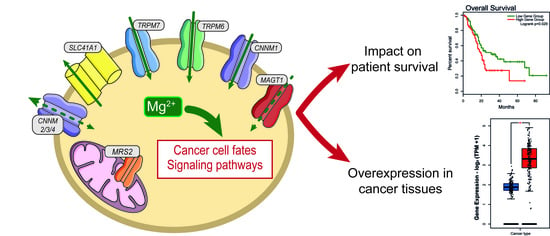
Mg2+ Transporters in Digestive Cancers
Abstract
:1. Introduction
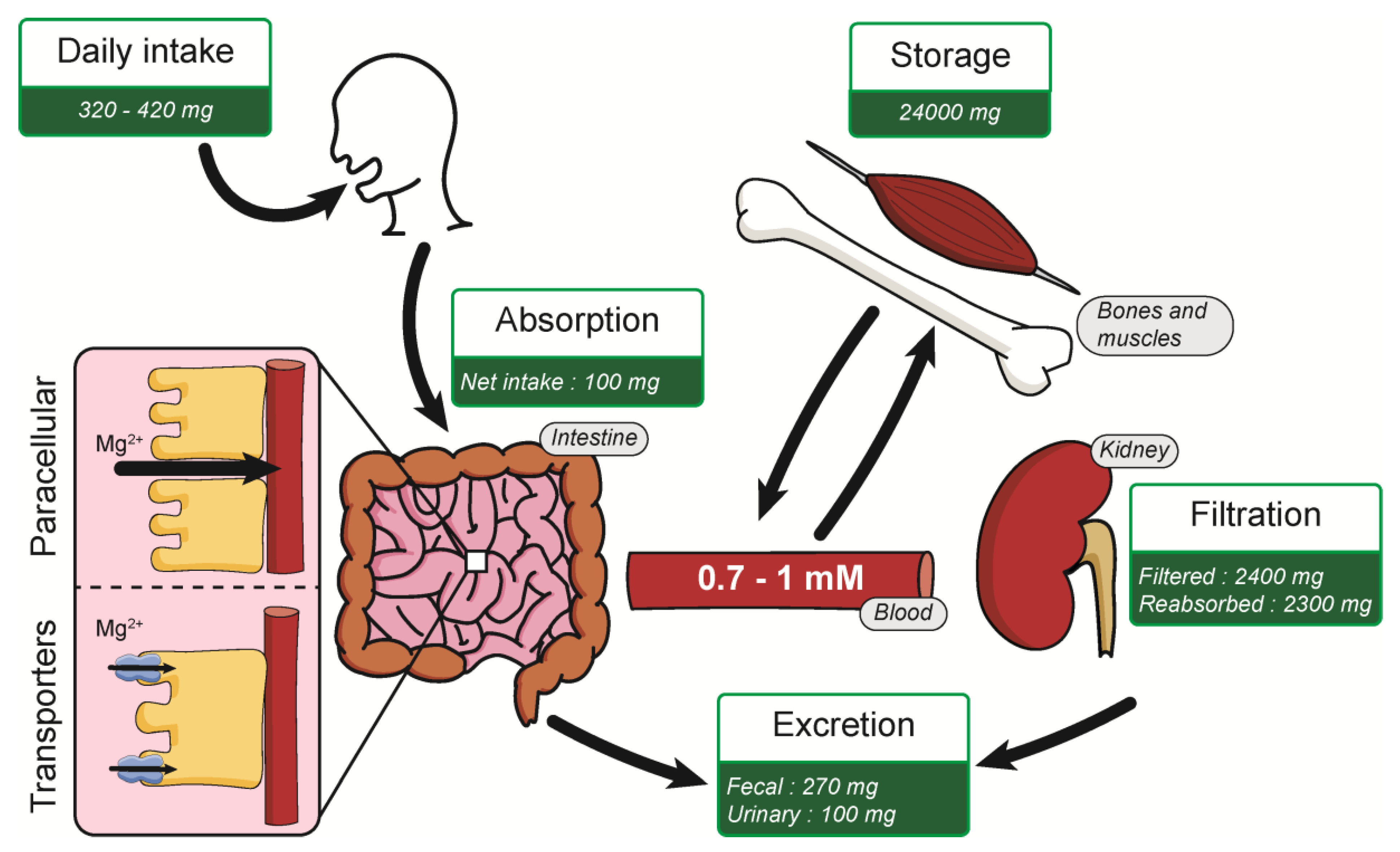
2. Magnesium
2.1. MRS2
2.2. TRPM7 and TRPM6
2.3. SLC41A1
2.4. MAGT1
2.5. CNNM Family
3. Mg2+ Intake and Digestive Cancers
4. Expression of Mg2+ Transporters in Digestive Cancers
4.1. Analysis of the Literature
4.2. Analysis of the Human Protein Atlas
4.3. Transcriptome Analysis in Datasets
4.3.1. Mg2+ Transporters Expression in Digestive Cancers
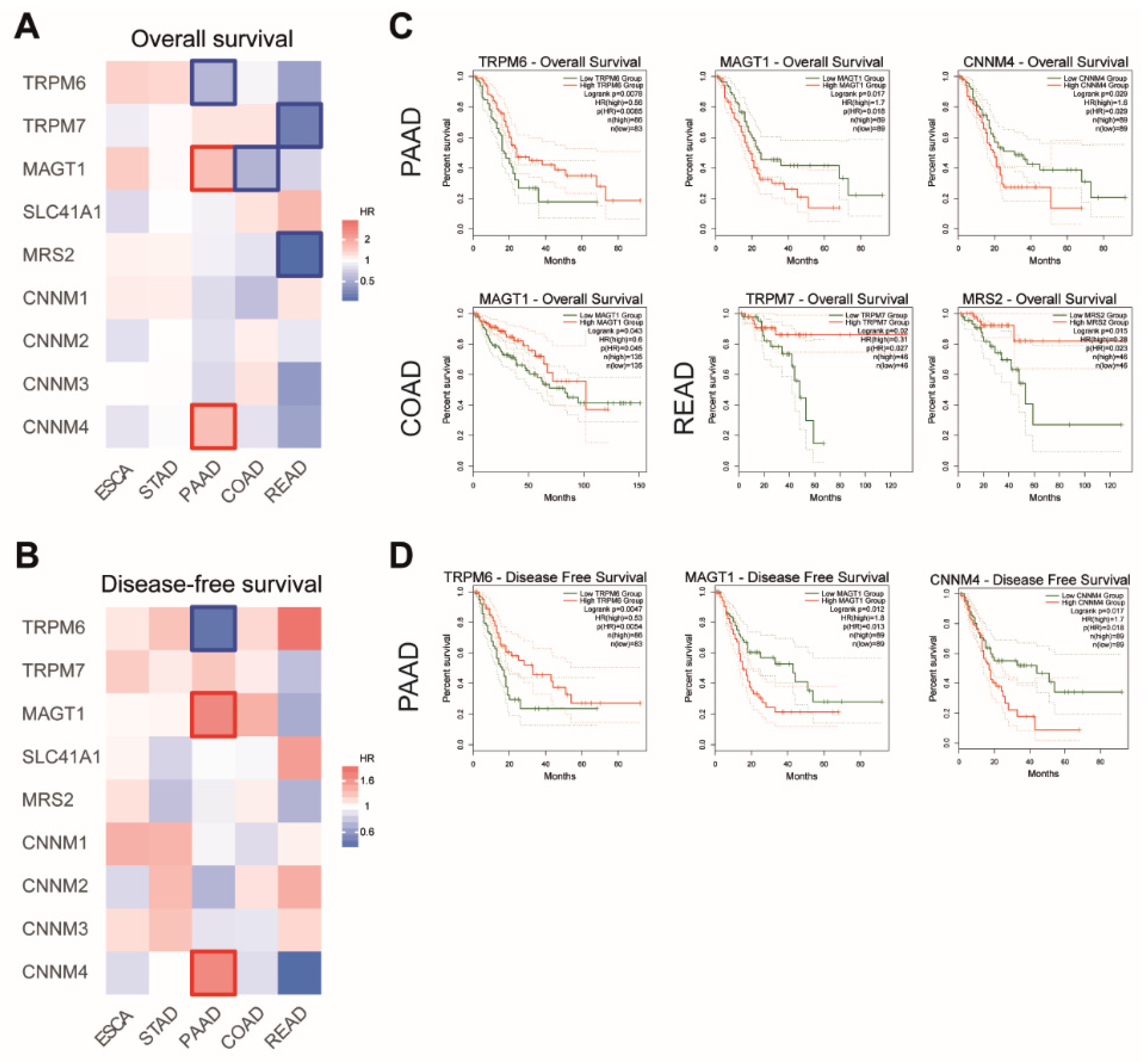
4.3.2. Mg2+ Transporters and Patient Survival
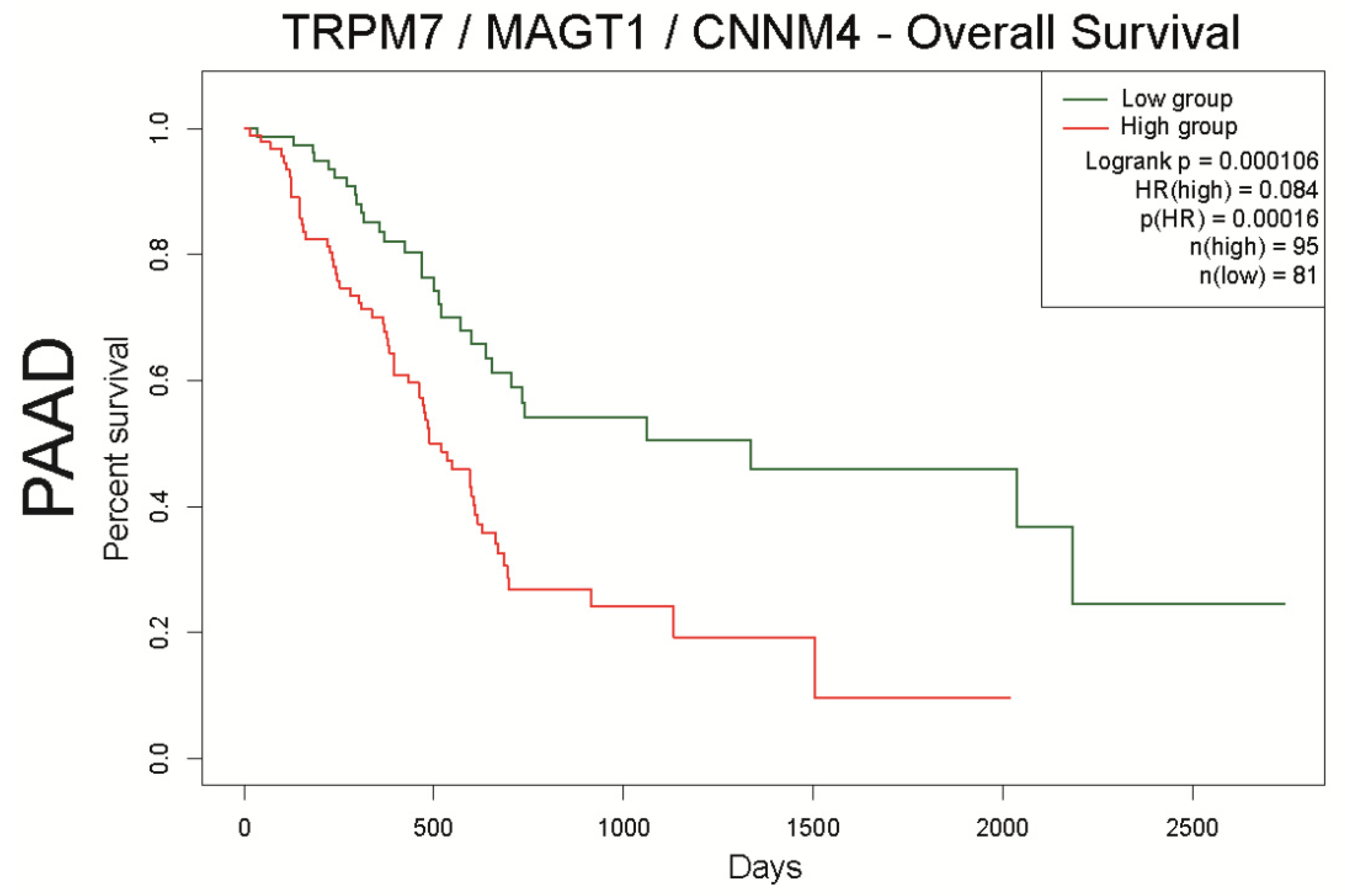
4.3.3. Mg2+ Transporters and Patient Survival
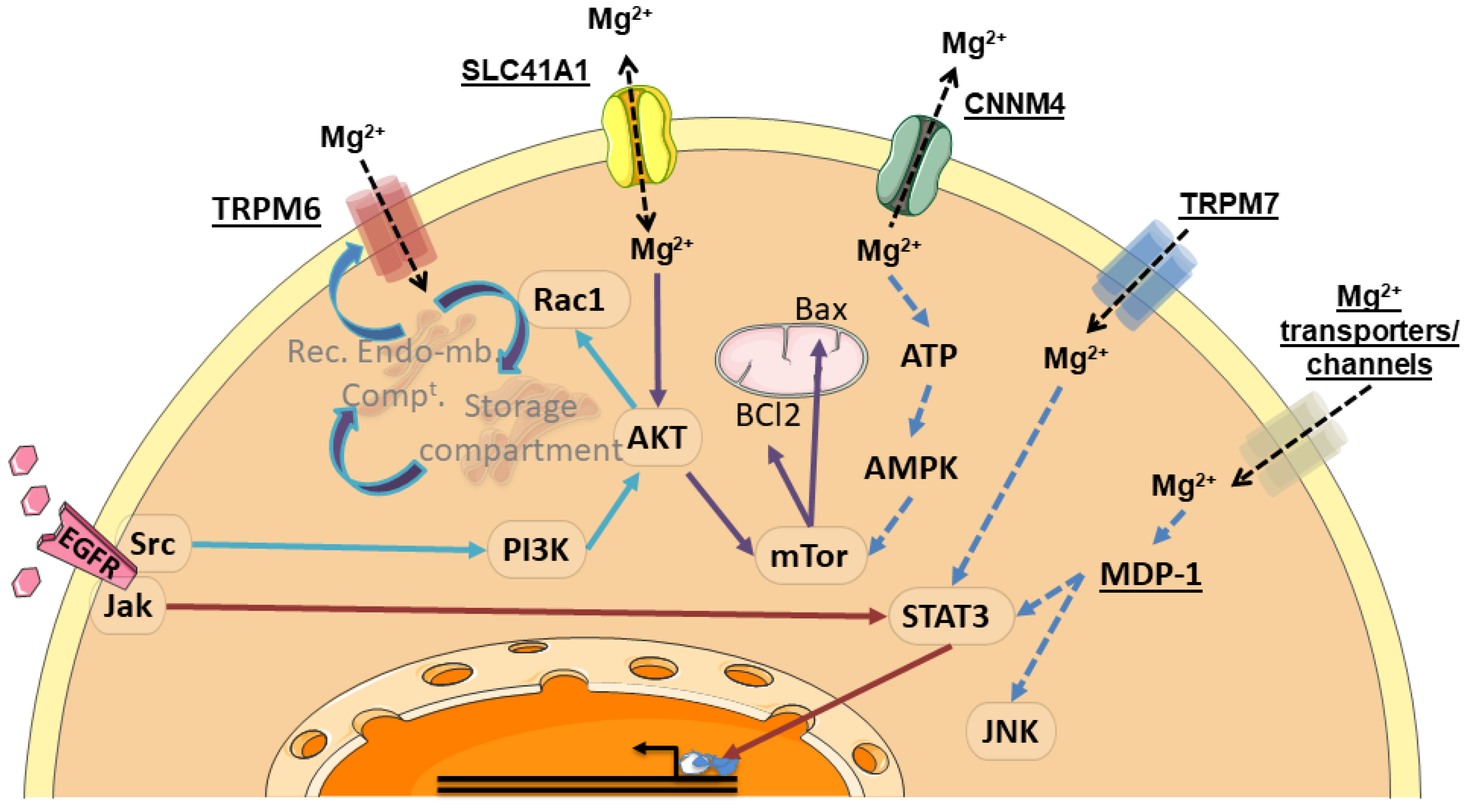
5. Regulation of Digestive Cancer Cell Fates by Magnesium Transporters
6. Mg2+-Regulated Signaling Pathways in Digestive Cancer Cells
7. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, M.; Soerjomataram, I.; Ferlay, J.; Forman, D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut 2015, 64, 381–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubenstein, J.H.; Shaheen, N.J. Epidemiology, diagnosis, and management of esophageal adenocarcinoma. Gastroenterology 2015, 149, 302–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eusebi, L.H.; Telese, A.; Marasco, G.; Bazzoli, F.; Zagari, R.M. Gastric cancer prevention strategies: A global perspective. J Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef]
- De Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef] [Green Version]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of pancreatic cancer: Global trends, etiology and risk factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [Green Version]
- Khomiak, A.; Brunner, M.; Kordes, M.; Lindblad, S.; Miksch, R.C.; Ohlund, D.; Regel, I. Recent discoveries of diagnostic, prognostic and predictive biomarkers for pancreatic cancer. Cancers 2020, 12, 3234. [Google Scholar] [CrossRef]
- Akimoto, N.; Ugai, T.; Zhong, R.; Hamada, T.; Fujiyoshi, K.; Giannakis, M.; Wu, K.; Cao, Y.; Ng, K.; Ogino, S. Rising incidence of early-onset colorectal cancer—A call to action. Nat. Rev. Clin. Oncol. 2020. [Google Scholar] [CrossRef]
- Thanikachalam, K.; Khan, G. Colorectal cancer and nutrition. Nutrients 2019, 11, 164. [Google Scholar] [CrossRef] [Green Version]
- De Baaij, J.H.; Hoenderop, J.G.; Bindels, R.J. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Rubin, H. Central role for magnesium in coordinate control of metabolism and growth in animal cells. Proc. Natl. Acad. Sci. USA 1975, 72, 3551–3555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, T.K.; Dickerson, R.E. 1 A crystal structures of B-DNA reveal sequence-specific binding and groove-specific bending of DNA by magnesium and calcium. J. Mol. Biol. 2000, 301, 915–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ban, C.; Junop, M.; Yang, W. Transformation of MutL by ATP binding and hydrolysis: A switch in DNA mismatch repair. Cell 1999, 97, 85–97. [Google Scholar] [CrossRef] [Green Version]
- Calsou, P.; Salles, B. Properties of damage-dependent DNA incision by nucleotide excision repair in human cell-free extracts. Nucleic Acids Res. 1994, 22, 4937–4942. [Google Scholar] [CrossRef] [Green Version]
- Caspi, R.; Billington, R.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Midford, P.E.; Ong, W.K.; Paley, S.; Subhraveti, P.; Karp, P.D. The MetaCyc database of metabolic pathways and enzymes—A 2019 update. Nucleic Acids Res. 2020, 48, D445–D453. [Google Scholar] [CrossRef] [Green Version]
- Lowenstein, F.W.; Stanton, M.F. Serum magnesium levels in the United States, 1971–1974. J. Am. Coll. Nutr. 1986, 5, 399–414. [Google Scholar] [CrossRef]
