Effectiveness of In-Hospital Cholecalciferol Use on Clinical Outcomes in Comorbid COVID-19 Patients: A Hypothesis-Generating Study
Abstract
:1. Introduction
2. Materials and Methods
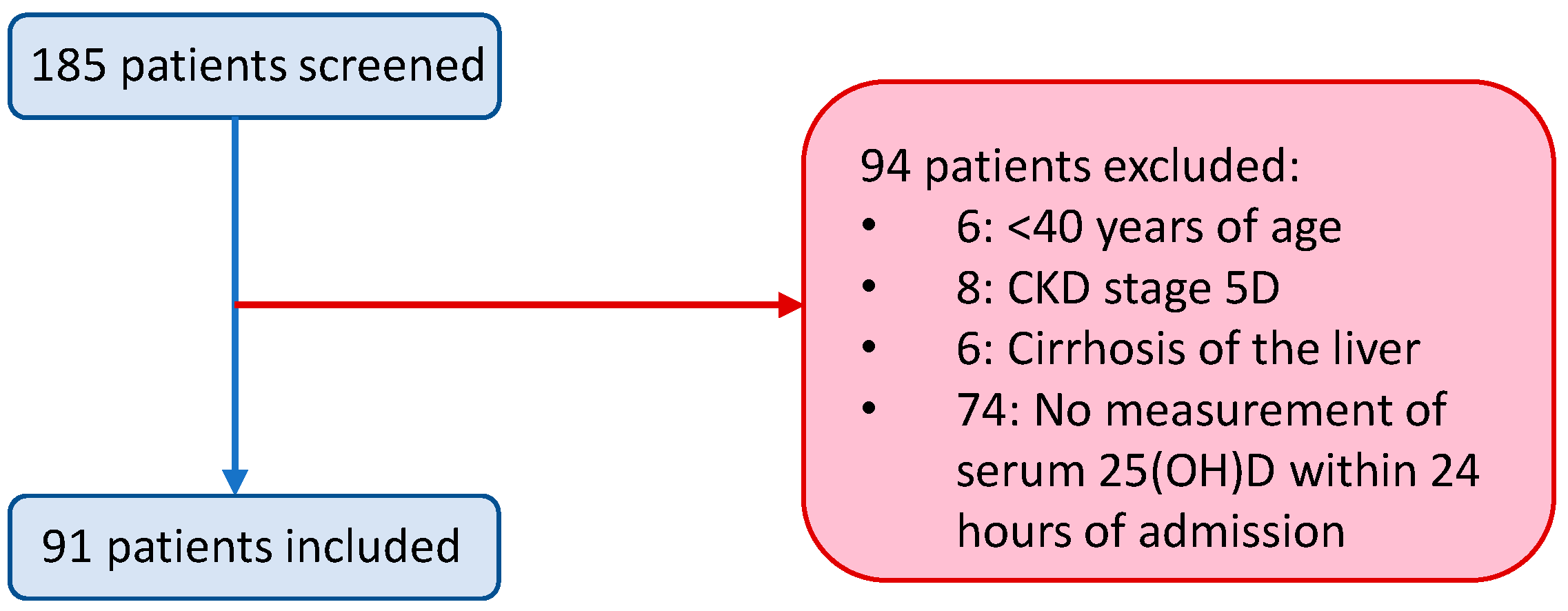
2.1. Study Population
2.2. Data Collection
2.3. Laboratory Analysis and X-ray
2.4. Treatment
2.5. Outcome Measures
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Drivers of Vitamin D Treatment
3.3. Association between Vitamin D Treatment and Clinical Outcome
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A New Coronavirus Associated with Human Respiratory Disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef] [Green Version]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- WHO. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 21 November 2020).
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet Lond. Engl. 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study. Lancet Lond. Engl. 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Fan, E.; Brodie, D.; Slutsky, A.S. Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. JAMA 2018, 319, 698–710. [Google Scholar] [CrossRef]
- ARDS Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- CDC Coronavirus Disease 2019 (COVID-19). Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html (accessed on 21 November 2020).
