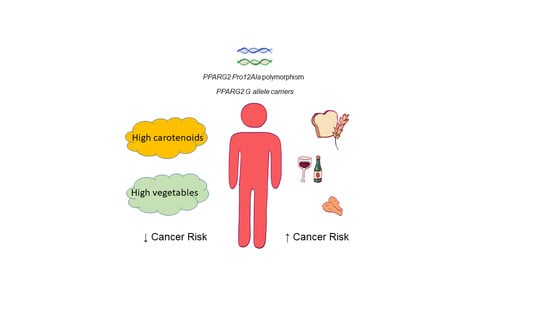
Diet and PPARG2 Pro12Ala Polymorphism Interactions in Relation to Cancer Risk: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Registration
2.2. Search Strategy
2.3. Study Inclusion and Exclusion Criteria
2.4. Data Extraction
2.5. Risk of Bias Assessment
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Blunder, M.; Liu, X.; Malainer, C.; Blazevic, T.; Schwaiger, S.; Rollinger, J.M.; Heiss, E.H.; et al. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARgamma): A review. Biochem. Pharmacol. 2014, 92, 73–89. [Google Scholar] [CrossRef]
- Kroker, A.J.; Bruning, J.B. Review of the Structural and Dynamic Mechanisms of PPARgamma Partial Agonism. PPAR Res. 2015, 2015, 816856. [Google Scholar] [CrossRef]
- Michalik, L.; Desvergne, B.; Wahli, W. Peroxisome-proliferator-activated receptors and cancers: Complex stories. Nat. Rev. Cancer 2004, 4, 61–70. [Google Scholar] [CrossRef]
- Peters, J.M.; Shah, Y.M.; Gonzalez, F.J. The role of peroxisome proliferator-activated receptors in carcinogenesis and chemoprevention. Nat. Rev. Cancer 2012, 12, 181–195. [Google Scholar] [CrossRef]
- Janani, C.; Ranjitha Kumari, B.D. PPAR gamma gene—A review. Diabetes Metab. Syndr. 2015, 9, 46–50. [Google Scholar] [CrossRef]
- Vidal-Puig, A.J.; Considine, R.V.; Jimenez-Linan, M.; Werman, A.; Pories, W.J.; Caro, J.F.; Flier, J.S. Peroxisome proliferator-activated receptor gene expression in human tissues. Effects of obesity, weight loss, and regulation by insulin and glucocorticoids. J. Clin. Investig. 1997, 99, 2416–2422. [Google Scholar] [CrossRef] [PubMed]
- Penumetcha, M.; Santanam, N. Nutraceuticals as Ligands of PPARgamma. PPAR Res. 2012, 2012, 858352. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Gupta, P.; Saini, A.S.; Kaushal, C.; Sharma, S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Rizos, C.V.; Elisaf, M.S.; Mikhailidis, D.P.; Liberopoulos, E.N. How safe is the use of thiazolidinediones in clinical practice? Expert Opin. Drug Saf. 2009, 8, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Ortuno Sahagun, D.; Marquez-Aguirre, A.L.; Quintero-Fabian, S.; Lopez-Roa, R.I.; Rojas-Mayorquin, A.E. Modulation of PPAR-gamma by Nutraceutics as Complementary Treatment for Obesity-Related Disorders and Inflammatory Diseases. PPAR Res. 2012, 2012, 318613. [Google Scholar] [CrossRef]
- Willson, T.M.; Lambert, M.H.; Kliewer, S.A. Peroxisome proliferator-activated receptor gamma and metabolic disease. Annu. Rev. Biochem. 2001, 70, 341–367. [Google Scholar] [CrossRef] [PubMed]
- Robbins, G.T.; Nie, D. PPAR gamma, bioactive lipids, and cancer progression. Front. Biosci. 2012, 17, 1816–1834. [Google Scholar] [CrossRef] [PubMed]
- Stumvoll, M.; Haring, H. The peroxisome proliferator-activated receptor-gamma2 Pro12Ala polymorphism. Diabetes 2002, 51, 2341–2347. [Google Scholar] [CrossRef]