- IMSC. Dietary reference intakes for calcium, phosphorus, magnesium, vitamin d, and fluoride. In Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; National Academies Press: Washington, DC, USA, 1997. [Google Scholar] [CrossRef]
- Lameris, A.L.; Huybers, S.; Kaukinen, K.; Makela, T.H.; Bindels, R.J.; Hoenderop, J.G.; Nevalainen, P.I. Expression profiling of claudins in the human gastrointestinal tract in health and during inflammatory bowel disease. Scand. J. Gastroenterol. 2013, 48, 58–69. [Google Scholar] [CrossRef]
- Amasheh, S.; Fromm, M.; Gunzel, D. Claudins of intestine and nephron—A correlation of molecular tight junction structure and barrier function. Acta Physiol. 2011, 201, 133–140. [Google Scholar] [CrossRef]
- Elin, R.J. Magnesium: The fifth but forgotten electrolyte. Am. J. Clin. Pathol. 1994, 102, 616–622. [Google Scholar] [CrossRef]
- Worthington, V. Nutritional quality of organic versus conventional fruits, vegetables, and grains. J. Altern. Complement. Med. 2001, 7, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Rosanoff, A. Changing crop magnesium concentrations: Impact on human health. Plant Soil 2012, 368, 139–153. [Google Scholar] [CrossRef]
- Fulgoni, V.L., 3rd; Keast, D.R.; Bailey, R.L.; Dwyer, J. Foods, fortificants, and supplements: Where do Americans get their nutrients? J. Nutr. 2011, 141, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- Workinger, J.L.; Doyle, R.P.; Bortz, J. Challenges in the diagnosis of magnesium status. Nutrients 2018, 10, 1202. [Google Scholar] [CrossRef]
- Sinert, R.; Zehtabchi, S.; Desai, S.; Peacock, P.; Altura, B.T.; Altura, B.M. Serum ionized magnesium and calcium levels in adult patients with seizures. Scand. J. Clin. Lab Invest. 2007, 67, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Eastham, K.M.; Wrightson, N.; Spencer, D.A. Hypomagnesaemia in cystic fibrosis patients referred for lung transplant assessment. J. Cyst. Fibros. 2007, 6, 360–362. [Google Scholar] [CrossRef] [Green Version]
- Liao, F.; Folsom, A.R.; Brancati, F.L. Is low magnesium concentration a risk factor for coronary heart disease? The Atherosclerosis Risk in Communities (ARIC) Study. Am. Heart J. 1998, 136, 480–490. [Google Scholar] [CrossRef]
- Barbagallo, M.; Dominguez, L.J. Magnesium metabolism in type 2 diabetes mellitus, metabolic syndrome and insulin resistance. Arch. Biochem. Biophys. 2007, 458, 40–47. [Google Scholar] [CrossRef]
- Rosanoff, A.; Dai, Q.; Shapses, S.A. Essential nutrient interactions: Does low or suboptimal magnesium status interact with vitamin d and/or calcium status? Adv. Nutr. 2016, 7, 25–43. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.L.; Maguire, M.E. Microbial magnesium transport: Unusual transporters searching for identity. Mol. Microbiol. 1998, 28, 217–226. [Google Scholar] [CrossRef]
- Zsurka, G.; Gregan, J.; Schweyen, R.J. The human mitochondrial Mrs2 protein functionally substitutes for its yeast homologue, a candidate magnesium transporter. Genomics 2001, 72, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Kolisek, M.; Zsurka, G.; Samaj, J.; Weghuber, J.; Schweyen, R.J.; Schweigel, M. Mrs2p is an essential component of the major electrophoretic Mg2+ influx system in mitochondria. EMBO J. 2003, 22, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Nadler, M.J.; Hermosura, M.C.; Inabe, K.; Perraud, A.L.; Zhu, Q.; Stokes, A.J.; Kurosaki, T.; Kinet, J.P.; Penner, R.; Scharenberg, A.M.; et al. LTRPC7 is a Mg. ATP-regulated divalent cation channel required for cell viability. Nature 2001, 411, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Ryazanov, A.G.; Pavur, K.S.; Dorovkov, M.V. Alpha-kinases: A new class of protein kinases with a novel catalytic domain. Curr. Biol. 1999, 9, R43–R45. [Google Scholar] [CrossRef] [Green Version]