- WHO. WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int/table (accessed on 21 November 2020).
- Mahase, E. Covid-19: Vaccine Candidate May Be More than 90% Effective, Interim Results Indicate. BMJ 2020, 371, m4347. [Google Scholar] [CrossRef]
- Shaffer, L. 15 Drugs Being Tested to Treat COVID-19 and How They Would Work. Nat. Med. 2020. [Google Scholar] [CrossRef]
- Quesada-Gomez, J.M.; Entrenas-Castillo, M.; Bouillon, R. Vitamin D Receptor Stimulation to Reduce Acute Respiratory Distress Syndrome (ARDS) in Patients with Coronavirus SARS-CoV-2 Infections. J. Steroid Biochem. Mol. Biol. 2020, 202, 105719. [Google Scholar] [CrossRef]
- Daneshkhah, A.; Agrawal, V.; Eshein, A.; Subramanian, H.; Roy, H.K.; Backman, V. Evidence for Possible Association of Vitamin D Status with Cytokine Storm and Unregulated Inflammation in COVID-19 Patients. Aging Clin. Exp. Res. 2020. [Google Scholar] [CrossRef]
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef] [Green Version]
- Malaguarnera, L. Vitamin D3 as Potential Treatment Adjuncts for COVID-19. Nutrients 2020, 12, 3512. [Google Scholar] [CrossRef]
- Autier, P.; Boniol, M.; Pizot, C.; Mullie, P. Vitamin D Status and Ill Health: A Systematic Review. Lancet Diabetes Endocrinol. 2014, 2, 76–89. [Google Scholar] [CrossRef]
- Aibana, O.; Huang, C.-C.; Aboud, S.; Arnedo-Pena, A.; Becerra, M.C.; Bellido-Blasco, J.B.; Bhosale, R.; Calderon, R.; Chiang, S.; Contreras, C.; et al. Vitamin D Status and Risk of Incident Tuberculosis Disease: A Nested Case-Control Study, Systematic Review, and Individual-Participant Data Meta-Analysis. PLoS Med. 2019, 16, e1002907. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D Supplementation to Prevent Acute Respiratory Tract Infections: Systematic Review and Meta-Analysis of Individual Participant Data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef] [Green Version]
- Sabetta, J.R.; DePetrillo, P.; Cipriani, R.J.; Smardin, J.; Burns, L.A.; Landry, M.L. Serum 25-Hydroxyvitamin d and the Incidence of Acute Viral Respiratory Tract Infections in Healthy Adults. PLoS ONE 2010, 5, e11088. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Endocrine Society Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
- Mithal, A.; Wahl, D.A.; Bonjour, J.-P.; Burckhardt, P.; Dawson-Hughes, B.; Eisman, J.A.; El-Hajj Fuleihan, G.; Josse, R.G.; Lips, P.; Morales-Torres, J.; et al. Global Vitamin D Status and Determinants of Hypovitaminosis D. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2009, 20, 1807–1820. [Google Scholar] [CrossRef] [Green Version]
- Isaia, G.; Giorgino, R.; Rini, G.B.; Bevilacqua, M.; Maugeri, D.; Adami, S. Prevalence of Hypovitaminosis D in Elderly Women in Italy: Clinical Consequences and Risk Factors. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2003, 14, 577–582. [Google Scholar] [CrossRef]
- Biesalski, H.K. Vitamin D Deficiency and Co-Morbidities in COVID-19 Patients—A Fatal Relationship? NFS J. 2020, 20, 10–21. [Google Scholar] [CrossRef]
