Habitual FODMAP Intake in Relation to Symptom Severity and Pattern in Patients with Irritable Bowel Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Questionnaires
2.2.1. IBS Severity Scoring System (IBS-SSS)
2.2.2. The Patient Health Questionnaire (PHQ-15)
2.2.3. Hospital Anxiety and Depression (HAD) scale
2.2.4. Visceral Sensitivity Index (VSI)
2.2.5. Multidimensional Fatigue Inventory-20 (MFI-20)
2.3. Dietary Assessment
2.4. Statistical Analysis
3. Results
3.1. Reported Diet Intake
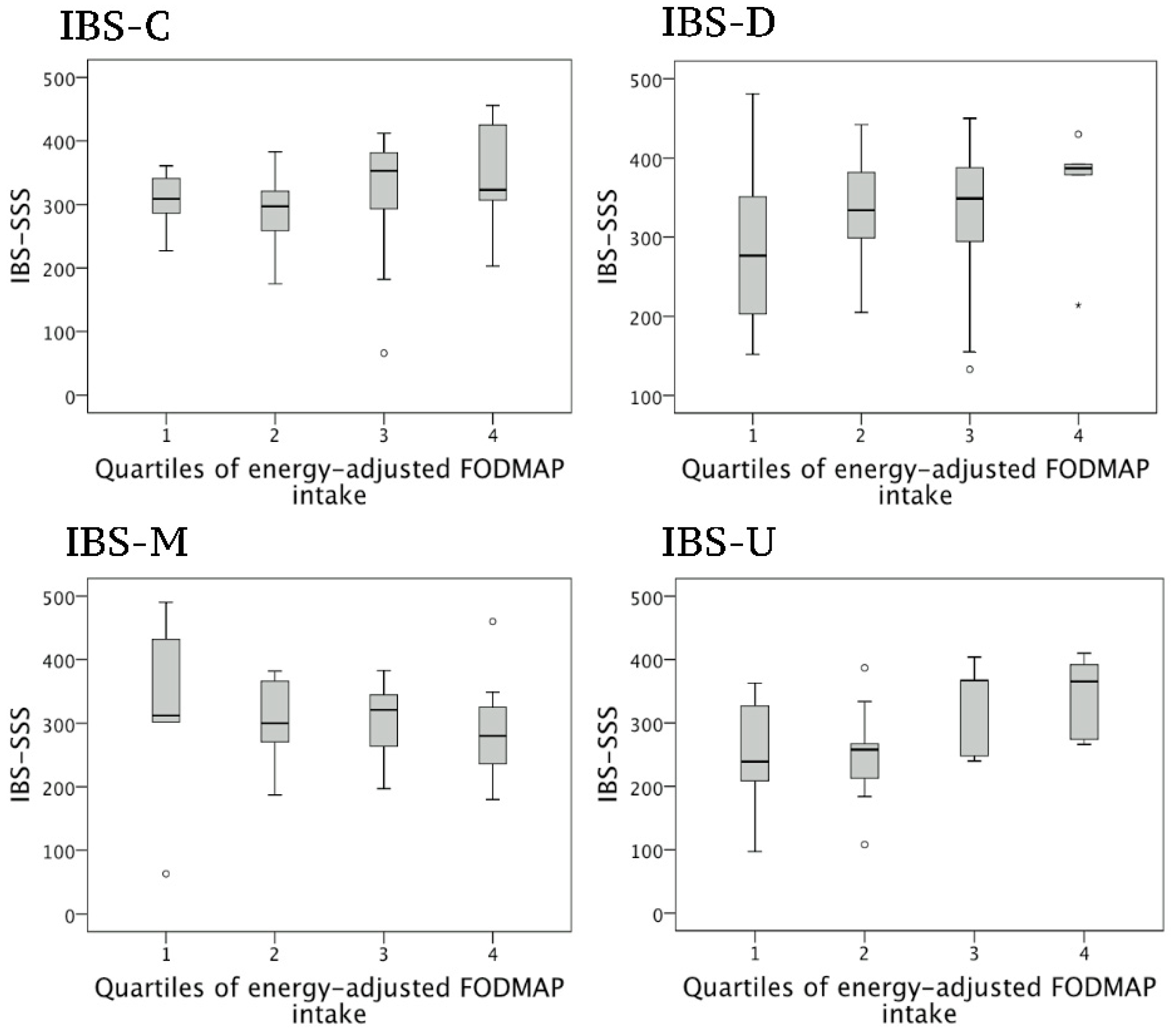
3.2. Correlations between FODMAP Intake and Symptom Severity in Women
3.3. Univariable Regression Analyses
3.4. Multivariable Regression Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Longstreth, G.F.; Thompson, W.G.; Chey, W.D.; Houghton, L.A.; Mearin, F.; Spiller, R.C. Functional bowel disorders. Gastroenterology 2006, 130, 1480–1491. [Google Scholar] [CrossRef] [PubMed]
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome Foundation global study. Gastroenterology 2021, 160, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.L.; Von Korff, M.; Whitehead, W.E.; Stang, P.; Saunders, K.; Jhingran, P.; Barghout, V.; Feld, A.D. Costs of care for irritable bowel syndrome patients in a health maintenance organization. Am. J. Gastroenterol. 2001, 96, 3122–3129. [Google Scholar] [CrossRef] [PubMed]
- Böhn, L.; Störsrud, S.; Törnblom, H.; Bengtsson, U.; Simrén, M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am. J. Gastroenterol. 2013, 108, 634–641. [Google Scholar] [CrossRef]
- Simrén, M.; Månsson, A.; Langkilde, A.M.; Svedlund, J.; Abrahamsson, H.; Bengtsson, U.; Björnsson, E.S. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion 2001, 63, 108–115. [Google Scholar] [CrossRef]
- Monsbakken, K.; Vandvik, P.; Farup, P. Perceived food intolerance in subjects with irritable bowel syndrome–etiology, prevalence and consequences. Eur. J. Clin. Nutr. 2006, 60, 667–672. [Google Scholar] [CrossRef]
- Hayes, P.A.; Fraher, M.H.; Quigley, E.M. Irritable bowel syndrome: The role of food in pathogenesis and management. Gastroenterol. Hepatol. 2014, 10, 164. [Google Scholar]
- Hayes, P.; Corish, C.; O’mahony, E.; Quigley, E. A dietary survey of patients with irritable bowel syndrome. J. Hum. Nutr. Diet. 2014, 27, 36–47. [Google Scholar] [CrossRef]
- Reding, K.W.; Cain, K.C.; Jarrett, M.E.; Eugenio, M.D.; Heitkemper, M.M. Relationship between patterns of alcohol consumption and gastrointestinal symptoms among patients with irritable bowel syndrome. Am. J. Gastroenterol. 2013, 108, 270. [Google Scholar] [CrossRef]