- Runnels, L.W.; Yue, L.; Clapham, D.E. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 2001, 291, 1043–1047. [Google Scholar] [CrossRef]
- Monteilh-Zoller, M.K.; Hermosura, M.C.; Nadler, M.J.; Scharenberg, A.M.; Penner, R.; Fleig, A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J. Gen. Physiol. 2003, 121, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Fonfria, E.; Murdock, P.R.; Cusdin, F.S.; Benham, C.D.; Kelsell, R.E.; McNulty, S. Tissue distribution profiles of the human TRPM cation channel family. J. Recept Signal Transduct. Res. 2006, 26, 159–178. [Google Scholar] [CrossRef]
- Schmitz, C.; Perraud, A.L.; Johnson, C.O.; Inabe, K.; Smith, M.K.; Penner, R.; Kurosaki, T.; Fleig, A.; Scharenberg, A.M. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell 2003, 114, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Ryazanova, L.V.; Rondon, L.J.; Zierler, S.; Hu, Z.; Galli, J.; Yamaguchi, T.P.; Mazur, A.; Fleig, A.; Ryazanov, A.G. TRPM7 is essential for Mg(2+) homeostasis in mammals. Nat. Commun. 2010, 1, 109. [Google Scholar] [CrossRef] [Green Version]
- Mittermeier, L.; Demirkhanyan, L.; Stadlbauer, B.; Breit, A.; Recordati, C.; Hilgendorff, A.; Matsushita, M.; Braun, A.; Simmons, D.G.; Zakharian, E.; et al. TRPM7 is the central gatekeeper of intestinal mineral absorption essential for postnatal survival. Proc. Natl. Acad. Sci. USA 2019, 116, 4706–4715. [Google Scholar] [CrossRef] [Green Version]
- Schlingmann, K.P.; Weber, S.; Peters, M.; Niemann Nejsum, L.; Vitzthum, H.; Klingel, K.; Kratz, M.; Haddad, E.; Ristoff, E.; Dinour, D.; et al. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat. Genet. 2002, 31, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Walder, R.Y.; Landau, D.; Meyer, P.; Shalev, H.; Tsolia, M.; Borochowitz, Z.; Boettger, M.B.; Beck, G.E.; Englehardt, R.K.; Carmi, R.; et al. Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat. Genet. 2002, 31, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Voets, T.; Nilius, B.; Hoefs, S.; van der Kemp, A.W.; Droogmans, G.; Bindels, R.J.; Hoenderop, J.G. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J. Biol. Chem. 2004, 279, 19–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wabakken, T.; Rian, E.; Kveine, M.; Aasheim, H.-C. The human solute carrier SLC41A1 belongs to a novel eukaryotic subfamily with homology to prokaryotic MgtE Mg2+ transporters. Biochem. Biophys. Res. Commun. 2003, 306, 718–724. [Google Scholar] [CrossRef]
- Goytain, A.; Quamme, G.A. Functional characterization of human SLC41A1, a Mg2+ transporter with similarity to prokaryotic MgtE Mg2+ transporters. Physiol. Genom. 2005, 21, 337–342. [Google Scholar] [CrossRef]
- Kolisek, M.; Nestler, A.; Vormann, J.; Schweigel-Rontgen, M. Human gene SLC41A1 encodes for the Na(+)/Mg(2+) exchanger. Am. J. Physiol. Cell. Physiol. 2012, 302, C318–C326. [Google Scholar] [CrossRef] [Green Version]
- Goytain, A.; Quamme, G.A. Identification and characterization of a novel mammalian Mg2+ transporter with channel-like properties. BMC Genom. 2005, 6, 48. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Clapham, D.E. Mammalian MagT1 and TUSC3 are required for cellular magnesium uptake and vertebrate embryonic development. Proc. Natl. Acad. Sci. USA 2009, 106, 15750–15755. [Google Scholar] [CrossRef] [Green Version]
- Li, F.Y.; Chaigne-Delalande, B.; Kanellopoulou, C.; Davis, J.C.; Matthews, H.F.; Douek, D.C.; Cohen, J.I.; Uzel, G.; Su, H.C.; Lenardo, M.J. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature 2011, 475, 471–476. [Google Scholar] [CrossRef]