- Mitchell, F. Vitamin-D and COVID-19: Do Deficient Risk a Poorer Outcome? Lancet Diabetes Endocrinol. 2020, 8, 570. [Google Scholar] [CrossRef]
- Martineau, A.R.; Forouhi, N.G. Vitamin D for COVID-19: A Case to Answer? Lancet Diabetes Endocrinol. 2020, 8, 735–736. [Google Scholar] [CrossRef]
- Laird, E.; Rhodes, J.; Kenny, R.A. Vitamin D and Inflammation: Potential Implications for Severity of Covid-19. Ir. Med. J. 2020, 113, 81. [Google Scholar]
- Ilie, P.C.; Stefanescu, S.; Smith, L. The Role of Vitamin D in the Prevention of Coronavirus Disease 2019 Infection and Mortality. Aging Clin. Exp. Res. 2020. [Google Scholar] [CrossRef]
- van der Wielen, R.P.; Löwik, M.R.; van den Berg, H.; de Groot, L.C.; Haller, J.; Moreiras, O.; van Staveren, W.A. Serum Vitamin D Concentrations among Elderly People in Europe. Lancet Lond. Engl. 1995, 346, 207–210. [Google Scholar] [CrossRef]
- Annweiler, G.; Corvaisier, M.; Gautier, J.; Dubée, V.; Legrand, E.; Sacco, G.; Annweiler, C. Vitamin D Supplementation Associated to Better Survival in Hospitalized Frail Elderly COVID-19 Patients: The GERIA-COVID Quasi-Experimental Study. Nutrients 2020, 12, 3377. [Google Scholar] [CrossRef]
- Murai, I. Effect of Vitamin D3 Supplementation vs. Placebo on Hospital Length of Stay in Patients with Severe COVID-19: A Multicenter, Double-Blind, Randomized Controlled Trial|MedRxiv. Available online: https://www.medrxiv.org/content/10.1101/2020.11.16.20232397v1 (accessed on 21 November 2020).
- Rastogi, A.; Bhansali, A.; Khare, N.; Suri, V.; Yaddanapudi, N.; Sachdeva, N.; Puri, G.D.; Malhotra, P. Short Term, High-Dose Vitamin D Supplementation for COVID-19 Disease: A Randomised, Placebo-Controlled, Study (SHADE Study). Postgrad. Med. J. 2020. [Google Scholar] [CrossRef]
- Entrenas Castillo, M.; Entrenas Costa, L.M.; Vaquero Barrios, J.M.; Alcalá Díaz, J.F.; López Miranda, J.; Bouillon, R.; Quesada Gomez, J.M. Effect of Calcifediol Treatment and Best Available Therapy versus Best Available Therapy on Intensive Care Unit Admission and Mortality among Patients Hospitalized for COVID-19: A Pilot Randomized Clinical Study. J. Steroid Biochem. Mol. Biol. 2020, 203, 105751. [Google Scholar] [CrossRef]
- Bowles, K.H.; McDonald, M.; Barrón, Y.; Kennedy, E.; O’Connor, M.; Mikkelsen, M. Surviving COVID-19 After Hospital Discharge: Symptom, Functional, and Adverse Outcomes of Home Health Recipients. Ann. Intern. Med. 2020. [Google Scholar] [CrossRef]
- Amrein, K.; Schnedl, C.; Holl, A.; Riedl, R.; Christopher, K.B.; Pachler, C.; Urbanic Purkart, T.; Waltensdorfer, A.; Münch, A.; Warnkross, H.; et al. Effect of High-Dose Vitamin D3 on Hospital Length of Stay in Critically Ill Patients with Vitamin D Deficiency: The VITdAL-ICU Randomized Clinical Trial. JAMA 2014, 312, 1520–1530. [Google Scholar] [CrossRef] [Green Version]
- Clinical Management of COVID-19. Available online: https://www.who.int/publications-detail-redirect/clinical-management-of-covid-19 (accessed on 20 November 2020).