- Wang, W.; Shao, Y.; Tang, S.; Cheng, X.; Lian, H.; Qin, C. Peroxisome proliferator-activated receptor-gamma (PPARgamma) Pro12Ala polymorphism and colorectal cancer (CRC) risk. Int. J. Clin. Exp. Med. 2015, 8, 4066–4072. [Google Scholar] [PubMed]
- Tang, W.; Chen, Y.; Wang, Y.; Gu, H.; Chen, S.; Kang, M. Peroxisome proliferator-activated receptor gamma (PPARG) polymorphisms and breast cancer susceptibility: A meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 12226–12238. [Google Scholar]
- Mansoori, A.; Amini, M.; Kolahdooz, F.; Seyedrezazadeh, E. Obesity and Pro12Ala Polymorphism of Peroxisome Proliferator-Activated Receptor-Gamma Gene in Healthy Adults: A Systematic Review and Meta-Analysis. Ann. Nutr. Metab. 2015, 67, 104–118. [Google Scholar] [CrossRef]
- Zhao, J.; Zhi, Z.; Song, G.; Wang, J.; Wang, C.; Ma, H.; Yu, X.; Sui, A.; Zhang, H. Peroxisome proliferator-activated receptor-gamma Pro12Ala polymorphism could be a risk factor for gastric cancer. Asian Pac. J. Cancer Prev. 2015, 16, 2333–2340. [Google Scholar] [CrossRef]
- Ruiz-Narvaez, E.A.; Kraft, P.; Campos, H. Ala12 variant of the peroxisome proliferator-activated receptor-gamma gene (PPARG) is associated with higher polyunsaturated fat in adipose tissue and attenuates the protective effect of polyunsaturated fat intake on the risk of myocardial infarction. Am. J. Clin. Nutr. 2007, 86, 1238–1242. [Google Scholar] [CrossRef]
- Memisoglu, A.; Hu, F.B.; Hankinson, S.E.; Manson, J.E.; De Vivo, I.; Willett, W.C.; Hunter, D.J. Interaction between a peroxisome proliferator-activated receptor gamma gene polymorphism and dietary fat intake in relation to body mass. Hum. Mol. Genet. 2003, 12, 2923–2929. [Google Scholar] [CrossRef]
- Zeggini, E.; Weedon, M.N.; Lindgren, C.M.; Frayling, T.M.; Elliott, K.S.; Lango, H.; Timpson, N.J.; Perry, J.R.; Rayner, N.W.; Freathy, R.M.; et al. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 2007, 316, 1336–1341. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.S.B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 12 December 2020).
- Murtaugh, M.A.; Ma, K.N.; Caan, B.J.; Sweeney, C.; Wolff, R.; Samowitz, W.S.; Potter, J.D.; Slattery, M.L. Interactions of peroxisome proliferator-activated receptor {gamma} and diet in etiology of colorectal cancer. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L.; Murtaugh, M.A.; Sweeney, C.; Ma, K.N.; Potter, J.D.; Caan, B.J.; Samowitz, W. PPARgamma, energy balance, and associations with colon and rectal cancer. Nutr. Cancer 2005, 51, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Gajalakshmi, V.; Wang, J.; Kuriki, K.; Suzuki, S.; Nakamura, S.; Akasaka, S.; Ishikawa, H.; Tokudome, S. Influence of the C161T but not Pro12Ala polymorphism in the peroxisome proliferator-activated receptor-gamma on colorectal cancer in an Indian population. Cancer Sci. 2005, 96, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Landi, S.; Moreno, V.; Gioia-Patricola, L.; Guino, E.; Navarro, M.; de Oca, J.; Capella, G.; Canzian, F.; Bellvitge Colorectal Cancer Study, G. Association of common polymorphisms in inflammatory genes interleukin (IL)6, IL8, tumor necrosis factor alpha, NFKB1, and peroxisome proliferator-activated receptor gamma with colorectal cancer. Cancer Res. 2003, 63, 3560–3566. [Google Scholar]
