D.; Lee, K.; Cutler, R.G.; Telljohann, R.S.; Egan, J.M.; Mattson, M.P. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus 2008, 18, 1085–1088. [Google Scholar] [CrossRef] [Green Version]
- Scarmeas, N.; Anastasiou, C.A.; Yannakoulia, M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018, 17, 1006–1015. [Google Scholar] [CrossRef]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R.; Simons-Morton, D.G. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef]
- Thabut, D.; D’Amico, G.; Tan, P.; De Franchis, R.; Fabricius, S.; Lebrec, D.; Bosch, J.; Bendtsen, F. Diagnostic performance of Baveno IV criteria in cirrhotic patients with upper gastrointestinal bleeding: Analysis of the F7 liver-1288 study population. J. Hepatol. 2010, 53, 1029–1034. [Google Scholar] [CrossRef]
- Tiniakos, D.G. Nonalcoholic fatty liver disease/nonalcoholic steatohepatitis: Histological diagnostic criteria and scoring systems. Eur. J. Gastroenterol. Hepatol. 2010, 22, 643–650. [Google Scholar] [CrossRef]
- Südhof, T.C. Neuroligins and neurexins link synaptic function to cognitive disease. Nature 2008, 455, 903–911. [Google Scholar] [CrossRef] [Green Version]
- Lu, B.; Nagappan, G.; Lu, Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. In Neurotrophic Factors; Springer: Berlin/Heidelberg, Germany, 2014; pp. 223–250. [Google Scholar]
- Gazdzinski, S.; Kornak, J.; Weiner, M.W.; Meyerhoff, D.J. Body mass index and magnetic resonance markers of brain integrity in adults. Ann. Neurol. 2008, 63, 652–657. [Google Scholar] [CrossRef] [Green Version]
- Gustafson, D.; Lissner, L.; Bengtsson, C.; Björkelund, C.; Skoog, I. A 24-year follow-up of body mass index and cerebral atrophy. Neurology 2004, 63, 1876–1881. [Google Scholar] [CrossRef]
- Eskelinen, M.H.; Ngandu, T.; Helkala, E.L.; Tuomilehto, J.; Nissinen, A.; Soininen, H.; Kivipelto, M. Fat intake at midlife and cognitive impairment later in life: A population-based CAIDE study. Int. J. Geriatr. Psychiatry A J. Psychiatry Late Life Allied Sci. 2008, 23, 741–747. [Google Scholar] [CrossRef]
- Nuttall, F.Q. Body mass index: Obesity, BMI, and health: A critical review. Nutr. Today 2015, 50, 117. [Google Scholar] [CrossRef] [Green Version]
- Kleiner, K.D.; Gold, M.S.; Frost-Pineda, K.; Lenz-Brunsman, B.; Perri, M.G.; Jacobs, W.S. Body mass index and alcohol use. J. Addict. Dis. 2004, 23, 105–118. [Google Scholar] [CrossRef]
- Van Skike, C.E.; Goodlett, C.; Matthews, D.B. Acute alcohol and cognition: Remembering what it causes us to forget. Alcohol 2019, 79, 105–125. [Google Scholar] [CrossRef]
- Bora, E.; Zorlu, N. Social cognition in alcohol use disorder: A meta-analysis. Addiction 2017, 112, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Bartley, P.C.; Rezvani, A.H. Alcohol and cognition-consideration of age of initiation, usage patterns and gender: A brief review. Curr. Drug Abus. Rev. 2012, 5, 87–97. [Google Scholar] [CrossRef]
- Stampfer, M.J.; Kang, J.H.; Chen, J.; Cherry, R.; Grodstein, F. Effects of moderate alcohol consumption on cognitive function in women. N. Engl. J. Med. 2005, 352, 245–253. [Google Scholar] [CrossRef]
- Ehrmann, J.; Aiglova, K.; Urban, O.; Cvekova, S.; Dvoran, P. Alcohol-related liver diseases (ALD). Vnitr. Lek. 2020, 66, 39–51. [Google Scholar]
- Karanfilian, B.V.; Park, T.; Senatore, F.; Rustgi, V.K. Minimal Hepatic Encephalopathy. Clin. Liver Dis. 2020, 24, 209–218. [Google Scholar] [CrossRef]
- Shoji, H.; Hagihara, H.; Takao, K.; Hattori, S.; Miyakawa, T. T-maze forced alternation and left-right discrimination tasks for assessing working and reference memory in mice. J. Vis. Exp. 2012, 60, e3300. [Google Scholar] [CrossRef]
- Deacon, R.M.; Rawlins, J.N. T-maze alternation in the rodent. Nat. Protoc. 2006, 1, 7–12. [Google Scholar] [CrossRef]