- Gibson, P.R.; Shepherd, S.J. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. J. Gastroenterol. Hepatol. 2010, 25, 252–258. [Google Scholar] [CrossRef]
- Wilson, B.; Whelan, K. Prebiotic inulin-type fructans and galacto-oligosaccharides: Definition, specificity, function, and application in gastrointestinal disorders. J. Gastroenterol. Hepatol. 2017, 32, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, S.M.; Wong, S.H.; Ser, H.-L.; Lee, L.-H. Role of low FODMAP diet and probiotics on gut microbiome in irritable bowel syndrome (IBS). Prog. Microbes Mol. Biol. 2020, 3, a0000069. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Whelan, K.; Irving, P.M.; Lomer, M.C. Comparison of symptom response following advice for a diet low in fermentable carbohydrates (FODMAPs) versus standard dietary advice in patients with irritable bowel syndrome. J. Hum. Nutr. Diet. 2011, 24, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Böhn, L.; Störsrud, S.; Liljebo, T.; Collin, L.; Lindfors, P.; Törnblom, H.; Simrén, M. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: A randomized controlled trial. Gastroenterology 2015, 149, 1399–1407. [Google Scholar] [CrossRef]
- Eswaran, S.L.; Chey, W.D.; Han-Markey, T.; Ball, S.; Jackson, K. A randomized controlled trial comparing the low FODMAP diet vs. modified NICE guidelines in US adults with IBS-D. Am. J. Gastroenterol. 2016, 111, 1824–1832. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Lomer, M.C.; Farquharson, F.M.; Louis, P.; Fava, F.; Franciosi, E.; Scholz, M.; Tuohy, K.M.; Lindsay, J.O.; Irving, P.M. A diet low in FODMAPs reduces symptoms in patients with irritable bowel syndrome and a probiotic restores bifidobacterium species: A randomized controlled trial. Gastroenterology 2017, 153, 936–947. [Google Scholar] [CrossRef]
- Nybacka, S.; Störsrud, S.; Liljebo, T.; Le Nevé, B.; Törnblom, H.; Simrén, M.; Winkvist, A. Within-and Between-Subject Variation in Dietary Intake of Fermentable Oligo-, Di-, Monosaccharides, and Polyols Among Patients with Irritable Bowel Syndrome. Curr. Dev. Nutr. 2019, 3, nzy101. [Google Scholar] [CrossRef]
- Le Nevé, B.; Derrien, M.; Tap, J.; Brazeilles, R.; Cools Portier, S.; Guyonnet, D.; Ohman, L.; Störsrud, S.; Törnblom, H.; Simrén, M. Fasting breath H2 and gut microbiota metabolic potential are associated with the response to a fermented milk product in irritable bowel syndrome. PLoS ONE 2019, 14, e0214273. [Google Scholar] [CrossRef]