- Siddiqui, M.; Manansala, J.S.; Abdulrahman, H.A.; Nasrallah, G.K.; Smatti, M.K.; Younes, N.; Althani, A.A.; Yassine, H.M. Immune Modulatory Effects of Vitamin D on Viral Infections. Nutrients 2020, 12, 2879. [Google Scholar] [CrossRef]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [Green Version]
- Meltzer, D.O.; Best, T.J.; Zhang, H.; Vokes, T.; Arora, V.; Solway, J. Association of Vitamin D Status and Other Clinical Characteristics With COVID-19 Test Results. JAMA Netw. Open 2020, 3, e2019722. [Google Scholar] [CrossRef]
- D’Avolio, A.; Avataneo, V.; Manca, A.; Cusato, J.; De Nicolò, A.; Lucchini, R.; Keller, F.; Cantù, M. 25-Hydroxyvitamin D Concentrations Are Lower in Patients with Positive PCR for SARS-CoV-2. Nutrients 2020, 12, 1359. [Google Scholar] [CrossRef]
- Maghbooli, Z.; Sahraian, M.A.; Ebrahimi, M.; Pazoki, M.; Kafan, S.; Tabriz, H.M.; Hadadi, A.; Montazeri, M.; Nasiri, M.; Shirvani, A.; et al. Vitamin D Sufficiency, a Serum 25-Hydroxyvitamin D at Least 30 Ng/ML Reduced Risk for Adverse Clinical Outcomes in Patients with COVID-19 Infection. PLoS ONE 2020, 15, e0239799. [Google Scholar] [CrossRef]
- Hastie, C.E.; Mackay, D.F.; Ho, F.; Celis-Morales, C.A.; Katikireddi, S.V.; Niedzwiedz, C.L.; Jani, B.D.; Welsh, P.; Mair, F.S.; Gray, S.R.; et al. Vitamin D Concentrations and COVID-19 Infection in UK Biobank. Diabetes Metab. Syndr. 2020, 14, 561–565. [Google Scholar] [CrossRef]
- Jain, A.; Chaurasia, R.; Sengar, N.S.; Singh, M.; Mahor, S.; Narain, S. Analysis of Vitamin D Level among Asymptomatic and Critically Ill COVID-19 Patients and Its Correlation with Inflammatory Markers. Sci. Rep. 2020, 10, 20191. [Google Scholar] [CrossRef]
- Pereira, M.; Dantas Damascena, A.; Galvão Azevedo, L.M.; de Almeida Oliveira, T.; da Mota Santana, J. Vitamin D Deficiency Aggravates COVID-19: Systematic Review and Meta-Analysis. Crit. Rev. Food Sci. Nutr. 2020, 1–9. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, X.; Fan, Q.; Liu, H.; Liu, X.; Liu, Z.; Zhang, Z. D-Dimer Levels on Admission to Predict in-Hospital Mortality in Patients with Covid-19. J. Thromb. Haemost. JTH 2020, 18, 1324–1329. [Google Scholar] [CrossRef]
- Polverino, F. Cigarette Smoking and COVID-19: A Complex Interaction. Am. J. Respir. Crit. Care Med. 2020, 202, 471–472. [Google Scholar] [CrossRef]
- Rossato, M.; Russo, L.; Mazzocut, S.; Vincenzo, A.D.; Fioretto, P.; Vettor, R. Current Smoking Is Not Associated with COVID-19. Eur. Respir. J. 2020, 55. [Google Scholar] [CrossRef]
- Kassi, E.N.; Stavropoulos, S.; Kokkoris, P.; Galanos, A.; Moutsatsou, P.; Dimas, C.; Papatheodorou, A.; Zafeiris, C.; Lyritis, G. Smoking Is a Significant Determinant of Low Serum Vitamin D in Young and Middle-Aged Healthy Males. Horm. Athens Greece 2015, 14, 245–250. [Google Scholar] [CrossRef] [Green Version]
