Fructus lycii: A Natural Dietary Supplement for Amelioration of Retinal Diseases
Abstract
:1. Introduction
2. Bioactive Components
3. Bioavailability of Bioactive Components
4. Fructus lycii and Retinal Diseases and Degeneration
4.1. F. lycii and Age-Related Macular Degeneration
4.2. F. lycii and Diabetic Retinopathy
4.3. F. lycii and Retinitis Pigmentosa
5. Limitations of the Existing Studies
6. Side Effects and Drug Interaction
7. Future Directions and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| F. lycii | Fructus lycii |
| L | Lycium |
| Z | Zeaxanthin |
| AMD | Age-related macular degeneration |
| DR | Diabetic retinopathy |
| RP | Retinitis pigmentosa |
| ROS | Reactive oxygen species |
| RPE | Retinal pigment epithelium |
| DNA | Deoxyribonucleic acid |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| TrxR1 | Thioredoxin reductase |
| PARP | Poly-ADP-ribose polymerase |
| ERG | Electroretinography |
| VEGF | Vascular endothelial growth factor |
| PEDF | Pigment epithelium-derived growth factor |
| mRNA | Messenger ribonucleic acid |
| BRB | Blood-retinal-barrier |
| AMPK | AMP-activated protein kinase |
| ROCK | Rho A associated protein kinase |
| PPAR | Peroxisome proliferator activated receptor |
| ER | Endoplasmic reticulum |
| TFAM | Transcription factor A, mitochondria |
| SR-B1 | Scavenger receptor class B type 1 |
| GSTP1 | Glutathione S-transferase pi 1 |
| BCO2 | β-carotene 9′,10′-oxygenase 2 |
| PCG-1 | Peroxisome proliferator-activated receptor γ coactivator-1 |
| NRF-1 | Nuclear respirator factor 1 |
| HIF | Hypoxia inducible factor |
| HSP | Heat shock protein |
| FOX03 | Forkhead O transcription factor 3 |
| NF-kB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| cGMP | Cyclic guanosine monophosphate |
References
- Stuart, G.A.; Smith, F.P. Chinese Materia Medica; American Presbyterian Mission Press: Shanghai, China, 1991; p. 250. [Google Scholar]
- Integrated Taxonomic Information System. Lycium barbarum L. Taxonomic Serial No: 503599; 2019; p. 22. Available online: https://www.itis.gov (accessed on 29 October 2020).
- Chi, Z.S. Chemical constituents of Fructus Lycii and Folium Lycii (I)-Nutrients in Fructus lycii and Folium lycii. Zhong Yao Tong Bao 1986, 11, 41. [Google Scholar]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China Part I; Chemical Industry Press: Beijing, China, 2015. [Google Scholar]
- Cao, Y.L.; Wu, P.J. Wolfberry Germplasm Resources in China; China Forestry Publishing House: Beijing, China, 2015. [Google Scholar]
- Potterat, O. Goji (Lycium barbarum and L Chinese): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Medica 2010, 76, 7–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.F.; Zhang, H.; Teh, S.S.; Wang, C.; Zhang, Y.; Hayford, F.; Wang, L.; Ma, T.; Dong, Z.; Zhang, Y.; et al. Goji berries as a potential natural antioxidant medicine: An insight into their molecular mechanisms of action. Oxid. Med. Cell. Longev. 2019, 2019, 2437397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulczyriski, B.; Gramza-Michalowska, A. Goji berry (Lycium barbarum): Composition and health effects-a review. Pol. J. Food Nutr. Sci. 2016, 66, 67–76. [Google Scholar] [CrossRef]
- Wang, C.; Chang, S.B.; Inbaraj, S.; Chen, B.H. Isolation of carotenoids, flavonoids and polysaccharides from Lycium barbarum L and evaluation of antioxidant activity. Food Chem. 2010, 120, 184–192. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, W.; Zhao, J.; Xi, W. Functional constituents and antioxidant activities of eight Chinese native goji genotypes. Food Chem. 2016, 200, 230–236. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, S. Determination of taurine in Lycium barbarum by high performance liquid chromatography with OPA-urea pre-column derivatization. Se Pu = Chin. J. Chromatogr. 1997, 15, 54–56. [Google Scholar]
- Tatania, K.; Alexander, E.; Alexander, D. Why Dietary Zeaxanthin? A Scientific Summary; Kemin Foods LC: Des Moines, IA, USA, 2012. [Google Scholar]
- Hempel, J.; Schädle, C.N.; Sprenger, J.; Heller, A.; Carle, R.; Schweiggerta, R.M. Ultrastructural deposition forms and bio accessibility of carotenoids and carotenoid esters from goji berries (Lycium barbarum L.). Food Chem. 2017, 218, 525–533. [Google Scholar] [CrossRef]
- Hempel, J.; Fischer, A.; Fischer, M.; Högel, J.; Bosy-Westphal, A.; Carle, R.; Schweiggert, R.M. Effect of aggregation forms on bioavailability of zeaxanthin in humans: A randomized cross-over study. Br. J. Nutr. 2017, 118, 698–706. [Google Scholar] [CrossRef] [Green Version]
- Bertoldi, D.; Cossignani, L.; Blasi, F.; Perini, M.; Barbero, A.; Pianezze, S.; Montesano, D. Characterization and geographical traceability of Italian goji berries. Food Chem. 2019, 275, 585–593. [Google Scholar] [CrossRef]
- Lu, Y.; Guo, S.; Zhang, F.; Yan, H.; Qian, D.W.; Wang, H.Q.; Jin, L.; Duan, J.A. Comparison of Functional Components and Antioxidant Activity of Lycium barbarum L. Fruits from Different Regions in China. Molecules 2019, 24, 2228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Galvez, A.; Martin, H.D.; Sies, H.; Stahl, W. Incorporation of carotenoids from paprika oleoresin into human chylomicrons. Br. J. Nutr. 2003, 89, 787–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, I.Y.F.; Tso, M.O.M.; Li, W.W.Y.; Lam, T.T. Absorption and tissue distribution of Z and L in rhesus monkeys after taking fructus lycii (Gou Qi Zi) extract. Investig. Ophthalmol. Vis. Sci. 2001, 42, 466–471. [Google Scholar]