- Blommaert, E.; Peanne, R.; Cherepanova, N.A.; Rymen, D.; Staels, F.; Jaeken, J.; Race, V.; Keldermans, L.; Souche, E.; Corveleyn, A.; et al. Mutations in MAGT1 lead to a glycosylation disorder with a variable phenotype. Proc. Natl. Acad. Sci. USA 2019, 116, 9865–9870. [Google Scholar] [CrossRef] [Green Version]
- Matsuda-Lennikov, M.; Biancalana, M.; Zou, J.; Ravell, J.C.; Zheng, L.; Kanellopoulou, C.; Jiang, P.; Notarangelo, G.; Jing, H.; Masutani, E.; et al. Magnesium transporter 1 (MAGT1) deficiency causes selective defects in N-linked glycosylation and expression of immune-response genes. J. Biol. Chem. 2019, 294, 13638–13656. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Shi, J.-D.; Yang, P.; Kumar, P.G.; Li, Q.-Z.; Run, Q.-G.; Su, Y.-C.; Scott, H.S.; Kao, K.-J.; She, J.-X. Molecular cloning and characterization of a novel gene family of four ancient conserved domain proteins (ACDP). Gene 2003, 306, 37–44. [Google Scholar] [CrossRef]
- Wang, C.Y.; Yang, P.; Shi, J.D.; Purohit, S.; Guo, D.; An, H.; Gu, J.G.; Ling, J.; Dong, Z.; She, J.X. Molecular cloning and characterization of the mouse Acdp gene family. BMC Genom. 2004, 5, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goytain, A.; Quamme, G.A. Functional characterization of ACDP2 (ancient conserved domain protein), a divalent metal transporter. Physiol. Genom. 2005, 22, 382–389. [Google Scholar] [CrossRef]
- Sponder, G.; Mastrototaro, L.; Kurth, K.; Merolle, L.; Zhang, Z.; Abdulhanan, N.; Smorodchenko, A.; Wolf, K.; Fleig, A.; Penner, R.; et al. Human CNNM2 is not a Mg(2+) transporter per se. Pflugers Arch. 2016, 468, 1223–1240. [Google Scholar] [CrossRef]
- Guo, D.; Ling, J.; Wang, M.H.; She, J.X.; Gu, J.; Wang, C.Y. Physical interaction and functional coupling between ACDP4 and the intracellular ion chaperone COX11, an implication of the role of ACDP4 in essential metal ion transport and homeostasis. Mol. Pain 2005, 1, 15. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, D.; Funato, Y.; Miura, J.; Sato, S.; Toyosawa, S.; Furutani, K.; Kurachi, Y.; Omori, Y.; Furukawa, T.; Tsuda, T.; et al. Basolateral Mg2+ extrusion via CNNM4 mediates transcellular Mg2+ transport across epithelia: A mouse model. PLoS Genet. 2013, 9, e1003983. [Google Scholar] [CrossRef] [Green Version]
- Arjona, F.J.; de Baaij, J.H.F. CrossTalk opposing view: CNNM proteins are not Na(+)/Mg(2+) exchangers but Mg(2+) transport regulators playing a central role in transepithelial Mg(2+) (re)absorption. J. Physiol. 2018, 596, 747–750. [Google Scholar] [CrossRef] [Green Version]
- Dai, Q.; Cantwell, M.M.; Murray, L.J.; Zheng, W.; Anderson, L.A.; Coleman, H.G. Dietary magnesium, calcium:magnesium ratio and risk of reflux oesophagitis, Barrett’s oesophagus and oesophageal adenocarcinoma: A population-based case-control study. Br. J. Nutr. 2016, 115, 342–350. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.C.; Dai, Q.; Zhu, X.; Peek, R.M., Jr.; Smalley, W.; Roumie, C.; Shrubsole, M.J. Associations between calcium and magnesium intake and the risk of incident gastric cancer: A prospective cohort analysis of the National Institutes of Health-American Association of Retired Persons (NIH-AARP) Diet and Health Study. Int. J. Cancer 2019. [Google Scholar] [CrossRef]
- Molina-Montes, E.; Wark, P.A.; Sánchez, M.-J.; Norat, T.; Jakszyn, P.; Luján-Barroso, L.; Michaud, D.S.; Crowe, F.; Allen, N.; Khaw, K.-T.; et al. Dietary intake of iron, heme-iron and magnesium and pancreatic cancer risk in the European prospective investigation into cancer and nutrition cohort. Int. J. Cancer 2012, 131, E1134–E1147. [Google Scholar] [CrossRef] [Green Version]