- Demelo-Rodríguez, P.; Cervilla-Muñoz, E.; Ordieres-Ortega, L.; Parra-Virto, A.; Toledano-Macías, M.; Toledo-Samaniego, N.; García-García, A.; García-Fernández-Bravo, I.; Ji, Z.; de-Miguel-Diez, J.; et al. Incidence of Asymptomatic Deep Vein Thrombosis in Patients with COVID-19 Pneumonia and Elevated D-Dimer Levels. Thromb. Res. 2020, 192, 23–26. [Google Scholar] [CrossRef]
- Léonard-Lorant, I.; Delabranche, X.; Séverac, F.; Helms, J.; Pauzet, C.; Collange, O.; Schneider, F.; Labani, A.; Bilbault, P.; Molière, S.; et al. Acute Pulmonary Embolism in Patients with COVID-19 at CT Angiography and Relationship to d-Dimer Levels. Radiology 2020, 296, E189–E191. [Google Scholar] [CrossRef] [Green Version]
- Bansal, A.; Singh, A.D.; Jain, V.; Aggarwal, M.; Gupta, S.; Padappayil, R.P.; Nadeem, M.; Joshi, S.; Mian, A.; Greathouse, T.; et al. The Association of D-Dimers with Mortality, Intensive Care Unit Admission or Acute Respiratory Distress Syndrome in Patients Hospitalized with Coronavirus Disease 2019 (COVID-19): A Systematic Review and Meta-Analysis. Heart Lung 2021, 50, 9–12. [Google Scholar] [CrossRef]
- Negrea, L. Active Vitamin D in Chronic Kidney Disease: Getting Right Back Where We Started From? Kidney Dis. 2019, 5, 59–68. [Google Scholar] [CrossRef]
- Al-Badr, W.; Martin, K.J. Vitamin D and Kidney Disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1555–1560. [Google Scholar] [CrossRef] [Green Version]
- Simonnet, A.; Chetboun, M.; Poissy, J.; Raverdy, V.; Noulette, J.; Duhamel, A.; Labreuche, J.; Mathieu, D.; Pattou, F.; Jourdain, M.; et al. High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Requiring Invasive Mechanical Ventilation. Obesity 2020, 28, 1195–1199. [Google Scholar] [CrossRef]
- Bettica, P.; Bevilacqua, M.; Vago, T.; Norbiato, G. High Prevalence of Hypovitaminosis D among Free-Living Postmenopausal Women Referred to an Osteoporosis Outpatient Clinic in Northern Italy for Initial Screening. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 1999, 9, 226–229. [Google Scholar] [CrossRef]
- Kyriakaki, A.; Fragkoulis, E. The Vitamin D Paradox: High Prevalence of Deficiency in Sunny Athens (Greece). Ann. Res. Hosp. 2019, 3. [Google Scholar] [CrossRef]
- Nota 96|Agenzia Italiana del Farmaco. Available online: https://aifa.gov.it (accessed on 1 December 2020).
- Vitamina, D. Analisi Dell’effetto Della Nota 96 nel Primo Trimestre di Applicazione. Available online: https://aifa.gov.it/-/nota-96-prescrizione-a-carico-del-ssn-di-farmaci-a-base-di-vitamina-d-e-analoghi (accessed on 1 December 2020).
- Get Vitamin D Supplements If You’re at High Risk from Coronavirus (COVID-19). Available online: https://www.nhs.uk/conditions/coronavirus-covid-19/people-at-higher-risk/get-vitamin-d-supplements/ (accessed on 9 December 2020).
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Lancet Lond. Engl. 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. STROBE initiative Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. Ann. Intern. Med. 2007, 147, W163–W194. [Google Scholar] [CrossRef] [Green Version]