- Cheng, C.Y.; Chung, W.Y.; Szeto, Y.T.; Benzie, I.F.F. Fasting plasma zeaxanthin response to Fructus barbarum L. (wolfberry; Kei Tze) in a food-based human supplementation trial. Br. J. Nutr. 2005, 93, 123–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucheli, P.; Vidal, K.; Shen, L.; Gu, Z.; Zhang, C.; Miller, L.E.; Wang, J. Goji berry effects on macular characteristics and plasma antioxidant levels. Optom. Vis. Sci. 2011, 88, 257–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breithaupt, D.E.; Weller, P.; Wolters, M.; Hahn, A. Comparison of plasma responses in human subjects after the ingestion of 3R,3R′-zeaxanthin dipalmitate from wolfberry (Lycium barbarum) and non-esterified 3R,3R′-zeaxanthin using chiral high-performance liquid chromatography. Br. J. Nutr 2004, 91, 707–713. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.F.; Chung, W.Y.; Wang, J.; Richelle, M.; Bucheli, P. Enhanced bioavailability of zeaxanthin in a milk-based formulation of wolfberry (Gou Qi Zi; Fructus barbarum L.). Br. J. Nutr. 2006, 96, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Rukeya, J.; Tao, W.; Sun, P.; Yi, Z. Bioactive compounds and antioxidant activity of wolfberry infusion. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Werklust, B.V.; Beheer, N. Carotenoid Esters for the Prevention and Treatment of Eye Diseases. Patent DE19950327, 20 April 2000. [Google Scholar]
- Leung, I.Y.F.; Ngai, J.; Lam, K.W.; Tso, M.O.M. Absorption of zeaxanthin in rats after feeding with purified zeaxanthin or a traditional Chinese medicine, Gou Qi Zi. Investig. Ophthalmol Vis. Sci. 1999, 40, S608. [Google Scholar]
- Zhong, V.W.; Horn, L.V.; Cornelis, M.C. Association of dietary cholesterol or egg consumption with incident cardiovascular disease and mortality. JAMA 2019, 32, 1081–1095. [Google Scholar] [CrossRef]
- Ulbricht, C.; Bryan, J.K.; Costa, D.; Culwell, S.; Giese, N.; Isaac, R.; Nummy, K.; Pham, T.; Rapp, C.; Rusie, E.; et al. An evidence-based systematic review of goji (Lycium spp.) by the natural standard research collaboration. J. Diet. Suppl. 2015, 12, 184–240. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, A.; Niro, S.; Alam, M.D.R.; Cinquanta, L.; Di Matteo, M.; Adiletta, G.; Panfili, G. Effect of a physical pre-treatment and drying on carotenoids of goji berries (Lycium barbarum L.). LWT - Food Sci. Technol. 2018, 92, 318–323. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, F.; Wu, M.; Yan, X.; Meng, X.; Song, Y. A rapid and effective approach for on-site assessment of total carotenoid content in wolfberry juice during processing. J. Sci. Food Agric. 2015, 95, 2951–2955. [Google Scholar] [CrossRef] [PubMed]
- Dos-Reis, B.A.; Kosinka-Cagnazzo, A.; Schmitt, R. Fermentation of plant material-effect on sugar content and stability of bioactive compounds. Pol. J. Food Nutr. Sci. 2014, 64, 235–241. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Liang, T.; Liu, Y.; Ding, G.; Zhang, F.; Ma, Z. Extraction, structural characterization and biological functions of Lycium barbarum polysaccharides: A review. Biomolecules 2019, 9, 389. [Google Scholar] [CrossRef] [Green Version]
- Cutler, R.G. Oxidative stress profiling: Part I. Its potential importance in the optimization of human health. Ann. N. Y. Acad. Sci. 2005, 1055, 93–135. [Google Scholar] [CrossRef]
- Khaliq, A.; Jervis-Evans, J.; McLeod, D.; Boulton, M. Oxygen modulates the response of the retinal pigment epithelium to basic fibroblast growth factor and epidermal growth factor by receptor regulation. Investig. Ophthalmol. Vis. Sci. 1996, 37, 436–443. [Google Scholar]
- Beatty, S.; Koh, H.; Henson, D.; Boulton, M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [Google Scholar] [CrossRef] [Green Version]
- Cui, B.; Liu, S.; Lin, X.; Wang, J.; Li, S.H.; Wang, Q.B.; Li, S.P. Effects of Lycium barbarum aqueous and ethanol extracts on high fat induced oxidative stress in rat liver tissue. Molecules 2011, 16, 9116–9128. [Google Scholar] [CrossRef]
- Huang, L.J.; Tian, G.Y.; Wang, Z.F.; Dong, J.B. Studies on the glycolconjugates and glycans from Lycium barbarum L in inhibiting low density lipoprotein (LDL) peroxidation. Acta Pharm Sin. 2001, 36, 108–111. [Google Scholar]
- Wu, S.; Ng, L.; Lin, C. Antioxidant activities of some common ingredients of traditional Chinese medicine, Angelica sinensis, Lycium barbarum and Poria cocos. Phytother. Res. 2004, 18, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, Y.; Liu, X. Effect of the Lycium barbarum polysaccharide on age-related oxidative stress in aged mice. J. Ehanopharmacol. 2007, 111, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, A. Evaluation of the antioxidant effects of polysaccharides extracted from Lycium barbarum. Med. Chem. Res. 2007, 15, 471–482. [Google Scholar] [CrossRef]
- Amagase, H.; Sun, B.; Borek, C. Lycium barbarum (goji) juice improves in vivo antioxidant biomarkers in serum of healthy adults. Nutr. Res. 2009, 29, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.P.; Ke, C.Y.; Kuo, C.C.; Lee, Y.-J. Effect of a complex lutein formula in an animal model for light-induced retinal degeneration. Chin. J. Physiol. 2016, 59, 202–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Li, W.; Qi, D.; Wang, D. Lycium barbarum polysaccharide protects against LPS-induced ARDS by inhibiting apoptosis, oxidative stress and inflammation in pulmonary endothelial cells. Free Radic Res. 2018, 52, 480–490. [Google Scholar] [CrossRef]
- Gong, G.P.; Dang, T.T.; Deng, Y.N.; Han, J.; Zou, Z.; Jing, S.; Zhang, Y.; Liu, Q.; Huang, L.; Wang, Z. Physiochemical properties and biological activities of polysaccharides from Lycium barbarum prepared by fractional precipitation. Int. J. Biol. Macromol. 2018, 109, 611–618. [Google Scholar] [CrossRef]
- Liang, B.; Jin, M.; Liu, H. Water-soluble polysaccharide from dried Lycium barbarum fruits: Isolation, structural features and antioxidant activity. Carbohydr Polym 2011, 83, 1947–1951. [Google Scholar] [CrossRef]
- Resnikoff, S.; Pascolini, D.; Etya’ale, D.; Kocur, I.; Pararajasegaram, R.; Pokharel, G.P.; Mariotti, S.P. Global data on visual impairment in the year 2002. Bull. World Health Organ. 2004, 82, 844–851. [Google Scholar]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.H.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020and 2040: A systemic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [Green Version]
- Dong, W.H.; Du, X.J.; Guo, D.D.; Dou, R. Protective effects of Lycium barbarum polysaccharide on damaged hRPE cells induced by blue light irradiation. Int. Eye Sci. 2013, 13, 2381–2384. [Google Scholar]