- Petersen, R.K.; Larsen, S.B.; Jensen, D.M.; Christensen, J.; Olsen, A.; Loft, S.; Nellemann, C.; Overvad, K.; Kristiansen, K.; Tjonneland, A.; et al. PPARgamma-PGC-1alpha activity is determinant of alcohol related breast cancer. Cancer Lett. 2012, 315, 59–68. [Google Scholar] [CrossRef]
- Paltoo, D.; Woodson, K.; Taylor, P.; Albanes, D.; Virtamo, J.; Tangrea, J. Pro12Ala polymorphism in the peroxisome proliferator-activated receptor-gamma (PPAR-gamma) gene and risk of prostate cancer among men in a large cancer prevention study. Cancer Lett. 2003, 191, 67–74. [Google Scholar] [CrossRef]
- Vogel, U.; Christensen, J.; Dybdahl, M.; Friis, S.; Hansen, R.D.; Wallin, H.; Nexo, B.A.; Raaschou-Nielsen, O.; Andersen, P.S.; Overvad, K.; et al. Prospective study of interaction between alcohol, NSAID use and polymorphisms in genes involved in the inflammatory response in relation to risk of colorectal cancer. Mutat. Res. 2007, 624, 88–100. [Google Scholar] [CrossRef]
- Kuriki, K.; Hirose, K.; Matsuo, K.; Wakai, K.; Ito, H.; Kanemitsu, Y.; Hirai, T.; Kato, T.; Hamajima, N.; Takezaki, T.; et al. Meat, milk, saturated fatty acids, the Pro12Ala and C161T polymorphisms of the PPARgamma gene and colorectal cancer risk in Japanese. Cancer Sci. 2006, 97, 1226–1235. [Google Scholar] [CrossRef]
- Kim, N.H.; Seol, J.E.; Kim, J.; Lee, B.H.; Hwang, D.Y.; Jeong, J.; Lee, H.J.; Ahn, Y.O.; Kim, D.H.; Lee, J.E. Red meat intake, CYP2E1 and PPARgamma polymorphisms, and colorectal cancer risk. Eur. J. Cancer Prev. 2019, 28, 304–310. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Q.; Yin, Y.; Yang, Z.; Li, W.; Liang, D.; Zhou, P. Association between Peroxisome Proliferator-activated Receptor Gamma Gene Polymorphisms and Atherosclerotic Diseases: A Meta-analysis of Case-control Studies. J. Atheroscler. Thromb. 2015, 22, 912–925. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Fan, X.; Tan, K.; Zhang, L.; Jian, L.; Yu, L. Peroxisome proliferators-activated receptor gamma polymorphisms and colorectal cancer risk. J. Cancer Res. Ther. 2018, 14, S306–S310. [Google Scholar] [CrossRef] [PubMed]
- Ryan-Harshman, M.; Aldoori, W. Diet and colorectal cancer: Review of the evidence. Can. Fam. Physician 2007, 53, 1913–1920. [Google Scholar] [PubMed]
- La Vecchia, C.; Ferraroni, M.; Mezzetti, M.; Enard, L.; Negri, E.; Franceschi, S.; Decarli, A. Attributable risks for colorectal cancer in northern Italy. Int. J. Cancer 1996, 66, 60–64. [Google Scholar] [CrossRef]
- The Human Protein Altas. Tissue Expression of PPARG- Summary—The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000132170-PPARG/tissue (accessed on 12 December 2020).