- Scragg, R. Limitations of Vitamin D Supplementation Trials: Why Observational Studies Will Continue to Help Determine the Role of Vitamin D in Health. J. Steroid Biochem. Mol. Biol. 2018, 177, 6–9. [Google Scholar] [CrossRef]
- Vena, A.; Giacobbe, D.R.; Di Biagio, A.; Mikulska, M.; Taramasso, L.; De Maria, A.; Ball, L.; Brunetti, I.; Loconte, M.; Patroniti, N.A.; et al. Clinical Characteristics, Management and in-Hospital Mortality of Patients with Coronavirus Disease 2019 in Genoa, Italy. Clin. Microbiol. Infect. 2020, 26, 1537–1544. [Google Scholar] [CrossRef]
- Kearns, M.D.; Binongo, J.N.G.; Watson, D.; Alvarez, J.A.; Lodin, D.; Ziegler, T.R.; Tangpricha, V. The Effect of a Single, Large Bolus of Vitamin D in Healthy Adults over the Winter and Following Year: A Randomized, Double-Blind, Placebo-Controlled Trial. Eur. J. Clin. Nutr. 2015, 69, 193–197. [Google Scholar] [CrossRef] [Green Version]
- Roffman, C.E.; Buchanan, J.; Allison, G.T. Charlson Comorbidities Index. J. Physiother. 2016, 62, 171. [Google Scholar] [CrossRef] [Green Version]

| Characteristic | Total Population (n = 91) | No Treatment with Vitamin D (n = 55) | Treatment with Vitamin D (n = 36) | p-Value |
|---|---|---|---|---|
| General | ||||
| Age (years) | 74 ± 13 | 74 ± 13 | 73 ± 13 | 0.817 |
| Male gender, n (%) | 50 (55) | 31 (56) | 19 (53) | 0.738 |
| Current smokers, n (%) | 21 (23) | 8 (15) | 13 (36) | 0.018 |
| BMI (kg/m2) | 26.6 ± 4.2 | 27 ± 5 | 26 ± 4 | 0.099 |
| Comorbidity burden, n (%) | ||||
| 0 | 9 (10) | 7 (13) | 2 (6) | |
| 1 | 32 (35) | 23 (42) | 9 (25) | 0.013 |
| 2 | 24 (26) | 14 (25) | 10 (28) | |
| ≥3 | 26 (29) | 11 (20) | 15 (42) | |
| Major comorbidities, n (%) | ||||
| Cardiovascular disease | 72 (79) | 43 (78) | 29 (81) | 0.786 |
| COPD | 17 (19) | 7 (13) | 10 (28) | 0.073 |
| CKD | 13 (14) | 2 (4) | 11 (31) | <0.001 |
| Active cancer | 11 (12) | 7 (13) | 4 (11) | 0.818 |
| Diabetes mellitus | 30 (33) | 18 (33) | 12 (33) | 0.952 |
| Hematological diseases | 8 (9) | 2 (4) | 6 (17) | 0.033 |
| Endocrine diseases | 14 (15) | 7 (13) | 7 (19) | 0.388 |
| Respiratory function/laboratory | ||||
| PaO2/FiO2 mmHg ratio | 280 (188–336) | 280 (173–328) | 280 (192–357) | 0.612 |
| PaO2 (mmHg) | 71 (65–81) | 71 (65–81) | 72 (63–83) | 0.606 |
| O2 saturation (%) | 96 (95–98) | 96 (95–98) | 96 (93–97) | 0.157 |
| 25(OH) vitamin D (nmol/L) | 35 (16–60) | 36 (19–77) | 24 (12–42) | 0.015 |
| Creatinine (µmol/L) | 88 (69–115) | 88 (71–113) | 88 (62–115) | 0.812 |
| Procalcitonin (µg/L) | 0.25 (0.08–0.52) | 0.21 (0.07–0.48) | 0.41 (0.13–0.60) | 0.490 |
| D-dimer (µg/L) | 610 (207–1264) | 317 (172–966) | 936 (533–1452) | 0.001 |
| LDH (U/L) | 365 (240–484) | 373 (264–503) | 317 (218–394) | 0.057 |
| Calcium (mmol/L) | 2.14 (2.07–2.21) | 2.14 (2.07–2.24) | 2.15 (2.07–2.19) | 0.842 |