- Francis, C.Y.; Morris, J.; Whorwell, P.J. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment. Pharmacol. Ther. 1997, 11, 395–402. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-15: Validity of a new measure for evaluating the severity of somatic symptoms. Psychosom. Med. 2002, 64, 258–266. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Labus, J.; Bolus, R.; Chang, L.; Wiklund, I.; Naesdal, J.; Mayer, E.; Naliboff, B. The Visceral Sensitivity Index: Development and validation of a gastrointestinal symptom-specific anxiety scale. Aliment. Pharmacol. Ther. 2004, 20, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Smets, E.; Garssen, B.; Bonke Bd De Haes, J. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psychosom. Res. 1995, 39, 315–325. [Google Scholar] [CrossRef]
- Liljebo, T.; Störsrud, S.; Andreasson, A. Presence of Fermentable Oligo-, Di-, Monosaccharides, and Polyols (FODMAPs) in commonly eaten foods: Extension of a database to indicate dietary FODMAP content and calculation of intake in the general population from food diary data. BMC Nutr. 2020, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, R.P.; Choe, J.-Y.; Patel, C.R. Intestinal Absorption of Fructose. Annu. Rev. Nutr. 2018, 38, 41–67. [Google Scholar] [CrossRef]
- Skoog, S.M.; Bharucha, A.E.; Zinsmeister, A.R. Comparison of breath testing with fructose and high fructose corn syrups in health and IBS. Neurogastroenterol. Motil. 2008, 20, 505–511. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Ralph, F.S.E.; Irving, P.M.; Whelan, K.; Lomer, M.C.E. Nutrient Intake, Diet Quality, and Diet Diversity in Irritable Bowel Syndrome and the Impact of the Low FODMAP Diet. J. Acad. Nutr. Diet. 2020, 120, 535–547. [Google Scholar] [CrossRef]
- Tigchelaar, E.F.; Mujagic, Z.; Zhernakova, A.; Hesselink, M.A.M.; Meijboom, S.; Perenboom, C.W.M.; Masclee, A.A.M.; Wijmenga, C.; Feskens, E.J.M.; Jonkers, D. Habitual diet and diet quality in Irritable Bowel Syndrome: A case-control study. Neurogastroenterol. Motil. 2017, 29, e13151. [Google Scholar] [CrossRef]
- Misselwitz, B.; Pohl, D.; Frühauf, H.; Fried, M.; Vavricka, S.R.; Fox, M. Lactose malabsorption and intolerance: Pathogenesis, diagnosis and treatment. United Eur. Gastroenterol. J. 2013, 1, 151–159. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Whelan, K. The low FODMAP diet: Recent advances in understanding its mechanisms and efficacy in IBS. Gut 2017, 66, 1517–1527. [Google Scholar] [CrossRef]