- Jansen, R.J.; Robinson, D.P.; Stolzenberg-Solomon, R.Z.; Bamlet, W.R.; de Andrade, M.; Oberg, A.L.; Rabe, K.G.; Anderson, K.E.; Olson, J.E.; Sinha, R.; et al. Nutrients from fruit and vegetable consumption reduce the risk of pancreatic cancer. J. Gastrointest. Cancer 2013, 44, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Dibaba, D.; Xun, P.; Yokota, K.; White, E.; He, K. Magnesium intake and incidence of pancreatic cancer: The VITamins and Lifestyle study. Br. J. Cancer 2015, 113, 1615–1621. [Google Scholar] [CrossRef]
- Schilling, K.; Larner, F.; Saad, A.; Roberts, R.; Kocher, H.M.; Blyuss, O.; Halliday, A.N.; Crnogorac-Jurcevic, T. Urine metallomics signature as an indicator of pancreatic cancer. Metallomics 2020. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Shrubsole, M.J.; Ness, R.M.; Schlundt, D.; Cai, Q.; Smalley, W.E.; Li, M.; Shyr, Y.; Zheng, W. The relation of magnesium and calcium intakes and a genetic polymorphism in the magnesium transporter to colorectal neoplasia risk. Am. J. Clin. Nutr. 2007, 86, 743–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorczyca, A.M.; He, K.; Xun, P.; Margolis, K.L.; Wallace, J.P.; Lane, D.; Thomson, C.; Ho, G.Y.; Shikany, J.M.; Luo, J. Association between magnesium intake and risk of colorectal cancer among postmenopausal women. Cancer Causes Control 2015, 26, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Sun, J.; Yu, J.; Wang, C.; Su, J. Dietary intakes of calcium, iron, magnesium, and potassium elements and the risk of colorectal cancer: A meta-analysis. Biol. Trace Elem. Res. 2019, 189, 325–335. [Google Scholar] [CrossRef]
- Polter, E.; Onyeaghala, G.C.; Lutsey, P.L.; Folsom, A.R.; Joshu, C.E.; Platz, E.A.; Prizment, A.E. Prospective association of serum and dietary magnesium with colorectal cancer incidence. Cancer Epidemiol. Biomark. Prev. 2019. [Google Scholar] [CrossRef]
- Wesselink, E.; Kok, D.E.; Bours, M.J.L.; de Wilt, J.H.; van Baar, H.; van Zutphen, M.; Geijsen, A.M.J.R.; Keulen, E.T.P.; Hansson, B.M.E.; van den Ouweland, J.; et al. Vitamin D, magnesium, calcium, and their interaction in relation to colorectal cancer recurrence and all-cause mortality. Am. J. Clin. Nutr. 2020. [Google Scholar] [CrossRef] [Green Version]
- Anderson, K.J.; Cormier, R.T.; Scott, P.M. Role of ion channels in gastrointestinal cancer. World J. Gastroenterol. 2019, 25, 5732–5772. [Google Scholar] [CrossRef]
- Stokłosa, P.; Borgström, A.; Kappel, S.; Peinelt, C. TRP channels in digestive tract cancers. Int. J. Mol. Sci. 2020, 21, 1877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakashima, S.; Shiozaki, A.; Ichikawa, D.; Hikami, S.; Kosuga, T.; Konishi, H.; Komatsu, S.; Fujiwara, H.; Okamoto, K.; Kishimoto, M.; et al. Transient receptor potential melastatin 7 as an independent prognostic factor in human esophageal squamous cell carcinoma. Anticancer Res. 2017, 37, 1161–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yee, N.S.; Zhou, W.; Liang, I.C. Transient receptor potential ion channel Trpm7 regulates exocrine pancreatic epithelial proliferation by Mg2+-sensitive Socs3a signaling in development and cancer. Dis. Model Mech. 2011, 4, 240–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yee, N.S.; Kazi, A.A.; Li, Q.; Yang, Z.; Berg, A.; Yee, R.K. Aberrant over-expression of TRPM7 ion channels in pancreatic cancer: Required for cancer cell invasion and implicated in tumor growth and metastasis. Biol. Open 2015, 4, 507–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rybarczyk, P.; Gautier, M.; Hague, F.; Dhennin-Duthille, I.; Chatelain, D.; Kerr-Conte, J.; Pattou, F.; Regimbeau, J.M.; Sevestre, H.; Ouadid-Ahidouch, H. Transient receptor potential melastatin-related 7 channel is overexpressed in human pancreatic ductal adenocarcinomas and regulates human pancreatic cancer cell migration. Int. J. Cancer 2012, 131, E851–E861. [Google Scholar] [CrossRef] [PubMed]