- Du, X.J.; Dong, W.H.; Bi, H.S.; Xie, X.F. The anti-aging effect of Lycium barbarum polysaccharide on human retinal pigment epithelial cell. Chin. J. Exp. Ophthalmol. 2013, 31, 739–743. [Google Scholar]
- Liu, L.; Lao, W.; Ji, Q.S.; Yang, Z.H.; Yu, G.C.; Zhong, J.X. Lycium barbarum polysaccharides protected human retinal pigment epithelial cells against oxidative stress-induced apoptosis. Int. J. Ophthalmol. 2015, 8, 11–16. [Google Scholar] [PubMed]
- Hsieh, F.C.; Hung, C.T.; Cheng, K.C.; Wu, C.Y.; Chen, Y.C.; Wu, Y.J.; Liu, W.; Chiu, C.C. Protective effects of Lycium barbarum extracts on UVB-Induced damage in human retinal pigment epithelial cells accompanied by attenuating ROS and DNA Damage. Oxid. Med. Cell Longev. 2018, 2018, 4814928. [Google Scholar] [CrossRef]
- Tang, L.; Bao, S.; Du, Y.; Jiang, Z.; Wuliji, A.O.; Ren, X.; Zhang, C.; Chu, H.; Kong, L.; Ma, H. Antioxidant effects of Lycium barbarum polysaccharides on photoreceptor degeneration in the light-exposed mouse retina. Biomed. Pharmacother. 2018, 103, 829–837. [Google Scholar] [CrossRef]
- Vidal, H.; Bucheli, P.; Moulin, J.; Wang, J.; Benyacoub, J. Effect of a dietary wolfberry preparation on immune and physical status in elderly. Eur. Geriatr. Med. 2014, 5, S244–S245. [Google Scholar] [CrossRef]
- Li, S.; Liu, N.; Lin, L. Macular pigment and serum zeaxanthin levels with goji berry supplement in early age-related macular degeneration. Int. J. Ophthalmol. 2018, 11, 970–975. [Google Scholar]
- AREDS 2 Research Group. Lutein plus zeaxanthin and omega 3 fatty acids for age-related macular degeneration: The age-related eye disease study 2 (AREDS 2) randomized clinical trial. JAMA 2013, 309, 2005–2015. [Google Scholar] [CrossRef]
- Bone, R.A.; Landrum, J.T.; Friedes, L.M.; Gomez, G.M.; Kilburn, M.D.; Menendez, E.; Vidal, I.; Wang, W. Distribution of lutein and zeaxanthin stereoisomers in the human retina. Exp. Eye Res. 1997, 64, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Bone, R.A.; Landrum, J.T.; Hime, G.W.; Cains, A.; Zamor, J. Stereochemistry of the human macular carotenoids. Invest. Ophthalmol. Vis. Sci. 1993, 34, 2033–2040. [Google Scholar]
- Britton, G. Structure and properties of carotenoids in relation to function. FASEB J. 1995, 9, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, A.; McGarvey, D.J.; Truscott, T.G.; Rancan, F.; Böhm, F. Singlet oxygen quenching by dietary carotenoids in a model membrane environment. Arch. Biochem. Biophys. 2003, 412, 47–54. [Google Scholar] [CrossRef]
- Leasher, J.L.; Bourne, R.R.; Flaxman, S.R.; Jonas, J.B.; Keeffe, J.; Naidoo, K.; Pesudovs, K.; Price, H.; White, R.A.; Wong, T.Y.; et al. Vision Loss Expert Group of the Global Burden of Disease Study. Global Estimates on the Number of People Blind or Visually Impaired by Diabetic Retinopathy: A Meta-analysis from 1990 to 2010. Diabetes Care 2016, 39, 1643–1649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meta-Analysis for Eye Disease (META-EYE) Study Group. Meta-analysis for eye disease (META-EYE) study group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowluru, R.A.; Chan, P.S. Oxidative stress and diabetic retinopathy. Exp. Diabetes Res. 2007, 207, 43603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, L.; Zhang, Y.; Jiang, Y.; Willard, L.; Ortiz, E.; Wark, L.; Medeiros, D.; Lin, D. Dietary wolfberry ameliorates retinal structure abnormalities in db/db mice at the early stage of diabetes. Exp. Biol. Med. 2011, 236, 1051–1063. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.K.; Lee, Y.J.; Colitz, C.M.; Chang, C.J.; Lin, C.T. The protective effects of Lycium barbarum and Chrysanthemum morifolum on diabetic retinopathies in rats. Vet. Ophthalmol 2012, 15 (Suppl. 2), 65–71. [Google Scholar] [CrossRef]
- Guo, J.; Xu, G.X.; Hou, Z.J.; Xu, J.B.; Huang, L.Y. Effect of Lycium barbarum polysaccharides on the retinal ultrastructure of streptozocin-induced diabetic rats. Zhongguo Zhong Xi Yi Jie He Za Zhi 2013, 33, 1404–1407. [Google Scholar]
- Yao, Q.; Yang, Y.; Lu, X.; Zhang, Q.; Luo, M.; Li, A.; Pan, Y. Lycium barbarum polysaccharides improve retinopathy in diabetic Sprague-dawley rats. Evid Based Complement. Altern Med. 2018, 2018, 7943212. [Google Scholar] [CrossRef] [Green Version]
- Leith, E.; Barber, A.J.; Xu, B.; Dice, C.; Ratz, M.J.; Tanase, D.; Strother, J.M. Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Diabetes 1998, 47, 815–820. [Google Scholar] [CrossRef]
- Barber, A.J.; Antonetti, A.; Gardner, T.W.; The Penn State Retina Research Group. Altered expression of retinal occludin and glial fibrillary acidic protein in experimental diabetes. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3561–3568. [Google Scholar]
- Yu, H.; Wark, L.; Ji, H.; Willard, L.; Jaing, Y.; Han, J.; He, H.; Ortiz, E.; Zhang, Y.; Medeiros, D.M.; et al. Dietary wolfberry up regulates carotenoid metabolic genes and enhances mitochondrial biogenesis in the retina of db/db diabetic mice. Mol. Nutr. Food Res. 2013, 57, 1158–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Chen, N.; Ma, Y. Effects of Lycium barbarum polysaccharides on retinal pathological change and expression of VEGF in retina of diabetic rats. Chin. J. Exp. Ophthalmol. 2014, 32, 334–339. [Google Scholar]
- Wang, Y.; Ding, L.; Li, Y.; Guan, C.; Guo, J. Lycium barbarum polysaccharides can reduce the oxidative damage of the retinal nerve cells in diabetic rats. Int. J. Clin. Exp. Med. 2017, 10, 5168–5174. [Google Scholar]
- Wang, J.; Yao, Y.; Liu, X.; Wang, K.; Zhou, Q.; Tang, Y. Protective effects of Lycium barbarum polysaccharides on blood-retinal barrier via ROCK1 pathway in diabetic rats. Am. J. Transl. Res. 2019, 11, 6304–6315. [Google Scholar]
- Song, M.K.; Salam, N.K.; Roufogalis, B.D.; Huang, T.H.W. Lycium barbarum (Goji Berry) extracts and its taurine component inhibit PPAR-γ-dependent gene transcription in human retinal pigment epithelial cells: Possible implications for diabetic retinopathy treatment. Biochem. Pharmacol. 2011, 82, 1209–1218. [Google Scholar] [CrossRef]