- Gibson, P.R.; Newnham, E.; Barrett, J.S.; Shepherd, S.; Muir, J.G. Fructose malabsorption and the bigger picture. Aliment. Pharmacol. Ther. 2007, 25, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.K.; Kraft, N.; Zimmerman, B.; Jackson, M.; Rao, S.S. Fructose intolerance in IBS and utility of fructose-restricted diet. J Clin Gastroenterol 2008, 42, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Fedewa, A.; Rao, S.S.C. Dietary fructose intolerance, fructan intolerance and FODMAPs. Curr. Gastroenterol. Rep. 2014, 16, 370. [Google Scholar] [CrossRef] [PubMed]
- Nybacka, S.; Öhman, L.; Störsrud, S.; Mybeck, M.; Böhn, L.; Wilpart, K.; Winkvist, A.; Bengtsson, U.; Törnblom, H.; Simrén, M. Neither self-reported atopy nor IgE-mediated allergy are linked to gastrointestinal symptoms in patients with irritable bowel syndrome. Neurogastroenterol. Motil. 2018, 30, e13379. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, M.; Visser, R.; Heitkemper, M. Diet triggers symptoms in women with irritable bowel syndrome: The patient’s perspective. Gastroenterol. Nurs. 2001, 24, 246–252. [Google Scholar] [CrossRef] [PubMed]
| IBS-C | IBS-D | IBS-M | IBS-U | Difference between Groups | |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | p-Value | |
| Women (n = 145) | (n = 36) | (n = 40) | (n = 33) | (n = 36) | |
| Age, y | 40.2 ± 15.6 | 35.6 ± 13.3 | 35.0 ± 13.2 | 39.7 ± 16.1 | 0.30 |
| Weight, kg | 65 ± 11.6 | 65 ± 12.7 | 65.7 ± 16.1 | 63.0 ± 9.1 | 0.84 |
| BMI (kg/m2) | 23.0 ± 3.3 | 23.6 ± 4.3 | 23.4 ± 4.7 | 22.5 ± 2.8 | 0.68 |
| Men (n = 44) | (n = 8) | (n = 14) | (n = 12) | (n = 10) | |
| Age, y | 40.5 ± 11.8 | 39.7 ± 16.0 | 34.1 ± 13.5 | 35.5 ± 12.3 | 0.65 |
| Weight, kg | 78.6 ± 11.3 | 80.5 ± 10.4 | 81.8 ± 9.6 | 85.4 ± 15.8 | 0.68 |
| BMI (kg/m2) | 23.3 ± 3.6 | 24.5 ± 3.5 | 25.2 ± 2.1 | 25.5 ± 4.3 | 0.52 |
| All (n = 189) | (n = 44) | (n = 54) | (n = 45) | (n = 46) | |
| IBS-SSS | 306 ± 81 | 302 ± 92 | 304 ± 84 | 291 ± 83 | 0.83 |
| Pain intensity | 52 ± 26 | 48 ± 27 | 54 ± 25 | 46 ± 24 | 0.48 |
| Pain frequency | 58 ± 34 | 52 ± 35 | 53 ± 33 | 54 ± 32 | 0.81 |
| Bloating severity | 61 ± 29 | 58 ± 27 | 58 ± 31 | 60 ± 28 | 0.92 |
| Bowel habit dissatisfaction | 65 ± 28 | 72 ± 23 | 73 ± 21 | 61 ± 29 | 0.065 |
| Daily life interference | 70 ± 23 | 72 ± 18 | 66 ± 26 | 69 ± 24 | 0.61 |
| VSI | 48.5 ± 17.5 | 43.7 ± 16.1 | 41.9 ± 15.9 | 48.1 ± 14.4 | 0.14 |
| PHQ | 12.2 ± 4.0 | 13.8 ± 5.6 | 14.3 ± 4.7 | 11.6 ± 4.3 | 0.025 * |
| HAD | 12.9 ± 8.1 | 14.2 ± 6.2 | 13.6 ± 7.0 | 12.1 ± 7.4 | 0.53 |
| MFI-20 | 59.1 ± 15.4 | 62.0 ± 16.2 | 60.7 ± 17.3 | 55.9 ± 16.2 | 0.32 |
| IBS-C | IBS-D | IBS-M | IBS-U | Difference between Groups | |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | p-Value | |