- Uhlen, M.; Fagerberg, L.; Hallstrom, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Adachi, M.; Kurotani, R.; Morimura, K.; Shah, Y.; Sanford, M.; Madison, B.B.; Gumucio, D.L.; Marin, H.E.; Peters, J.M.; Young, H.A.; et al. Peroxisome proliferator activated receptor gamma in colonic epithelial cells protects against experimental inflammatory bowel disease. Gut 2006, 55, 1104–1113. [Google Scholar] [CrossRef]
- Salgia, M.M.; Elix, C.C.; Pal, S.K.; Jones, J.O. Different roles of peroxisome proliferator-activated receptor gamma isoforms in prostate cancer. Am. J. Clin. Exp. Urol. 2019, 7, 98–109. [Google Scholar]
- Discacciati, A.; Orsini, N.; Wolk, A. Body mass index and incidence of localized and advanced prostate cancer—A dose-response meta-analysis of prospective studies. Ann. Oncol. 2012, 23, 1665–1671. [Google Scholar] [CrossRef]
- Nakles, R.E.; Kallakury, B.V.; Furth, P.A. The PPARgamma agonist efatutazone increases the spectrum of well-differentiated mammary cancer subtypes initiated by loss of full-length BRCA1 in association with TP53 haploinsufficiency. Am. J. Pathol. 2013, 182, 1976–1985. [Google Scholar] [CrossRef]
- Dong, J.T. Anticancer activities of PPARgamma in breast cancer are context-dependent. Am. J. Pathol. 2013, 182, 1972–1975. [Google Scholar] [CrossRef]
- Zaytseva, Y.Y.; Wallis, N.K.; Southard, R.C.; Kilgore, M.W. The PPARgamma antagonist T0070907 suppresses breast cancer cell proliferation and motility via both PPARgamma-dependent and -independent mechanisms. Anticancer. Res. 2011, 31, 813–823. [Google Scholar] [PubMed]
- Jiang, W.G.; Douglas-Jones, A.; Mansel, R.E. Expression of peroxisome-proliferator activated receptor-gamma (PPARgamma) and the PPARgamma co-activator, PGC-1, in human breast cancer correlates with clinical outcomes. Int. J. Cancer 2003, 106, 752–757. [Google Scholar] [CrossRef] [PubMed]

| Cancer Site | Study Design * | First Author/Publication Year | Study Location | Age ** | Race | Cases/Controls | Women (%) | Modifiable Factors | Case/CC | Case/CG | Case /GG | Control/CC | Control/CG | Control /GG |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Colon/rectum | N CC | Vogel 2007 [29] | Denmark | 59/56 | NR | 355/753 | 44% | Alcohol | 252 | 96 | 7 | 550 | 190 | 13 |
| Colon/rectum | PB CC | Murtaugh 2005 [23] and Slattery 2005 [24] | USA | 30–79 | NH White, Hispanic, Black, Asian, and Native American | 1577/1971 (colon) 794/1001 (rectum) | 45% (colon) 42% (rectum) | Dietary fats, energy, dietary antioxidants and food items ≠ | 1234 606 | 343 188 | 1493 790 | 478 211 | ||
| Colon/rectum | HB CC | Landi 2003 [26] | Spain | <58–>75 | NR | 377/326 | 43% | Vitamin A | 311 | 46 | 3 | 243 | 61 | 5 |
| Colon/rectum | HB CC | Jiang 2005 [25] | India | 20–75 | Asian | 301/291 | 36% | Fish intake | 240 | 57 | 4 | 230 | 57 | 4 |
| Colon/rectum | HB CC | Kuriki 2006 [30] | Japan | 57.9 (study 1) 58.9 (study 2) | NR | 128/238 (study 1) 257/771 (study 2) | 48% (study 1) 37% (study 2) | Meat, milk, and other food items ≠ | 120 248 | 7 9 | 221 732 | 17 37 | ||
| Colon/rectum | HB CC | Kim 2018 [31] | Korea | 58.2 | Asian | 971/658 | 44% | Red meat intake | 886 | 82 | 3 | 607 | 51 | 0 |