- Benedek, M.; Panzierer, L.; Jauk, E.; Neubauer, A.C. Creativity on tap? Effects of alcohol intoxication on creative cognition. Conscious. Cogn. 2017, 56, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, A.F.; Colflesh, G.J.; Wiley, J. Uncorking the muse: Alcohol intoxication facilitates creative problem solving. Conscious. Cogn. 2012, 21, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Janssen, C.I.; Jansen, D.; Mutsaers, M.P.; Dederen, P.J.; Geenen, B.; Mulder, M.T.; Kiliaan, A.J. The Effect of a High-Fat Diet on Brain Plasticity, Inflammation and Cognition in Female ApoE4-Knockin and ApoE-Knockout Mice. PLoS ONE 2016, 11, e0155307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pancani, T.; Anderson, K.L.; Brewer, L.D.; Kadish, I.; DeMoll, C.; Landfield, P.W.; Blalock, E.M.; Porter, N.M.; Thibault, O. Effect of high-fat diet on metabolic indices, cognition, and neuronal physiology in aging F344 rats. Neurobiol. Aging 2013, 34, 1977–1987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benton, D.; Donohoe, R.T. The influence on cognition of the interactions between lecithin, carnitine and carbohydrate. Psychopharmacology 2004, 175, 84–91. [Google Scholar] [CrossRef] [PubMed]
- D’Anci, K.E.; Watts, K.L.; Kanarek, R.B.; Taylor, H.A. Low-carbohydrate weight-loss diets. Effects on cognition and mood. Appetite 2009, 52, 96–103. [Google Scholar] [CrossRef]
- Jacob, A.; Wang, P. Alcohol Intoxication and Cognition: Implications on Mechanisms and Therapeutic Strategies. Front. Neurosci. 2020, 14, 102. [Google Scholar] [CrossRef] [Green Version]




| Diet | Regular | High Protein | High Carbohydrate | High Fat |
|---|---|---|---|---|
| Ingredient | g/L | g/L | g/L | g/L |
| Casein | 41.4 | 57.6 | 41.4 | 41.4 |
| DL-methionine | 0.3 | 0.4 | 0.3 | 0.3 |
| L-cystine | 0.5 | 0.65 | 0.5 | 0.5 |
| Cellulose | 10 | 10 | 10 | 10 |
| Maltose dextrin | 25.6 | 66 | 83.6 | 24.05 |
| Corn oil | 8.5 | 2.5 | 2.5 | 31.1 |
| Olive oil | 28.4 | 8.4 | 8.4 | 28.4 |
| Safflower oil | 2.7 | 2.7 | 2.7 | 2.7 |
| Mineral mix | 8.75 | 8.75 | 8.75 | 8.75 |
| Vitamin mix | 2.5 | 2.5 | 2.5 | 2.5 |
| Choline bitartrate | 0.53 | 0.53 | 0.53 | 0.53 |
| Xanthan gum | 3 | 3 | 3 | 3 |
| Variables (Mean) | BMI < 22 (n = 17) | BMI ≥ 22 (n = 26) | p Value |
|---|---|---|---|
| Male n (%) | 14 (82) | 20 (77) | |
| Age | 53.2 (10.2) | 50.0 (8.0) | 0.659 |
| BMI | 21.9 (4.6) | 24.9 (3.0) | 0.001 |
| Education period (years) | 9.3 (4.0) | 10.8 (4.0) | 0.302 |
| Hemoglobin (g/dL) | 11.7 (2.0) | 12.4 (2.5) | 0.368 |
| Albumin (g/dL) | 3.8 (0.6) | 3.6 (0.8) | 0.298 |
| Total bilirubin (mg/dL) | 0.9 (0.5) | 1.7 (1.3) | 0.023 |
| AST (IU/L) | 54.6 (38.7) | 69.7 (50.8) | 0.302 |
| ALT (IU/L) | 25.0 (17.8) | 36.8 (30.0) | 0.151 |
| ALP (IU/L) | 129.5 (47.7) | 135.8 (62.5) | 0.726 |
| γ-GT (IU/L) | 434.7 (455.2) | 333.0 (382.1) | 0.434 |
| Variable | Normal Control (n = 1000) | BMI < 22 (n = 17) | BMI ≥ 22 (n = 26) | p-Value |
|---|---|---|---|---|
| Visuospatial function | ||||
| Digit span forward | 7.7 (1.4) | 6.7 (1.8) | 7.5 (1.6) | 0.039 |
| Digit span backward | 4.3 (1.0) | 3.8 (1.3) | 4.3 (1.5) | 0.138 |
| Stroop test | ||||
| Color reading correct | 85.0 (10) | 72.4 (27.1) | 83.8 (26.0) | 0.079 |
| Color reading time | 120.0 (5.0) | 119.3 (3.4) | 116.4 (9.4) | 0.076 |
| Color reading correct response rate | 1.0 (0.1) | 1.0 (0.1) | 1.0 (0.1) | 0.432 |
| Color reading time per items | 45.1 (20.2) | 24.2 (26.5) | 43.6 (32.4) | 0.006 |
| Interference scores | 55.5 (20.0) | 33.9 (31.9) | 52.3 (33.9) | 0.017 |
| K-MMSE | ||||
| K-MMSE, language | 7.8 (0.5) | 7.4 (1.4) | 7.9 (0.4) | 0.037 |
| Language | ||||
| K-BNT | 13.1 (1.3) | 11.8 (2.7) | 13.0 (1.8) | 0.017 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.R.; Kim, H.S.; Yoon, S.J.; Lee, N.Y.; Gupta, H.; Raja, G.; Gebru, Y.A.; Youn, G.S.; Kim, D.J.; Ham, Y.L.; et al. Nutritional Status and Diet Style Affect Cognitive Function in Alcoholic Liver Disease. Nutrients 2021, 13, 185. https://doi.org/10.3390/nu13010185
Choi YR, Kim HS, Yoon SJ, Lee NY, Gupta H, Raja G, Gebru YA, Youn GS, Kim DJ, Ham YL, et al. Nutritional Status and Diet Style Affect Cognitive Function in Alcoholic Liver Disease. Nutrients. 2021; 13(1):185. https://doi.org/10.3390/nu13010185
Chicago/Turabian StyleChoi, Ye Rin, Hyeong Seop Kim, Sang Jun Yoon, Na Young Lee, Haripriya Gupta, Ganesan Raja, Yoseph Asmelash Gebru, Gi Soo Youn, Dong Joon Kim, Young Lim Ham, and et al. 2021. "Nutritional Status and Diet Style Affect Cognitive Function in Alcoholic Liver Disease" Nutrients 13, no. 1: 185. https://doi.org/10.3390/nu13010185