| Women (n = 145) | (n = 36) | (n = 40) | (n = 33) | (n = 36) | |
| Energy, kcal | 1943 ± 487 | 1982 ± 497 | 2076 ± 546 | 2132 ± 462 | 0.36 |
| Protein, E% | 17.4 ± 3.2 | 16.4 ± 3.4 | 16.1 ± 3.6 | 15.4 ± 2.7 | 0.087 |
| Carbohydrates, E% | 38.0 ± 8.5 | 40.8 ± 6.6 | 41.3 ± 9.8 | 40.8 ± 6.9 | 0.31 |
| Fat, E% | 38.7 ± 8.5 | 38.2 ± 6.1 | 38.2 ± 8.5 | 38.5 ± 6.1 | 0.99 |
| Alcohol, E% | 4.0 ± 4.6 | 2.3 ± 2.9 | 2.4 ± 3.2 | 3.5 ± 2.7 | 0.10 |
| Dietary fiber, E% | 1.8 ± 0.6 | 2.1 ± 0.8 | 1.9 ± 0.5 | 1.9 ± 0.7 | 0.39 |
| Dietary fiber, g | 17.8 ± 7.4 | 20.6 ± 8.8 | 19.6 ± 7.0 | 20.0 ± 7.0 | 0.43 |
| Total FODMAPs, g | 19.0 ± 8.2 | 17.0 ± 8.3 | 23.9 ± 13.2 | 20.1 ± 12.1 | 0.054 |
| Galacto-oligosaccharides, g | 0.5 ± 0.4 | 0.5 ± 0.4 | 0.6 ± 0.6 | 0.4 ± 0.2 | 0.25 |
| Fructans, g | 2.0 ± 0.9 | 2.3 ± 1.2 | 2.8 ± 1.6 | 2.5 ± 1.1 | 0.08 |
| Polyols, g | 1.4 ± 1.6 | 0.9 ± 1.3 | 1.5 ± 1.7 | 0.9 ± 1.0 | 0.14 |
| Lactose, g | 10.0 ± 6.2 | 7.6 ± 6.6 | 13.6 ± 8.1 | 10.0 ± 8.3 | 0.009 * |
| Excess fructose, g | 5.1 ± 3.7 | 5.6 ± 5.7 | 5.4 ± 7.8 | 6.3 ± 7.2 | 0.87 |
| Men (n = 44) | (n = 8) | (n = 14) | (n = 12) | (n = 10) | |
| Energy, kcal | 2292 ± 354 | 2309 ± 515 | 2432 ± 710 | 2369 ± 516 | 0.93 |
| Protein, E% | 16.9 ± 1.4 | 15.1 ± 2.4 | 18.4 ± 5.1 | 19.0 ± 8.2 | 0.21 |
| Carbohydrates, E% | 38.6 ± 8.8 | 44.5 ± 6.4 | 37.6 ± 8.4 | 43.0 ± 5.0 | 0.065 |
| Fat, E% | 42.1 ± 6.9 | 32.0 ± 6.8 | 36.7 ± 8.8 | 34.5 ± 7.9 | 0.037 * |
| Alcohol, E% | 0.9 ± 1.7 | 6.1 ± 6.0 | 5.7 ± 5.4 | 1.5 ± 2.0 | 0.017 * |
| Dietary fiber, E% | 1.5 ± 0.4 | 2.0 ± 0.7 | 1.6 ± 0.3 | 1.9 ± 0.8 | 0.182 |
| Dietary fiber, g | 18.1 ± 6.7 | 23.2 ± 9.3 | 18.5 ± 5.5 | 21.4 ± 8.3 | 0.343 |
| Total FODMAPs, g | 19.9 ± 9.1 | 19.6 ± 12.2 | 21.7 ± 18.4 | 28.9 ± 12.0 | 0.38 |
| Galacto-oligosaccharides, g | 0.5 ± 0.2 | 0.8 ± 0.6 | 0.5 ± 0.6 | 0.6 ± 0.5 | 0.52 |
| Fructans, g | 2.3 ± 1.2 | 3.2 ± 2.1 | 2.4 ± 1.0 | 3.3 ± 1.9 | 0.40 |
| Polyols, g | 1.0 ± 1.5 | 0.5 ± 0.7 | 0.8 ± 1.4 | 1.7 ± 2.5 | 0.36 |
| Lactose, g | 11.1 ± 7.8 | 9.5 ± 8.6 | 11.1 ± 10.5 | 14.5 ± 12.6 | 0.69 |
| Excess fructose, g | 5.1 ± 5.0 | 5.6 ± 4.4 | 6.9 ± 11.8 | 8.8 ± 5.1 | 0.69 |
| All (n = 189) | (n = 44) | (n = 54) | (n = 45) | (n = 46) | |
| Total FODMAPs, g/1000 kcal | 9.73 ± 3.96 | 8.83 ± 4.91 | 10.90 ± 5.99 | 10.13 ± 5.57 | 0.25 |
| Galacto-oligosaccharides, g/1000 kcal | 0.24 ± 0.21 | 0.30 ± 0.30 | 0.27 ± 0.31 | 0.21 ± 0.15 | 0.27 |
| Fructans, g/1000 kcal | 1.03 ± 0.41 | 1.24 ± 0.67 | 1.24 ± 0.60 | 1.24 ± 0.62 | 0.28 |