| Prostate | N CC | Paltoo 2003 [28] | Finland | 60.5 | NR | 193/188 | 0% | Dietary fat | 121 | 64 | 8 | 128 | 54 | 6 |
| Breast | N CC | Peterson 2012 [27] | Denmark | 57/57 | NR | 798/798 | 100% | Alcohol | 616 | 167 | 15 | 569 | 209 | 20 |
| OR/IRR (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|
| PPARG2 Pro12Ala Allele Polymorphism | ||||||||
| First Author/Publication Year | Age | Women (%) | Study Location | Cancer Site | Stratified Categories | CC | CG + GG | p-Value for Interaction |
| Petersen 2012 [27] | 57/57 | 100% | Denmark | Breast cancer | Alcohol intake | |||
| Per 10 g alcohol/day | 1.13 (1.04–1.23) | 0.95 (0.83–1.08) | 0.02 | |||||
| Vogel 2007 [29] | 59/56 | 44% | Denmark | Colorectal cancer | Alcohol intake | |||
| Per 10 g alcohol/day | 1.03 (0.96–1.10) | 1.22 (1.07–1.39) | 0.02 | |||||
| Jiang 2005 [25] | 20–75 | 36% | India | Colorectal cancer | Fish intake | |||
| Low | Reference | 1.14 (0.72–1.81) | 0.36 | |||||
| High | 0.74 (0.46–1.18) | 0.51 (0.20–1.27) | ||||||
| Kuriki 2006 [30] (study 2) | 58.9 | 37% | Japan | Colorectal cancer | Fish | |||
| Low | Reference | 0.69 (0.23–2.10) | 0.49 | |||||
| Middle | 0.93 (0.66–1.29) | 1.09 (0.34–3.47) | ||||||
| High | 0.91 (0.61–1.34) | 0.34 (0.04–2.81) | ||||||
| Fried foods | ||||||||
| Low | Reference | 0.23 (0.03–1.82) | 0.01 | |||||
| Middle | 0.94 (0.65–1.34) | 0.20 (0.03–1.56) | ||||||
| High | 0.77 (0.51–1.16) | 2.04 (0.74–5.63) | ||||||
| Deep-fried foods | ||||||||
| Low | Reference | 0.45 (0.10–2.05) | 0.17 | |||||
| Middle | 0.81 (0.59–1.13) | 0.59 (0.19–1.77) | ||||||
| High | 0.68 (0.44–1.03) | 1.33 (0.32–5.49) | ||||||
| OR (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|
| PPARG2 Pro12Ala Allele Polymorphism | ||||||||
| First Author/Publication Year | Age (Range) | Women (%) | Study Location | Stratified Categories | CC | CG + GG | CC | CG + GG |
| Landi 2003 [26] | <58–>75 | 43% | Spain | Colorectal Cancer | ||||
| Vitamin A intake | ||||||||
| Low | Reference | 0.25 (0.12–0.53) | ||||||
| High | Reference | 1.08 (0.53–2.20) * | ||||||
| Murtaugh 2005 [23] | 30–79 | 45% (colon) | USA | Colon Cancer | Rectal Cancer | |||
| 42% (rectum) | Beta-Carotene | |||||||
| High | Reference | 0.71 (0.52–0.96) | Reference | 1.08 (0.73–1.61) | ||||
| Middle | 0.94 (0.77–1.16) | 0.85 (0.65–1.13) | 1.03 (0.77–1.37) | 0.43 (0.94–2.18) | ||||
| Low | 0.82 (0.65–1.02) | 0.82 (0.61–1.10) | 1.15 (0.83–1.58) | 1.28 (0.83–1.97) | ||||
| Lutein | ||||||||
| High | Reference | 0.63 (0.44–0.89) | Reference | 1.06 (0.72–1.57) | ||||
| Middle | 0.89 (0.72–1.10) | 0.82 (0.61–1.10) | 0.95 (0.72–1.27) | 1.19 (0.79–1.79) | ||||
| Low | 0.90 (0.71–1.13) | 0.90 (0.71–1.13) * | 0.90 (0.64–1.25) | 1.15 (0.73–1.79) | ||||
| Lycopene | ||||||||
| High | Reference | 0.75 (0.52–1.06) | Reference | 0.89 (0.61–1.30) | ||||
| Middle | 1.02 (0.82–1.27) | 0.96 (0.72–1.29) | 0.81 (0.61–1.08) | 1.27 (0.85–1.92) | ||||
| Low | 1.00 (0.80–1.26) | 0.89 (0.67–1.19) | 1.10 (0.81–1.48) | 1.37 (0.89–2.09) | ||||
| Vitamin C | ||||||||
| High | Reference | 0.79 (0.58–1.06) | Reference | 1.33 (0.90–1.97) | ||||
| Middle | 1.05 (0.86–1.28) | 0.90 (0.68–1.19) | 1.19 (0.89–1.59) | 1.31 (0.87–1.98) | ||||