- Rybarczyk, P.; Vanlaeys, A.; Brassart, B.; Dhennin-Duthille, I.; Chatelain, D.; Sevestre, H.; Ouadid-Ahidouch, H.; Gautier, M. The transient receptor potential melastatin 7 channel regulates pancreatic cancer cell invasion through the Hsp90alpha/uPA/MMP2 pathway. Neoplasia 2017, 19, 288–300. [Google Scholar] [CrossRef]
- Xie, J.; Cheng, C.S.; Zhu, X.Y.; Shen, Y.H.; Song, L.B.; Chen, H.; Chen, Z.; Liu, L.M.; Meng, Z.Q. Magnesium transporter protein solute carrier family 41 member 1 suppresses human pancreatic ductal adenocarcinoma through magnesium-dependent Akt/mTOR inhibition and bax-associated mitochondrial apoptosis. Aging 2019, 11, 2681–2698. [Google Scholar] [CrossRef]
- Xie, B.; Zhao, R.; Bai, B.; Wu, Y.; Xu, Y.; Lu, S.; Fang, Y.; Wang, Z.; Maswikiti, E.P.; Zhou, X.; et al. Identification of key tumorigenesis-related genes and their microRNAs in colon cancer. Oncol. Rep. 2018, 40, 3551–3560. [Google Scholar] [CrossRef]
- Ibrahim, S.; Dakik, H.; Vandier, C.; Chautard, R.; Paintaud, G.; Mazurier, F.; Lecomte, T.; Gueguinou, M.; Raoul, W. Expression profiling of calcium channels and calcium-activated potassium channels in colorectal cancer. Cancers 2019, 11, 561. [Google Scholar] [CrossRef] [Green Version]
- Pugliese, D.; Armuzzi, A.; Castri, F.; Benvenuto, R.; Mangoni, A.; Guidi, L.; Gasbarrini, A.; Rapaccini, G.L.; Wolf, F.I.; Trapani, V. TRPM7 is overexpressed in human IBD-related and sporadic colorectal cancer and correlates with tumor grade. Dig. Liver Dis. 2020. [Google Scholar] [CrossRef]
- Su, F.; Wang, B.F.; Zhang, T.; Hou, X.M.; Feng, M.H. TRPM7 deficiency suppresses cell proliferation, migration, and invasion in human colorectal cancer via regulation of epithelial-mesenchymal transition. Cancer Biomark. 2019, 26, 451–460. [Google Scholar] [CrossRef]
- Zheng, K.; Yang, Q.; Xie, L.; Qiu, Z.; Huang, Y.; Lin, Y.; Tu, L.; Cui, C. Overexpression of MAGT1 is associated with aggressiveness and poor prognosis of colorectal cancer. Oncol. Lett. 2019, 18, 3857–3862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funato, Y.; Yamazaki, D.; Mizukami, S.; Du, L.; Kikuchi, K.; Miki, H. Membrane protein CNNM4-dependent Mg2+ efflux suppresses tumor progression. J. Clin. Investig. 2014, 124, 5398–5410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Digre, A.; Lindskog, C. The Human Protein Atlas-Spatial localization of the human proteome in health and disease. Protein Sci. 2021, 30, 218–233. [Google Scholar] [CrossRef] [PubMed]
- Jonckheere, N.; Auwercx, J.; Hadj Bachir, E.; Coppin, L.; Boukrout, N.; Vincent, A.; Neve, B.; Gautier, M.; Trevino, V.; van Seuningen, I. Unsupervised hierarchical clustering of pancreatic adenocarcinoma dataset from TCGA defines a mucin expression profile that impacts overall survival. Cancers 2020, 12, 3309. [Google Scholar] [CrossRef]
- Ren, J.; Du, Y.; Li, S.; Ma, S.; Jiang, Y.; Wu, C. Robust network-based regularization and variable selection for high-dimensional genomic data in cancer prognosis. Genet. Epidemiol. 2019, 43, 276–291. [Google Scholar] [CrossRef]
- Trapani, V.; Wolf, F.I. Dysregulation of Mg(2+) homeostasis contributes to acquisition of cancer hallmarks. Cell Calcium 2019, 83, 102078. [Google Scholar] [CrossRef]
- Kim, B.J.; Park, E.J.; Lee, J.H.; Jeon, J.-H.; Kim, S.J.; So, I. Suppression of transient receptor potential melastatin 7 channel induces cell death in gastric cancer. Cancer Sci. 2008, 99, 2502–2509. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, X.; Yan, P.; Han, Y.; Sun, S.; Wu, K.; Fan, D. Human mitochondrial Mrs2 protein promotes multidrug resistance in gastric cancer cells by regulating p27, cyclin D1 expression and cytochrome C release. Cancer Biol. Ther. 2009, 8, 607–614. [Google Scholar] [CrossRef] [Green Version]