| Polyols, g/1000 kcal | 0.70 ± 0.83 | 0.41 ± 0.60 | 0.60 ± 0.69 | 0.50 ± 0.72 | 0.23 |
| Lactose, g/1000 kcal | 5.15 ± 3.02 | 4.18 ± 4.26 | 6.14 ± 3.94 | 5.00 ± 4.20 | 0.11 |
| Excess fructose, g/1000 kcal | 2.59 ± 1.98 | 2.69 ± 2.33 | 2.64 ± 3.72 | 3.18 ± 3.11 | 0.75 |
| All | IBS-C | IBS-D | IBS-M | IBS-U | |
|---|---|---|---|---|---|
| IBS-SSS total | 0.217 ** | 0.246 | 0.257 | −0.159 | 0.518 ** |
| Pain intensity | 0.153 | 0.276 | −0.069 | 0.088 | 0.258 |
| Pain frequency | 0.168 * | 0.081 | 0.220 | −0.061 | 0.386 * |
| Bloating severity | 0.104 | 0.232 | 0.214 | −0.229 | 0.188 |
| Bowel habit dissatisfaction | 0.124 | 0.090 | 0.088 | −0.160 | 0.323 |
| Daily life interference | 0.159 | 0.229 | 0.086 | 0.090 | 0.347 * |
| VSI | −0.058 | −0.075 | 0.100 | −0.120 | −0.015 |
| PHQ | 0.091 | 0.175 | 0.148 | 0.059 | 0.090 |
| HAD | 0.095 | 0.278 | −0.036 | 0.148 | 0.047 |
| MFI-20 | 0.046 | 0.132 | 0.010 | 0.037 | −0.014 |
| Independent Variable | All | IBS-C | IBS-D | IBS-M | IBS-U | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | B | p-Value | R2 | B | p-Value | R2 | B | p-Value | R2 | B | p-Value | R2 | B | p-Value | |
| Age | 0.009 | 0.545 | 0.208 | 0.051 | −1.226 | 0.141 | 0.003 | 0.363 | 0.692 | 0.000 | −0.022 | 0.982 | 0.049 | −1.199 | 0.141 |
| BMI | 0.001 | −0.633 | 0.709 | 0.021 | −3.664 | 0.368 | 0.000 | −0.396 | 0.902 | 0.023 | −2.949 | 0.340 | 0.046 | 5.128 | 0.174 |
| Energy, kcal | 0.029 | −0.030 | 0.042 * | 0.009 | −0.033 | 0.261 | −0.022 | −0.012 | 0.698 | 0.012 | −0.035 | 0.249 | −0.001 | −0.031 | 0.330 |
| CVw kcal | 0.001 | 0.242 | 0.683 | 0.036 | 1.178 | 0.266 | 0.030 | −1.246 | 0.283 | 0.023 | −1.114 | 0.404 | 0.104 | 2.374 | 0.055 |
| CVw FODMAP | 0.003 | −0.199 | 0.532 | 0.017 | −0.654 | 0.447 | 0.023 | −0.569 | 0.355 | 0.066 | −0.992 | 0.156 | 0.072 | 0.846 | 0.115 |
| EA FODMAP | 0.043 | 16.32 | 0.012 * | 0.039 | 15.09 | 0.250 | 0.046 | 18.89 | 0.186 | 0.009 | −7.735 | 0.607 | 0.233 | 33.77 | 0.003 * |
| EA GOS | 0.000 | −1,154 | 0.859 | 0.052 | −16.19 | 0.182 | 0.007 | −6.438 | 0.605 | 0.000 | −1.246 | 0.933 | 0.047 | 18.38 | 0.204 |
| EA FOS | 0.000 | 0.068 | 0.992 | 0.013 | −8.814 | 0.514 | 0.005 | −5.303 | 0.679 | 0.009 | 7.296 | 0.602 | 0.019 | 10.71 | 0.420 |
| EA polyols | 0.001 | 2.238 | 0.731 | 0.000 | 1.034 | 0.938 | 0.003 | −4.255 | 0.745 | 0.022 | 10.09 | 0.415 | 0.000 | 0.101 | 0.995 |
| EA lactose | 0.000 | −0.606 | 0.927 | 0.009 | −8.583 | 0.589 | 0.006 | −6.460 | 0.629 | 0.028 | −14.06 | 0.370 | 0.070 | 18.44 | 0.124 |