- Song, M.K.; Roufogalis, B.D.; Huang, T.H.W. Reversal of the caspase-dependent apoptotic cytotoxicity pathway by taurine from Lycium barbarum (Goji Berry) in human retinal pigment epithelial cells: Potential benefit in diabetic retinopathy. Evid. Based Complement. Altern. Med. 2012, 2012, 323784. [Google Scholar] [CrossRef] [Green Version]
- Pavan, B.; Capuzzo, A.; Forlani, G. High glucose-induced barrier impairment of human retinal pigment epithelium is ameliorated by treatment with Goji berry extracts through modulation of cAMP levels. Exp. Eye Res. 2014, 120, 50–54. [Google Scholar] [CrossRef]
- El Rami, H.; Barham, R.; Sun, J.K.; Silva, P.S. Evidence-Based Treatment of Diabetic Retinopathy. Semin. Ophthalmol. 2017, 32, 67–74. [Google Scholar] [CrossRef]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight 2017, 2, e93751. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, H.; Bao, S.; Wang, N.; Gillies, M.C. Diabetic macular edema: New concepts in patho-physiology and treatment. Cell Biosci. 2014, 4, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dörfel, M.J.; Huber, O. Modulation of tight junction structure and function by kinases and phosphatases targeting occludin. J. Biomed. Biotechnol. 2012, 2012, 807356. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Breslin, J.W.; Zhu, J.; Yuan, S.Y.; Wu, M.H. Rho and ROCK signaling in VEGF-induced microvascular endothelial hyperpermeability. Microcirculation 2006, 13, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Herzlich, A.A.; Tuo, J.; Chan, C.C. Peroxisome proliferator-activated receptor and age-related macular degeneration. PPAR Res. 2008, 2008, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tawfik, A.; Sanders, T.; Kahook, K.; Akeel, S.; Elmarakby, A.; Al-Shabrawey, M. Suppression of retinal peroxisome proliferator-activated receptor γ in experimental diabetes and oxygen-induced retinopathy: Role of NADPH oxidase. Investig. Ophthalmol. Vis. Sci. 2009, 50, 878–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takatani, T.; Takahashi, Y.; Uozumi, Y.; Matsuda, T.; Ito, T.; Schaffer, S.W.; Fujio, Y.; Azuma, J. Taurine prevents the ischemia-induced apoptosis in cultured neonatal rat cardiomyocytes through Akt/caspase 9 pathway. Biochem. Biophys. Commun. 2004, 316, 484–489. [Google Scholar] [CrossRef]
- Wu, Q.D.; Wang, J.H.; Fennessy, F.; Redmond, P.H.; Bouchier-Hayes, D. Taurine prevents high glucose induced human vascular cell endothelial apoptosis. Am. J. Physiol. 1999, 227, C1229–C1238. [Google Scholar]
- Tomi, M.; Terayama, T.; Isobe, T.; Egami, F.; Morito, A.; Kurachi, M.; Ohtsuki, S.; Kang, Y.S.; Terasaki, T.; Hosoya, K. Function and regulation of taurine transport at the inner blood–retinal barrier. Microvasc. Res. 2007, 73, 100–106. [Google Scholar] [CrossRef]
- Pasantes-Morales, H.; Klethi, J.; Ledig, M.; Mandel, P. Free amino acids of chicken and rat retina. Brain Res. 1972, 41, 494–497. [Google Scholar] [CrossRef]
- Vilchis, C.; Salceda, R. Effect of diabetes on levels and uptake of putative amino acid neurotransmitters in rat retina and retinal pigment epithelium. Neurochem. Res. 1996, 21, 1167–1171. [Google Scholar] [CrossRef]
- McCarty, M.F. Exploiting complementary therapeutic strategies for the treatment of type II diabetes and prevention of its complications. Med. Hypotheses 1997, 49, 143–152. [Google Scholar] [CrossRef]
- Yu, X.; Xu, Z.; Mi, M.; Xu, H.; Zhu, J.; Wei, N.; Chen, K.; Zhang, Q.; Zeng, K.; Wang, J.; et al. Dietary taurine supplementation ameliorates diabetic retinopathy via anti-excitotoxicity of glutamate in streptozotocin-induced Sprague-Dawley rats. Neurochem. Res. 2008, 33, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Song, M.K.; Salam, N.K.; Roufogalis, B.D.; Huang, T.H.W. The effect of taurine in Lycium barbarum (goji berry) on diabetic retinopathy in human retinal pigment epithelial cells: Activation of PPAR-gamma. Paed Diabetes 2011, 12, 53. [Google Scholar]
- Kowluru, R.A.; Mishra, M. Oxidative stress, mitochondrial damage and diabetic retinopathy. Biochem. Biophys. Acta 2015, 1852, 2474–2483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowluru, R.A.; Mohammad, G.; dos Santos, J.M.; Zhong, Q. Abrogation of MMP-9 gene protects against the development of retinopathy in diabetic mice by preventing mitochondrial damage. Diabetes 2011, 60, 3023–3033. [Google Scholar] [CrossRef] [Green Version]
- Lobo, G.P.; Isken, A.; Hoff, S.; Babino, D.; Lintig, J.V. BCO2 acts as a carotenoid scavenger and gatekeeper for the mitochondrial apoptotic pathway. Development 2012, 139, 2966–2967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivitiz, W.I.; Yorek, M.A. Mitochondrial dysfunction in diabetes: From molecular mechanisms to functional significance and therapeutic opportunities. Antioxid. Redox Signal. 2010, 12, 537–577. [Google Scholar] [CrossRef] [Green Version]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- Newton, F.; Megaw, R. Mechanisms of photoreceptor death in retinitis pigmentosa. Genetic 2020, 11, 1120. [Google Scholar]
- Chen, Y.; Yang, M.; Wang, Z.J. (Z)-7,40 -Dimethoxy-6-hydroxy-aurone-4-O-β-glucopyranoside mitigates retinal degeneration in Rd10 mouse model through inhibiting oxidative stress and inflammatory responses. Cutan. Ocul. Toxicol. 2019, 39, 36–42. [Google Scholar] [CrossRef]
- Bennett, J.; Tanabe, T.; Sun, D.; Zeng, Y.; Kjeldbye, H.; Gouras, P.; Maguire, A.M. Photoreceptor cell rescue in retinal degeneration (rd) mice by in vivo gene therapy. Nat. Med. 1996, 2, 649–654. [Google Scholar] [CrossRef] [PubMed]
- MacLaren, R.E.; Pearson, R.A.; MacNeil, A.; Douglas, R.H.; Salt, T.E.; Akimoto, M.; Swaroop, A.; Sowden, J.C.; Ali, R.R. Retinal repair by transplantation of photoreceptor precursors. Nature 2006, 444, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Otani, A.; Dorrell, M.I.; Kinder, K.; Moreno, S.K.; Nusinowitz, S.; Banin, E.; Heckenlively, J.; Friedlander, M. Rescue of retinal degeneration by intravitreally injected adult bone marrow-derived lineage-negative hematopoietic stem cells. J. Clin. Investig. 2004, 114, 765–774. [Google Scholar] [CrossRef]