| Phosphate (mmol/L) | 0.95 (0.78–1.13) | 0.93 (0.74–1.13) | 0.97 (0.78–1.16) | 0.167 |
| CRP (mg/L) | 64 (30–133) | 69 (33–148) | 55 (25–88) | 0.091 |
| Current treatment, n (%) | ||||
| Hydroxychloroquine | 68 (75) | 35 (64) | 33 (92) | 0.003 |
| Azithromycin | 70 (77) | 39 (71) | 31 (86) | 0.094 |
| Glucocorticoids | 41 (45) | 24 (44) | 17 (47) | 0.738 |
| Tocilizumab | 13 (14) | 6 (11) | 7 (19) | 0.258 |
| Lopinavir/ritonavir | 30 (33) | 9 (16) | 21 (58) | <0.001 |
| Other antibiotics | 71 (78) | 47 (86) | 24 (67) | 0.035 |
| Characteristics | Total Population (n = 91) | No Comorbidities (n = 9) | One Comorbidity (n = 32) | Two Comorbidities (n = 24) | ≥3 Comorbidities (n = 26) | p-Value |
|---|---|---|---|---|---|---|
| General | ||||||
| Age (years) | 74 ± 13 | 63 ± 13 | 73 ± 14 | 77 ± 10 | 75 ± 12 | 0.021 |
| Male gender, n (%) | 50 (55) | 7 (78) | 17 (53) | 12 (50) | 14 (54) | 0.416 |
| Current smokers, n (%) | 21 (23) | 4 (44) | 6 (19) | 2 (8) | 9 (35) | 0.892 |
| BMI (kg/m2) | 26.6 ± 4.2 | 27.5 ± 2.7 | 26.6 ± 5.1 | 27.4 ± 4.0 | 25.6 ± 4.0 | 0.284 |
| Major comorbidities, n (%) | ||||||
| Cardiovascular disease | 72 (79) | 0 (0) | 26 (81) | 21 (88) | 25 (96) | <0.001 |
| COPD | 17 (19) | 0 (0) | 0 (0) | 8 (33) | 9 (35) | <0.001 |
| CKD | 13 (14) | 0 (0) | 0 (0) | 3 (13) | 10 (39) | <0.001 |
| Active cancer | 11 (12) | 0 (0) | 1 (3) | 2 (8) | 8 (31) | 0.001 |
| Diabetes mellitus | 30 (33) | 0 (0) | 4 (13) | 9 (38) | 17 (65) | <0.001 |
| Hematological diseases | 8 (9) | 0 (0) | 1 (3) | 1 (4) | 6 (23) | 0.008 |
| Endocrine diseases | 14 (15) | 0 (0) | 0 (0) | 4 (17) | 10 (39) | <0.001 |
| Respiratory function/laboratory | ||||||
| PaO2/FiO2 mmHg ratio | 280 (188–336) | 323 (269–381) | 295 (191–338) | 277 (194–349) | 214 (163–304) | 0.067 |
| PaO2 (mmHg) | 71 (65–81) | 79 (76–114) | 73 (63–82) | 70 (63–78) | 66 (63–77) | 0.062 |
| O2 saturation (%) | 96 (95–98) | 98 (96–99) | 96 (95–98) | 96 (94–97) | 97 (92–98) | 0.612 |
| 25(OH) vitamin D (nmol/L) | 35 (16–60) | 49 (38–59) | 24 (14–59) | 32 (15–70) | 36 (17–54) | 0.417 |
| Creatinine (µmol/L) | 88 (69–115) | 72 (65–95) | 94 (77–116) | 86 (73–116) | 80 (64–127) | 0.862 |
| Procalcitonin (µg/L) | 0.25 (0.08–0.52) | 0.17 (0.10–0.96) | 0.25 (0.07–0.64) | 0.26 (0.08–0.41) | 0.27 (0.10–1.20) | 0.851 |
| D-dimer (µg/L) | 610 (207–1264) | 234 (155–699) | 544 (177–963) | 544 (243–1029) | 1165 (513–2314) | 0.001 |
| LDH (U/L) | 365 (240–484) | 239 (217–382) | 372 (269–483) | 316 (250–483) | 391 (227–559) | 0.134 |
| Calcium (mmol/L) | 2.14 (2.07–2.21) | 2.14 (1.97–2.19) | 2.15 (2.07–2.24) | 2.14 (2.08–2.19) | 2.12 (2.00–2.22) | 0.166 |
| Phosphate (mmol/L) | 0.95 (0.78–1.13) | 0.79 (0.58–0.97) | 0.95 (0.78–1.13) | 0.97 (0.92–1.24) | 0.90 (0.65–1.16) | 0.307 |
| CRP (mg/L) | 64 (30–133) | 54 (33–125) | 86 (40–193) | 62 (23–145) | 56 (23–87) | 0.134 |