- Yee, N.S.; Zhou, W.; Lee, M.; Yee, R.K. Targeted silencing of TRPM7 ion channel induces replicative senescence and produces enhanced cytotoxicity with gemcitabine in pancreatic adenocarcinoma. Cancer Lett. 2012, 318, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Vanlaeys, A.; Fouquet, G.; Kischel, P.; Hague, F.; Pasco-Brassart, S.; Lefebvre, T.; Rybarczyk, P.; Dhennin-Duthille, I.; Brassart, B.; Ouadid-Ahidouch, H.; et al. Cadmium exposure enhances cell migration and invasion through modulated TRPM7 channel expression. Arch. Toxicol. 2020, 94, 735–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefebvre, T.; Rybarczyk, P.; Bretaudeau, C.; Vanlaeys, A.; Cousin, R.; Brassart-Pasco, S.; Chatelain, D.; Dhennin-Duthille, I.; Ouadid-Ahidouch, H.; Brassart, B.; et al. TRPM7/RPSA complex regulates pancreatic cancer cell migration. Front. Cell Dev. Biol. 2020, 8, 549. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, S.; Cazzaniga, A.; Trapani, V.; Cappadone, C.; Farruggia, G.; Merolle, L.; Wolf, F.I.; Iotti, S.; Maier, J.A.M. Magnesium homeostasis in colon carcinoma LoVo cells sensitive or resistant to doxorubicin. Sci. Rep. 2015, 5, 16538. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, A.; Moscheni, C.; Trapani, V.; Wolf, F.I.; Farruggia, G.; Sargenti, A.; Iotti, S.; Maier, J.A.M.; Castiglioni, S. The different expression of TRPM7 and MagT1 impacts on the proliferation of colon carcinoma cells sensitive or resistant to doxorubicin. Sci. Rep. 2017, 7, 40538. [Google Scholar] [CrossRef] [Green Version]
- Luongo, F.; Pietropaolo, G.; Gautier, M.; Dhennin-Duthille, I.; Ouadid-Ahidouch, H.; Wolf, F.I.; Trapani, V. TRPM6 is essential for magnesium uptake and epithelial cell function in the colon. Nutrients 2018, 10, 784. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Furuya, H.; Faouzi, M.; Zhang, Z.; Monteilh-Zoller, M.; Kawabata, K.G.; Horgen, F.D.; Kawamori, T.; Penner, R.; Fleig, A. Inhibition of TRPM7 suppresses cell proliferation of colon adenocarcinoma in vitro and induces hypomagnesemia in vivo without affecting azoxymethane-induced early colon cancer in mice. Cell Commun. Signal. 2017, 15. [Google Scholar] [CrossRef]
- Yamazaki, D.; Hasegawa, A.; Funato, Y.; Tran, H.N.; Mori, M.X.; Mori, Y.; Sato, T.; Miki, H. Cnnm4 deficiency suppresses Ca(2+) signaling and promotes cell proliferation in the colon epithelia. Oncogene 2019, 38, 3962–3969. [Google Scholar] [CrossRef]
- Zou, Z.G.; Rios, F.J.; Montezano, A.C.; Touyz, R.M. TRPM7, magnesium, and signaling. Int. J. Mol. Sci. 2019, 20, 1877. [Google Scholar] [CrossRef] [Green Version]
- Lemmon, M.A.; Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef] [Green Version]
- Du, Z.; Lovly, C.M. Mechanisms of receptor tyrosine kinase activation in cancer. Mol. Cancer 2018, 17, 58. [Google Scholar] [CrossRef]
- Arjona, F.J.; Latta, F.; Mohammed, S.G.; Thomassen, M.; van Wijk, E.; Bindels, R.J.M.; Hoenderop, J.G.J.; de Baaij, J.H.F. SLC41A1 is essential for magnesium homeostasis in vivo. Pflügers Arch. Eur. J. Physiol. 2019, 471, 845–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell. Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, H.X.; Xiong, Y.; Guan, K.L. Nutrient sensing, metabolism, and cell growth control. Mol. Cell 2013, 49, 379–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Deng, L.; Chen, B.; Huang, W.; Lin, X.; Chen, G.; Tzeng, C.-M.; Ying, M.; Lu, Z. Magnesium-dependent phosphatase (MDP) 1 is a potential suppressor of gastric cancer. Curr. Cancer Drug Targets 2019, 19, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Desai, B.N.; Navarro, B.; Donovan, A.; Andrews, N.C.; Clapham, D.E. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science 2008, 322, 756–760. [Google Scholar] [CrossRef] [Green Version]