- Komeima, K.; Rogers, B.S.; Lu, L.; Campochiaro, P.A. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 2006, 103, 11300–11305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Zhang, J.; Xiang, Z.; Xu, D.; So, K.F.; Vardi, N.; Xu, Y. Lycium barbarum Polysaccharides Protect Retina in rd1 Mice During Photoreceptor Degeneration. Investig. Opthalmol. Vis. Sci. 2018, 59, 597. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Xiao, J.; Peng, B.; Xing, F.; So, K.F.; Tipoe, G.L.; Lin, B. Retinal structure and function preservation by polysaccharides of wolfberry in a mouse model of retinal degeneration. Sci. Rep. 2014, 4, 7601. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Zhao, Q.; Gao, H.; Peng, X.; Wen, Y.; Dai, G. Lycium barbarum polysaccharides attenuates N-methy-N-nitrosourea-induced photoreceptor cell apoptosis in rats through regulation of poly (ADP-ribose) polymerase and caspase expression. J. Ethnopharmacol. 2016, 191, 125–134. [Google Scholar] [CrossRef]
- Chan, H.H.; Lam, H.I.; Choi, K.Y.; Li, S.Z.C.; Lakshmanan, Y.; Yu, W.Y.; Chang, R.C.C.; Lai, J.S.M.; So, K.F. Delay of cone degeneration in retinitis pigmentosa using a 12-month treatment with Lycium barbarum supplement. J. Ethnopharmacol. 2019, 236, 336–344. [Google Scholar] [CrossRef]
- Xue, C.; Rosen, R.; Jordan, A.; Hu, D.N. Management of ocular diseases using lutein and zeaxanthin: What have we learned from experimental animal studies? J. Ophthlamol. 2015, 2015, 523027. [Google Scholar] [CrossRef]
- Amagase, H.; Farnsworth, N.R. A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (Goji). Food Res. Int. 2011, 44, 1702–1717. [Google Scholar] [CrossRef]
- Adams, M.; Wiedenmann, M.; Tittel, G.; Bauer, R. HPLC-MS trace analysis of atropine in Lycium barbarum berries. Phytochem. Anal. 2006, 17, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Monzon Ballarin, S.; Lopez-Matas, M.A.; Saenz Abad, D.; Perez-Cinto, N.; Carnes, J. Anaphylaxis associated with the ingestion of goji berries (Lycium barbarum). J. Investig. Allergol. Clin. Immunol. 2011, 21, 567–570. [Google Scholar] [PubMed]
- Arroyo-Martinez, Q.; Saenz, M.J.; Arias, F.A. Lycium barbarum: A new hepatotoxic “natural” agent? Dig. Liver Dis. 2011, 43, 749. [Google Scholar] [CrossRef] [PubMed]
- Larramendi, C.H.; Garcia-Abujeta, J.L.; Vicario, S.; Garcia-Endrino, A.; Lopez, M.A.; Garcia-Sedeno, M.D.; Carnes, J. Goji berries (Lycium barabarum) risk of allergic reactions in individuals with food allergy. J. Investig. Allergol. Clin. Immunol. 2012, 21, 345–350. [Google Scholar]
- Carnes, J.; de Larramendi, C.H.; Ferrer, A.; Huertas, A.J.; Lopez-Matas, M.A.; Pagan, J.A.; Navarro, L.A.; Garcia-Abujeta, J.L.; Vicario, S.; Pena, M. Recently introduced foods as new allergenic sources: Sensitization to Goji berries (Lycium barbarum). Food Chem. 2013, 137, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.; Hung, A.; Hui, A.C.; Chan, T.Y.K. Warfarin overdose due to possible effects of Lycium barbarum. Food Chem. Toxicol. 2008, 46, 1860–1862. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C.A.; Ferro, C.L.; Bursua, A.J.; Gerber, B.S. Possible interaction between Lycium barbarum (goji) and warfarin. Pharmacotherapy 2012, 32, e50–e53. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, L.; Xie, B. Bleeding due to a probable interaction between warfarin and Gou qi zi (Lycium barbarum L.). Toxicol. Rep. 2015, 2, 1209–1212. [Google Scholar] [CrossRef] [Green Version]

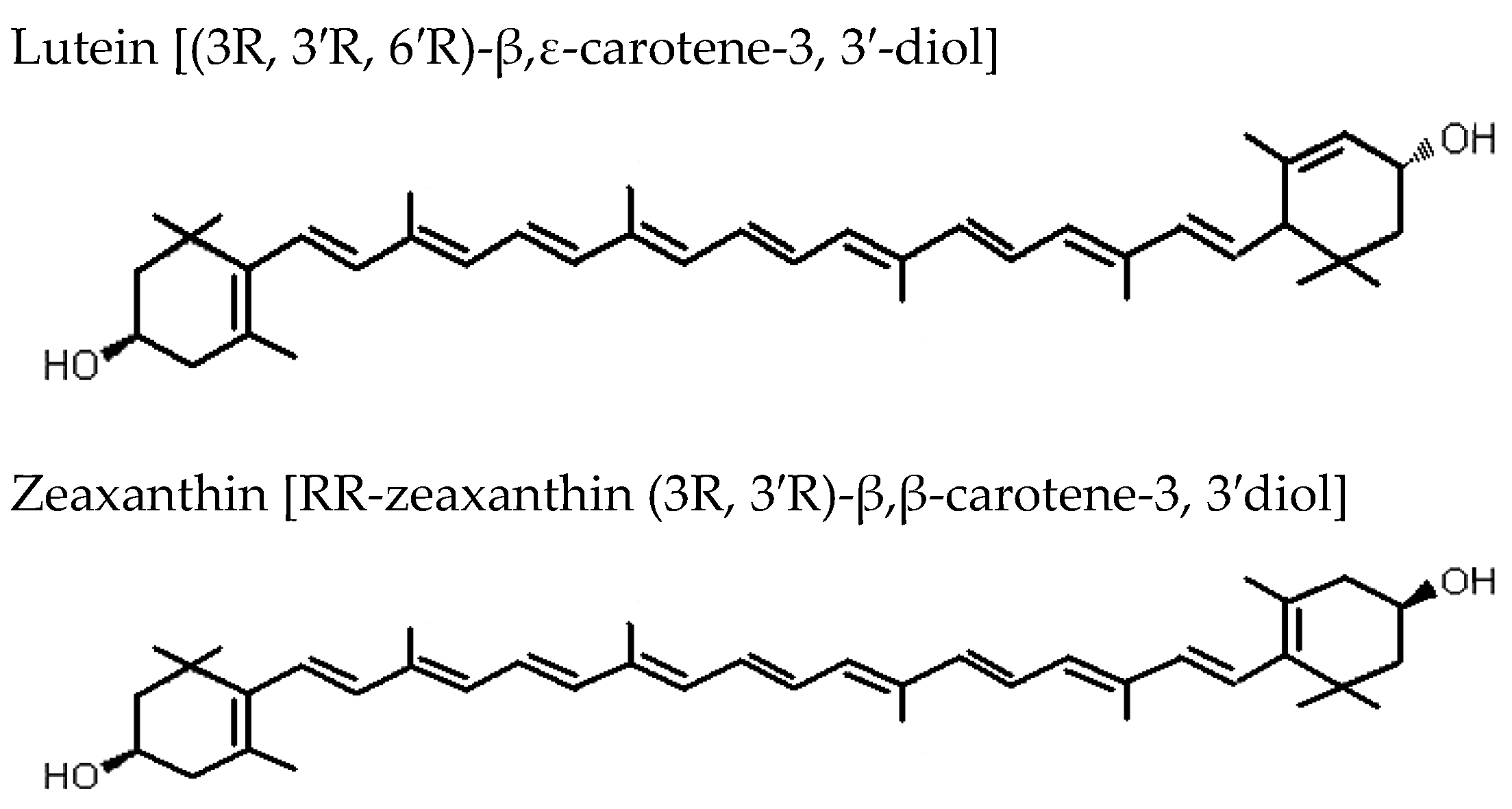
| Authors (year) | Type of Study | Subjects | F. lycii Formulation | Aim | Conclusion |
|---|---|---|---|---|---|
| HUMAN STUDIES (HEALTHY SUBJECTS) | |||||
| Breithaupt et al. (2004) [21] | Single-blinded, crossover study (3-week of depletion period) | N = 12, Males = 6; Females = 6 | A Z-standardized dose (5 mg) suspended in yoghurt, esterified (3R,3R’-Z dipalmitate from F. lycii) and non-esterified 3R,3R’-Z forms | Bioavailability of esterified Z versus non-esterified Z | Administration of both esterified and non-esterified Z increased plasma Z levels; however, levels were significantly higher with former suggesting an enhanced bioavailability of esterified form of Z. |
| Cheng et al. (2005) [19] | Single-blinded, placebo-controlled study | N = 27, supplementation group = 14; age and gender-matched controls = 13 | Whole F. lycii (15g/d, equivalent to about 3mg Z) for 28 days | Change in fasting plasma Z levels | Following supplementation, there was 2.5-fold rise in plasma Z levels suggesting that Z in whole F. lycii was bioavailable and that modest daily intake will markedly increases fasting plasma Z levels. |
| Benzie et al. (2006) [22] | Double-blinded, crossover study (3-5 week wash out period) | N = 12, Males = 5, Females = 7 | A Z-standardized dose (15 mg) from freeze dried powder of F. lycii homogenized in 3 forms: warm skimmed milk (40°); hot skimmed milk (80°); hot water (80°, control) | Bioavailability of different formulations of F. lycii | Homogenization of F. lycii in hot skimmed milk resulted in a formulation that has a 3-fold enhanced bioavailability of Z compared with both the ‘classical’ hot water and warm skimmed milk treatment of berries. |
| Bucheli et al. (2011) [20] | Randomized, double masked, placebo controlled study | N = 150, supplementation group = 75, placebo group = 75 | Milk based formulation of F. lycii (equivalent to 10mg/d Z) for 90 days | Effect on plasma Z levels | Daily dietary supplementation of F. lycii increased plasma Z levels by 26% and plasma antioxidant capacity by 57% in elderly subjects aged 65–70 years. |