| Low | 0.88 (0.70–1.10) | 0.86 (0.64–1.15) | 1.28 (0.92–1.77) | 1.46 (0.94–2.27) | ||||
| Total tocopherol | ||||||||
| High | Reference | 0.96 (0.71–1.30) | Reference | 1.02 (0.72–1.42) | ||||
| Middle | 1.02 (0.83–1.26) | 0.85 (0.63–1.15) | 0.96 (0.71–1.29) | 1.36 (0.92–2.03) | ||||
| Low | 0.96 (0.75–1.23) | 0.82 (0.60–1.12) | 1.21 (0.85–1.73) | 1.55 (0.92–2.64) | ||||
| OR (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|
| PPARG2 Pro12Ala Allele Polymorphism | ||||||||
| First Author/Publication Year | Age (Range) | Women (%) | Study Location | Stratified Categories | CC | CG + GG | CC | CG + GG |
| Murtaugh 2005 [23] | 30–79 | 45% (colon) 42% (rectum) | USA | Colon Cancer | Rectal Cancer | |||
| Western dietary pattern | ||||||||
| Low | Reference | 0.71 (0.52–0.96) | Reference | 1.40 (0.93–2.10) | ||||
| Middle | 1.22 (1.00–1.49) | 1.17 (0.89–1.54) | 0.98 (0.74–1.30) | 1.19 (0.79–1.79) | ||||
| High | 1.27 (1.00–1.62) | 1.18 (0.87–1.63) | 1.13 (0.84–1.52) | 1.17 (0.78–1.75) | ||||
| Prudent dietary pattern | ||||||||
| High | Reference | 0.66 (0.49–0.89) | Reference | 1.29 (0.87–1.92) | ||||
| Middle | 1.00 (0.82–1.23) | 0.92 (0.69–1.22) | 0.99 (0.74–1.34) | 0.95 (0.61–1.47) | ||||
| Low | 1.02 (0.81–1.28) | 1.07 (0.79–1.45) * | 1.07 (0.78–1.45) | 1.36 (0.91–2.04) | ||||
| Vegetables | ||||||||
| High | Reference | 0.72 (0.54–0.96) | Reference | 1.16 (0.79–1.71) | ||||
| Middle | 0.91 (0.75–1.12) | 0.81 (0.61–1.08) | 0.95 (0.71–1.26) | 0.93 (0.62–1.40) | ||||
| Low | 0.94 (0.75–1.17) | 0.96 (0.71–1.30) | 0.87 (0.64–1.19) | 1.28 (0.83–1.97) | ||||
| Refined grain | ||||||||
| Low | Reference | 0.70 (0.53–0.94) | Reference | 1.68 (1.11–2.54) | ||||
| Middle | 1.07 (0.88–1.29) | 0.95 (0.72–1.24) | 1.04 (0.75–1.43) | 1.2 (0.78–1.59) | ||||
| High | 1.08 (0.88–1.33) | 1.17 (0.87–1.58) * | 1.12 (0.83–1.51) | 1.53 (1.03–2.28) | ||||
| Whole grain | ||||||||
| High | Reference | 0.74 (0.57–0.97) | Reference | 1.24 (0.81–1.89) | ||||
| Middle | 0.98 (0.81–1.19) | 0.96 (0.73–1.28) | 1.07 (0.81–1.43) | 1.21 (0.79–1.84) | ||||
| Low | 0.92 (0.74–1.13) | 0.85 (0.63–1.15) | 1.32 (0.93–1.87) | 1.22 (0.78–1.92) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, L.; Bobe, G.; Arani, G.; Zhang, Y.; Zhang, Z.; Shannon, J.; Takata, Y. Diet and PPARG2 Pro12Ala Polymorphism Interactions in Relation to Cancer Risk: A Systematic Review. Nutrients 2021, 13, 261. https://doi.org/10.3390/nu13010261
Tran L, Bobe G, Arani G, Zhang Y, Zhang Z, Shannon J, Takata Y. Diet and PPARG2 Pro12Ala Polymorphism Interactions in Relation to Cancer Risk: A Systematic Review. Nutrients. 2021; 13(1):261. https://doi.org/10.3390/nu13010261
Chicago/Turabian StyleTran, Lieu, Gerd Bobe, Gayatri Arani, Yang Zhang, Zhenzhen Zhang, Jackilen Shannon, and Yumie Takata. 2021. "Diet and PPARG2 Pro12Ala Polymorphism Interactions in Relation to Cancer Risk: A Systematic Review" Nutrients 13, no. 1: 261. https://doi.org/10.3390/nu13010261
APA StyleTran, L., Bobe, G., Arani, G., Zhang, Y., Zhang, Z., Shannon, J., & Takata, Y. (2021). Diet and PPARG2 Pro12Ala Polymorphism Interactions in Relation to Cancer Risk: A Systematic Review. Nutrients, 13(1), 261. https://doi.org/10.3390/nu13010261