| EA excess fructose | 0.045 | 16.09 | 0.011 * | 0.029 | 12.49 | 0.318 | 0.042 | 15.33 | 0.213 | 0.000 | −0.071 | 0.996 | 0.199 | 34.52 | 0.007 * |
| All | IBS-C | IBS-D | IBS-M | IBS-U | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted R2 | B | p-Value | Adjusted R2 | B | p-Value | Adjusted R2 | B | p-Value | Adjusted R2 | B | p-Value | Adjusted R2 | B | p-Value | |
| Full model 1 EA FODMAP | 0.246 | 5.092 | 0.362 | 0.338 | 7.752 | 0.523 | 0.174 | 14.82 | 0.251 | 0.273 | −28.42 | 0.023 | 0.313 | 21.89 | 0.047 |
| Energy intake | −0.013 | 0.362 | −0.010 | 0.696 | 0.004 | 0.882 | −0.016 | 0.526 | −0.026 | 0.286 | |||||
| Age | 0.116 | 0.791 | −0.104 | 0.910 | 1.434 | 0.161 | −0.333 | 0.712 | −0.853 | 0.259 | |||||
| BMI | −2.668 | 0.106 | −4.163 | 0.320 | −1.945 | 0.528 | −3.692 | 0.192 | −0.151 | 0.976 | |||||
| PHQ | 8.217 | 0.000 | 13.16 | 0.001 | 7.784 | 0.007 | 8.457 | 0.008 | 5.938 | 0.040 | |||||
| Full model 2 EA FODMAP minus lactose | 0.241 | 1.860 | 0.744 | 0.327 | −1.676 | 0.886 | 0.137 | −1.873 | 0.876 | 0.123 | −12.15 | 0.386 | 0.336 | 26.10 | 0.029 |
| Energy intake | −0.014 | 0.243 | −0.014 | 0.607 | 0.000 | 0.988 | −0.005 | 0.860 | −0.023 | 0.349 | |||||
| Age | 0.142 | 0.746 | −0.008 | 0.993 | 1.545 | 0.139 | −0.240 | 0.810 | −0.993 | 0.191 | |||||
| BMI | −2.685 | 0.111 | −4.246 | 0.330 | −1.851 | 0.567 | −3.350 | 0.277 | −1.055 | 0.823 | |||||
| PHQ | 8.243 | 0.001 | 13.49 | 0.001 | 8.343 | 0.005 | 9.118 | 0.014 | 7.212 | 0.010 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nybacka, S.; Störsrud, S.; Lindqvist, H.M.; Törnblom, H.; Simrén, M.; Winkvist, A. Habitual FODMAP Intake in Relation to Symptom Severity and Pattern in Patients with Irritable Bowel Syndrome. Nutrients 2021, 13, 27. https://doi.org/10.3390/nu13010027
Nybacka S, Störsrud S, Lindqvist HM, Törnblom H, Simrén M, Winkvist A. Habitual FODMAP Intake in Relation to Symptom Severity and Pattern in Patients with Irritable Bowel Syndrome. Nutrients. 2021; 13(1):27. https://doi.org/10.3390/nu13010027
Chicago/Turabian StyleNybacka, Sanna, Stine Störsrud, Helen M. Lindqvist, Hans Törnblom, Magnus Simrén, and Anna Winkvist. 2021. "Habitual FODMAP Intake in Relation to Symptom Severity and Pattern in Patients with Irritable Bowel Syndrome" Nutrients 13, no. 1: 27. https://doi.org/10.3390/nu13010027
APA StyleNybacka, S., Störsrud, S., Lindqvist, H. M., Törnblom, H., Simrén, M., & Winkvist, A. (2021). Habitual FODMAP Intake in Relation to Symptom Severity and Pattern in Patients with Irritable Bowel Syndrome. Nutrients, 13(1), 27. https://doi.org/10.3390/nu13010027