| Hempel et al. (2017) [14] | Randomized, single-blinded, two-way crossover study | N = 16, Males =8, Females = 8 | Z extracted from F. lycii in two forms: H-aggregated Z and J-aggregated Z (equivalent to 10 mg Z) | Bioavailability of H aggregated versus J-aggregated Z in vivo (humans) and in-vitro (INFOGEST digestion protocol) | Overall, J-aggregated Z showed marginally higher bioavailability (23%) than H-aggregated Z in both humans and in-vitro models but bioaccessibility (micellization rate) were seen higher with H-aggregated Z. Combined effect of aggregation and esterification represents a small magnitude of effect on Z bioavailability in humans. |
| IN-VITRO STUDIES | |||||
| Hempel et al. (2017) [13] | In-vitro study | In-vitro digestion model | Fresh (both unripe and fully ripe) and dried berry fruits of F. lycii | Ultrastructure of F. lycii during fruit ripening; carotenoid profiling of unripe and ripe berry fruits; in-vitro bioaccessibility of xanthophyll carotenoids in dried F. lycii versus fresh spinach | There was ripening induced modification in carotenoid profile (high amounts of xanthophyll esters, mainly Z dipalmitate) and deposition (tubular chromoplasts presumably containing liquid-crystalline state of Z). F. lycii might represent a more potent source of xanthophyll carotenoid than green leafy vegetable due to enhanced liberation and bioaccessibility of Z from these berries. |
| Sun et al. (2017) [23] | In-vitro study | F. lycii Infusion | 5 g of F. lycii in 150 mL of F. lycii infusion in hot water at different temperatures (60 °C, 70 °C, 80 °C, 90 °C, 100 °C), for different lengths of time (15, 30, 60, 120, 150, 180 min), and for different infusion times (1, 2, 3, 4 times) | Assessment of bioactive compounds and antioxidant activity of F. lycii infusions | The bioactive compounds and antioxidant activity of F. lycii infusion increased with increased infusion temperature and time but were equivalent with preparation conditions of 100 °C for 1~3 h, 90 °C for 2~3 h and 80 °C for 2.5~3 h. The antioxidant activity was contributed by polyphenols followed by flavonoids, carotenoids and polysaccharides. |
| SNo | Authors (year) | Study Subjects | Administration of F. lycii | Impact on Variables | Conclusion |
|---|---|---|---|---|---|
| A | STUDIES IN ANIMAL MODELS/CELL LINES | ||||
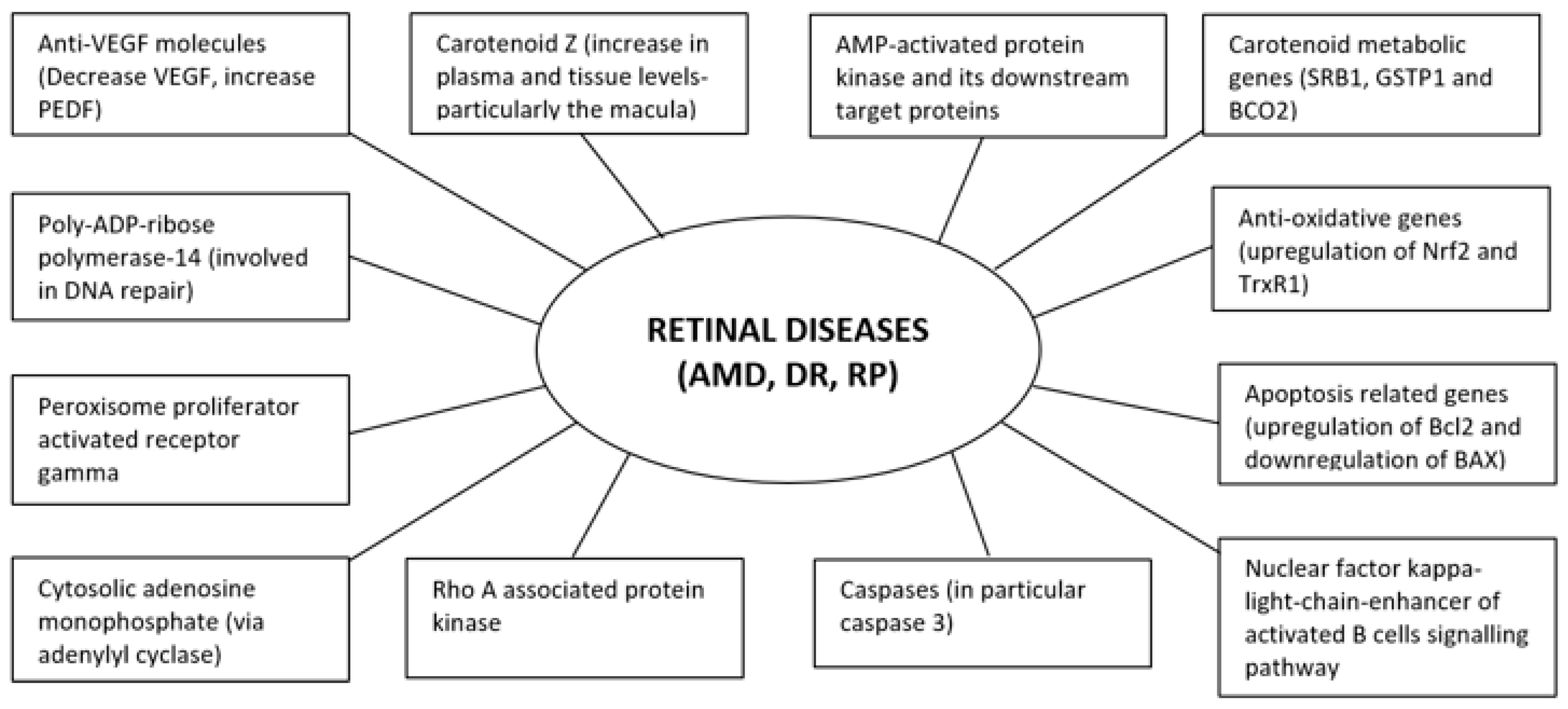
| 1 | Dong et al. (2013) [47] | Human RPE cells (blue light) | Pretreatment with F. lycii in 3 concentrations (0.01 mg/mL, 0.1 mg/mL, 1 mg/mL) | Decreased levels of ROS and apoptotic cells | F. lycii pre-treatment can protect RPE cells against blue light induced damage via inhibiting ROS over generation and subsequent apoptosis in RPE cells. |
| 2 | Du et al. (2013) [48] | Human RPE cells and porcine photoreceptor outer segments | F. lycii in culture medium in 3 concentrations (0.01 mg/mL, 0.1 mg/mL, 1 mg/mL) | (1) RPE cells—increased proliferation and ability to phagocytose photoreceptor outer segments (2) decreased photoreceptor outer segment-induced lipofuscin accumulation in RPE cells | F. lycii treatment enhanced the ability of RPE cells to phagocytose photoreceptor outer segments along with proliferation and removal of lipofuscin. |
| 3 | Liu et al. (2015) [49] | Human RPE cell line (exposed to H2O2) | Pretreatment with different concentrations of F. lycii (0, 10, 50, 100, 500, 1000, 5000 ug/mL) | (1) Prevented loss of cell viability with maximal effect at 500 ug/mL (2) Reduced cell apoptosis (3) Inhibited up-regulation of Bcl-2 and down-regulation of Bax gene | F. lycii protected RPE cells against H2O2-induced acute oxidative stress injury (apoptotic cell death). This is probably attributable to increase in Bcl-2/Bax due to up-regulation of Bcl-2 and down-regulation of Bax expression. |
| 4 | Hsieh et al. (2018) [50] | Human RPE cell line (exposed to UVB) | Pretreatment with aqueous and ethanol extracts of F. lycii (from 0–200 ug/mL for 2 h) | (1) Prevented loss of cell viability (2) Reduced endogenous ROS levels (3) Reduced cell apoptosis (4) Attenuated loss of mitochondrial membrane potential (5) Dose dependent protection against DNA damage as measured by γH2AX levels (6) Prevented G2/M-arrest | F. lycii demonstrated protective effect on oxidative-induced apoptosis of RPE cells exerted via antioxidant property and protective activity on growth arrest as well as DNA damage. Ethanol extract showed stronger antioxidant effect when compared to aqueous extract. |
| 5 | Cheng et al. (2018) [41] | Rats, Male Sprague-Dawley (exposed to white light) | Diet supplemented with F. lycii 250 mg/kg for 54 days, submicron (particle size = 100 ± 70 nm) or blended (particle size = 3.58 ± 3.8 µm) type | (1) Maintained outer nuclear layer thickness (2) Preserved a and b waves on ERG, decreased MDA levels, higher total glutathione levels | F. lycii has protective effect on light induced retinal degeneration via its antioxidant property as evident by lower MDA and higher total glutathione levels. Submicron type provided better protection than blended type probably due to improvement in pharmacokinetics. |
| 6 | Tang et al. (2018) [51] | Mice, BALB/cJ (exposed to white light) | F. lycii (150 mg/kg, low dose, 300 mg/kg, high dose) once per day for 7 days | (1) Attenuated cell nuclei loss in outer nuclear layer (2) Ameliorated light induced damage in the form of decrease ROS production and increased rhodopsin (3) Prevented decrease in a- and b- wave amplitudes (6) Increased mRNA levels of TrxR1 and Nrf2 and decreased mRNA levels of PARP14 | F. lycii protected photoreceptor cells against light-induced retinal damage probably due to decrease ROS production and up-regulation of anti-oxidative genes (Nrf2 and TrxR1). The resultant decrease in oxidative stress leads to reduction in mitochondrial damage and in apoptosis of photoreceptors. |
| B | STUDIES IN HUMAN SUBJECTS | ||||
| 7 | Bucheli et al. (2011) [20] | 150 healthy subjects (65–70 years) | F. lycii (milk based formulation, 13.7 g/d) for 90 days, Placebo-controlled, double-masked, randomized | Subjects with F. lycii supplementation demonstrated: (1) Decreased hypopigmentation and soft drusen accumulation in macula (2) Increased plasma Z levels by 26% (3) Increased antioxidant capacity by 57% | F. lycii supplementation was associated with prevention of early AMD features, such as macular hypopigmentation and soft drusen accumulation, due to its antioxidant activity. |