| Current treatment, n (%) | ||||||
| Hydroxychloroquine | 68 (75) | 7 (78) | 22 (69) | 19 (79) | 20 (77) | 0.636 |
| Azithromycin | 70 (77) | 6 (67) | 26 (81) | 20 (83) | 18 (69) | 0.698 |
| Glucocorticoids | 41(45) | 2 (22) | 13 (41) | 13 (54) | 13 (50) | 0.146 |
| Tocilizumab | 13 (14) | 0 (0) | 2 (6) | 4 (17) | 7 (27) | 0.011 |
| Lopinavir/ritonavir | 30 (33) | 3 (33) | 7 (22) | 7 (29) | 13 (50) | 0.074 |
| Other antibiotics | 71 (78) | 7 (78) | 25 (78) | 19 (79) | 20 (77) | 0.944 |
| Cholecalciferol | 36 (40) | 2 (22) | 9 (28) | 10 (42) | 15 (58) | 0.013 |
| Variables | Crude Analyses (OR, 95% CI and p-Value) | Model 1 (OR, 95% CI and p-Value) | |
|---|---|---|---|
| Vitamin D treatment | (0 = no; 1 = yes) | 0.57 (0.24–1.34), p = 0.20 | 0.45 (0.20–1.22), p = 0.13 |
| Comorbidity burden | (1-unit increase) | 1.17 (0.76–1.78), p = 0.48 | 1.29 (0.82–2.01), p = 0.27 |
| Comorbidity Burden | Crude Effect Modification Analysis * (OR, 95% CI and p-Value) | Adjusted Effect Modification Analysis ** (OR, 95% CI and p-Value) |
|---|---|---|
| 0 | 3.26 (0.48–22.2) | 3.59 (0.52–24.8) |
| 1 | 1.16 (0.36–3.76) | 1.32 (0.39–4.43) |
| 2 | 0.41 (0.16–1.05) | 0.49 (0.18–1.32) |
| ≥3 | 0.15 (0.034–0.64) | 0.18 (0.04–0.83) |
| P for effect modification by comorbidity burden | 0.033 | 0.039 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannini, S.; Passeri, G.; Tripepi, G.; Sella, S.; Fusaro, M.; Arcidiacono, G.; Torres, M.O.; Michielin, A.; Prandini, T.; Baffa, V.; et al. Effectiveness of In-Hospital Cholecalciferol Use on Clinical Outcomes in Comorbid COVID-19 Patients: A Hypothesis-Generating Study. Nutrients 2021, 13, 219. https://doi.org/10.3390/nu13010219
Giannini S, Passeri G, Tripepi G, Sella S, Fusaro M, Arcidiacono G, Torres MO, Michielin A, Prandini T, Baffa V, et al. Effectiveness of In-Hospital Cholecalciferol Use on Clinical Outcomes in Comorbid COVID-19 Patients: A Hypothesis-Generating Study. Nutrients. 2021; 13(1):219. https://doi.org/10.3390/nu13010219
Chicago/Turabian StyleGiannini, Sandro, Giovanni Passeri, Giovanni Tripepi, Stefania Sella, Maria Fusaro, Gaetano Arcidiacono, Marco Onofrio Torres, Alberto Michielin, Tancredi Prandini, Valeria Baffa, and et al. 2021. "Effectiveness of In-Hospital Cholecalciferol Use on Clinical Outcomes in Comorbid COVID-19 Patients: A Hypothesis-Generating Study" Nutrients 13, no. 1: 219. https://doi.org/10.3390/nu13010219
APA StyleGiannini, S., Passeri, G., Tripepi, G., Sella, S., Fusaro, M., Arcidiacono, G., Torres, M. O., Michielin, A., Prandini, T., Baffa, V., Aghi, A., Egan, C. G., Brigo, M., Zaninotto, M., Plebani, M., Vettor, R., Fioretto, P., Rossini, M., Vignali, A., ... Bertoldo, F. (2021). Effectiveness of In-Hospital Cholecalciferol Use on Clinical Outcomes in Comorbid COVID-19 Patients: A Hypothesis-Generating Study. Nutrients, 13(1), 219. https://doi.org/10.3390/nu13010219