- Tejpar, S.; Piessevaux, H.; Claes, K.; Piront, P.; Hoenderop, J.G.J.; Verslype, C.; Van Cutsem, E. Magnesium wasting associated with epidermal-growth-factor receptor-targeting antibodies in colorectal cancer: A prospective study. Lancet Oncol. 2007, 8, 387–394. [Google Scholar] [CrossRef]
- Costa, A.; Tejpar, S.; Prenen, H.; Van Cutsem, E. Hypomagnesaemia and targeted anti-epidermal growth factor receptor (EGFR) agents. Target Oncol. 2011, 6, 227–233. [Google Scholar] [CrossRef]
- Thebault, S.; Alexander, R.T.; Tiel Groenestege, W.M.; Hoenderop, J.G.; Bindels, R.J. EGF increases TRPM6 activity and surface expression. J. Am. Soc. Nephrol. 2009, 20, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Ravell, J.C.; Chauvin, S.D.; He, T.; Lenardo, M. An update on XMEN disease. J. Clin. Immunol. 2020, 40, 671–681. [Google Scholar] [CrossRef]
- Ravell, J.C.; Matsuda-Lennikov, M.; Chauvin, S.D.; Zou, J.; Biancalana, M.; Deeb, S.J.; Price, S.; Su, H.C.; Notarangelo, G.; Jiang, P.; et al. Defective glycosylation and multisystem abnormalities characterize the primary immunodeficiency XMEN disease. J. Clin. Investig. 2020, 130, 507–522. [Google Scholar] [CrossRef] [Green Version]
- King, D.E.; Mainous, A.G., 3rd; Geesey, M.E.; Woolson, R.F. Dietary magnesium and C-reactive protein levels. J. Am. Coll. Nutr. 2005, 24, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Danbolt, N.C.; Zhou, Y.; Furness, D.N.; Holmseth, S. Strategies for immunohistochemical protein localization using antibodies: What did we learn from neurotransmitter transporters in glial cells and neurons. Glia 2016, 64, 2045–2064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deason-Towne, F.; Perraud, A.L.; Schmitz, C. The Mg2+ transporter MagT1 partially rescues cell growth and Mg2+ uptake in cells lacking the channel-kinase TRPM7. FEBS Lett. 2011, 585, 2275–2278. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Cancer | Transporter | Expression in Cancerous Tissues (Compared to Normal Tissues) | Technique | Reference |
|---|---|---|---|---|
| ESAC | TRPM7 | Upregulated | IHC | [73] |
| PDAC | TRPM7 | Upregulated | IHC | [74,75,76,77] |
| SLC41A1 | Downregulated | In silico + Protein Atlas qRT-PCR Western-Blot | [78] | |
| CRC | TRPM6 | Downregulated (RNA) Upregulated (Protein) | In silico qRT-PCR IHC | [79,80,81] |
| TRPM7 | Upregulated | In silico qRT-PCR IF IHC | [81,82] | |
| MAGT1 | Upregulated | In silico qRT-PCR | [83] | |
| CNNM4 | Downregulated | IHC | [84] |
| Transporter | GC | PDAC | CRC |
|---|---|---|---|
| TRPM7 | 33% | 0% | 0% |
| MAGT1 | 44.4% | 22.2% | 83.3% |
| SLC41A1 | 91.7% | 100% | 100% |
| MRS2 | 75% | 91.7% | 75% |
| CNNM: | |||
| CNNM1 CNNM3 CNNM4 | 12.5% 72.7% 50% | 36.4% 100% 58.3% | 16.7% 100% 83.3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auwercx, J.; Rybarczyk, P.; Kischel, P.; Dhennin-Duthille, I.; Chatelain, D.; Sevestre, H.; Van Seuningen, I.; Ouadid-Ahidouch, H.; Jonckheere, N.; Gautier, M. Mg2+ Transporters in Digestive Cancers. Nutrients 2021, 13, 210. https://doi.org/10.3390/nu13010210
Auwercx J, Rybarczyk P, Kischel P, Dhennin-Duthille I, Chatelain D, Sevestre H, Van Seuningen I, Ouadid-Ahidouch H, Jonckheere N, Gautier M. Mg2+ Transporters in Digestive Cancers. Nutrients. 2021; 13(1):210. https://doi.org/10.3390/nu13010210
Chicago/Turabian StyleAuwercx, Julie, Pierre Rybarczyk, Philippe Kischel, Isabelle Dhennin-Duthille, Denis Chatelain, Henri Sevestre, Isabelle Van Seuningen, Halima Ouadid-Ahidouch, Nicolas Jonckheere, and Mathieu Gautier. 2021. "Mg2+ Transporters in Digestive Cancers" Nutrients 13, no. 1: 210. https://doi.org/10.3390/nu13010210