| 8 | Vidal et al. (2014) [52] | 150 healthy subjects (65–70 years) | F. lycii (milk based formulation, 13.7 g/d) for 90 days, Placebo-controlled, double-masked, randomized | Subjects with F. lycii supplementation demonstrated: (1) Increased plasma anti-oxidant capacity (2) Higher IgG antibody response, sero-conversion and protection rates following influenza vaccine (3) Improved syndrome of Yin deficiency | F. lycii supplementation reinforced immune defenses in elderly subjects, probably attributable to its antioxidant property, thus decreasing the likelihood of developing AMD. |
| 9 | Li et al. (2018) [53] | 114 subjects with AMD (51 to 92 years) | F. lycii supplementation (25 g/day for 90 days) Prospective, randomized controlled trial | AMD subjects with F. lycii supplementation demonstrated (1) Three-fold increased serum Z but not lutein (2) Increased macular pigment optical density (3) significant increased best corrected visual acuity | F. lycii supplementation increased serum Z, macular pigment as well as visual function (visual acuity) in patients with early AMD without causing any detectable adverse effects. |
| SNo | Authors (year) | Experimental Model | F. lycii Administration | Impact on Study Variables | Conclusion |
|---|---|---|---|---|---|
| A ANIMAL MODELS | |||||
| 1 | Tang et al. (2011) [62] | Mice (Spontaneous diabetes) | F. lycii diet 1% (Kcal), duration = 8 weeks | Structural: maintained thickness of whole retina and integrity of RPE and ganglion cells Biochemical: decreased ER stress biomarkers-BiP, PERK, ATF6 and caspase 12, restored AMPK & FOX03α; increased antioxidant enzymes (thioredoxin & MnSOD) | F. lycii ameliorated retinal structure abnormalities at early stage of diabetes by mitigating cellular oxidative stress and/or ER stress, at least partly due to carotenoid component Z. |
| 2 | Hu et al. (2012) [63] | Rats (Streptozotocin induced DM) | F. lycii (Dried powder decocted in distilled water) 5 g/kg/d orally by gavage, duration = 8 weeks | Structural: maintained thickness of retina, absent abnormal vascular tufts and cavities in photoreceptor segment Functional: reversal of reduction in a- and b-wave amplitude | F. lycii exhibited protective effect against degenerative and apoptotic changes in the retina as well as loss of retinal function secondary to diabetes. |
| 3 | Guo et al. (2013) [64] | Rats (Streptozotocin induced DM) | F. lycii (gastrogavage), duration = 24 weeks | Structural: decreased pathological changes in mitochondria, reduced neural cell apoptosis and cell degeneration (changes limited to inner nuclear layer if present) | F. lycii decreased pathological changes in mitochondria as well as apoptosis of neurons and associated glial cells in diabetic retina. |
| 4 | Yu et al. (2013) [68] | Mice (Spontaneous diabetes) | F. lycii diet, duration = 8 weeks | Structural (RPE): Ameliorated mitochondrial dispersion along with relocation of mitochondria, increased pigment granules Biochemical: Activated AMPK-α2 in mitochondria and nuclei, increased expression of carotenoid metabolic genes (SRB1, GSTP1, BCO2), mitochondrial biogenesis (PGC-1α, NRF1 &TFAM), decreased cell stress responses (HIF-1α &VEGF -hypoxia related; HSP60-mitochondrial stress related). | F. lycii up-regulated carotenoid metabolic genes (via activation of AMPK-α2) along with enhanced mitochondrial biogenesis and reduced cellular stress responses. This resulted in reversal of mitochondrial dysfunction with consequential neuroprotection of the diabetic retina. |
| 5 | Li et al. (2014) [69] | Rats (Streptozotocin induced DM) | F. lycii (Intragastric administration, 250 mg/kg) | Structural: Decreased abnormality in shape and diameter as well leakage of retinal vessels, as suggested by lowered Evan blue content in retina Biochemical: decreased levels of VEGF expression. | F. lycii alleviated DM-induced retinal vasculopathy via protection of blood retinal barrier resulting in decreased vascular leakage of diabetic retina. |
| 6 | Wang et al. (2017) [70] | Rats (Streptozotocin induced DM) | F. lycii (Intragastric administration, 0.5 mL 6% once a day), duration = 24 weeks, Placebo-controlled | Structural: Prevented pathological changes in photoreceptors and ganglion cells, reduced mitochondrial changes in bipolar and Müller cells (shorter and reduced cristae) Biochemical: higher SOD activity, reduced MDA expression level, reduced VEGF mRNA and protein levels. | F. lycii alleviated DM-induced retinal neuropathy by reducing oxidative damage to mitochondrial pathway (particularly in bipolar and Muller cells) through its anti-oxidative effect. Consequently, there was reduction in apoptosis of neural tissue in diabetic retina. |
| 7 | Yao et al. (2018) [65] | Rats (Streptozotocin induced DM) | F. lycii (oral administration of 400 mg/kg/d or 200 mg/kg/d), duration = 20 weeks | Structural: increased retinal thickness, less disorganization of photoreceptor segments, reduced numbers of pyknotic nuclei, morphologically normal retinal capillaries (reduced thickness of basement membrane, increased vessel lumen, close attachment to endothelial cells) Functional: blunting of decreased amplitude of a-wave, b-wave, and oscillatory potentials, improved blood flow in CRV Biochemical: reversed of diabetes induced elevation of VEGF and GFAP and suppression of PEDF. | F. lycii protected retinal function and morphology of diabetic retina probably through reinstallation of the balance between angiogenic (GFAP and VEGF) and anti-angiogenic (PEDF) factors indicating that F. lycii may be a potential therapeutic agent for DR. |
| 8 | Wang et al. (2019) [71] | Rats (Streptozotocin induced DM) | F. lycii (250 mg/kg/day by oral gavage), duration = 12 weeks | Structural: prevented changes in overall retinal thickness, decreased structure disturbance of photoreceptor membranous disks, less twisted capillaries, improved cell morphology Biochemical: increased expression of P-occludin and decreased expression of ROCK1, P-MLC, VEGF | F. lycii demonstrated protective effects on blood-retinal barrier in diabetic rats by regulating the Rho/ROCK1 signaling pathway. |
| B HUMAN CELL LINES | |||||
| 9 | Song et al. (2011) [72] | Retinal ARPE-19 cell line incubated in high glucose | F. lycii extraction (methanol, ethanol, water, methanol/water) | Biochemical: Enhanced PPAR-γ luciferase activity along with PPAR-γ mRNA and protein expression (dose dependent effect), down-regulated the mRNA of pro-inflammatory mediators encoding MMP-9, fibronectin and protein expression of COX-2 and iNOS. | F. lycii extract and its associated taurine content enhanced PPAR-γ activation in retinal cells, providing a rationale for its use in prevention of DR. Methanol extract showed highest PPAR-γ activation when compared to other extracts. |
| 10 | Song et al. (2012) [73] | Retinal ARPE-19 cell line exposed to high glucose | F. lycii extract rich in taurine | Structural: Enhanced cell viability and decreased number of apoptotic cell Functional: attenuated high glucose induced apoptosis Biochemical: down-regulated caspase-3 protein expression and caspase-3 enzymatic activity. | F. lycii extract rich in taurine exhibited a cytoprotective effect against glucose exposure in a human RPE cell line in a dose dependent manner, probably due to reversal of caspase-dependent apoptotic cytotoxic pathway. |
| 11 | Pavan et al. (2014) [74] | Human RPE cells treated with 25mM glucose | Methanolic extract from F. lycii | Functional changes: Reversed decrease in trans epithelial electrical resistance, increased activity of cytosolic adenylyl cyclase Biochemical: increased intracellular cAMP levels | High glucose-induced barrier impairment of human RPE was ameliorated by treatment with F. lycii extracts through modulation of cAMP levels. |
| SNo | Authors (year) | Animal Model | F. lycii Administration | Impact on Variables | Conclusion |
|---|---|---|---|---|---|
| A | STUDIES IN ANIMAL MODELS/CELL LINES | ||||
| 1 | Wang et al. (2014) [102] | Mice (Wild-type rd10) | F. lycii (1 mg/kg, oral administration) post natal day 14, 25, 29 and 41 | Structural: preserved outer retinal thickness and morphology of rods and cones, maintained ramified shape of microglial cells Functional: decreased latency and increased amplitude of b wave (photopic), larger scotopic a and b waves, improved visual behavior Biochemical: decreased levels of TNFα, IL-6β, CCL2, HIF-1α, activated caspase 3/7 and Bax, increased GSH/GSSG ratio | F. lycii exhibited long term neuroprotective effect on morphology and function of photoreceptors that was exerted through multiple pathways, such as antioxidation, antiinflammation and anti-apoptosis. |
| 2 | Zhu et al. (2016) [103] | Rats (Male Sprague-Dawley), N-Methyl-N-nitrosourea injection of 60 mg/kg | F. lycii (100, 200 and 400 mg/kg, gastric gavage) for 8 days or 14 days | Structural: Improved arrangement of outer nuclear and photoreceptor cell layer, maintained outer retinal thickness of central retina, decreased apoptotic cell ratio Biochemical: significant down-regulation of 9,3,7 of pro and cleaved caspases, increased nuclear levels of PARP and cleaved PARP protein | F. lycii attenuated methyl-N-nitrosourea induced photoreceptor cell apoptosis and protected retinal structure through regulation of PARP and caspase expression. |
| 3 | Liu et al. (2018) [101] | Mice (Wild-type rd1) | F. lycii (10 mg/kg, Intra-peritoneal injection) post natal day 4 to day 14, 20 or 24 | Structural: Improved photoreceptor survival, restored morphology of rod and cone bipolar cells Functional: enhanced light evoked responses (rate, sensitivity and speed) of ganglion cells, increased b-wave amplitude, decreased abnormally high spontaneous spiking, protected ON pathway at early stages and OF pathway at later stages, improved visual behavior | F. lycii improved visual processing at multiple stages of information transmission via protecting morphology and function of photoreceptor and bipolar cells, function of ganglion cells, and perhaps, function of higher brain centers as suggested by enhanced visual behavior. |
| B | STUDIES IN HUMAN SUBJECTS | ||||
| 4 | Chan et al. (2019) [104] | Human subjects (n = 42) Double masked, placebo controlled | Oral administration of F. lycii in the form of granules (2 packs per day, each containing 5 g net wt) for 12 months | Structural: Preserved macular thickness Functional: Absence of deterioration of VA, both high contrast (90%) as well as low contrast (10%), no significant difference in visual field sensitivity or in any parameter of full field elctroretinogram | There was preservation of VA and macular structure following F. lycii supplementation over 12 month period, which is probably attributable to delay or minimization of cone degeneration due to neuroprotective effect of F. lycii on the retina. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neelam, K.; Dey, S.; Sim, R.; Lee, J.; Au Eong, K.-G. Fructus lycii: A Natural Dietary Supplement for Amelioration of Retinal Diseases. Nutrients 2021, 13, 246. https://doi.org/10.3390/nu13010246
Neelam K, Dey S, Sim R, Lee J, Au Eong K-G. Fructus lycii: A Natural Dietary Supplement for Amelioration of Retinal Diseases. Nutrients. 2021; 13(1):246. https://doi.org/10.3390/nu13010246
Chicago/Turabian StyleNeelam, Kumari, Sonali Dey, Ralene Sim, Jason Lee, and Kah-Guan Au Eong. 2021. "Fructus lycii: A Natural Dietary Supplement for Amelioration of Retinal Diseases" Nutrients 13, no. 1: 246. https://doi.org/10.3390/nu13010246
APA StyleNeelam, K., Dey, S., Sim, R., Lee, J., & Au Eong, K.-G. (2021). Fructus lycii: A Natural Dietary Supplement for Amelioration of Retinal Diseases. Nutrients, 13(1), 246. https://doi.org/10.3390/nu13010246





