Cordyceps militaris Fruit Body Extract Decreases Testosterone Catabolism and Testosterone-Stimulated Prostate Hypertrophy
Abstract
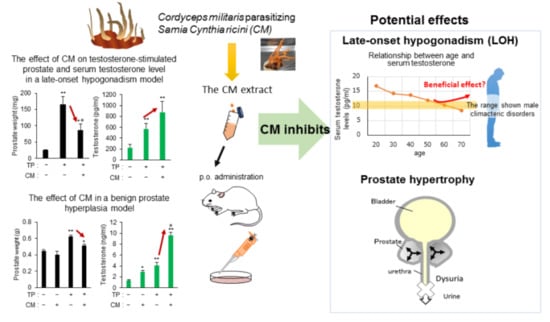
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Extract from Cordyceps militaris Fruit Body (CM)
2.2. NMR Experiments and Analysis
2.3. Animals and Tissue Preparation
2.4. Measurement of Testosterone and DHT
2.5. RNA Extraction and Quantitative RT-PCR
2.6. Isolation of Primary Rat Testicular Cells
2.7. Cell Culture and Evaluation of Androgen Secretion
2.8. Cell Viability and Proliferation Assays
2.9. Western Blotting
2.10. Statistical Analysis
3. Results
3.1. Quantification of the Amino Acids and Sugars in the Extract Components from Cordyceps militaris Fruit Bodies
3.2. The Effect of CM in a Rat Model of LOH
3.3. The Effect of CM on BPH in Rats
3.4. The Effect of CM on the Proliferation and Androgen Receptor (AR) Activation of Prostate Cells
3.5. The Effect of CM on the Secretion of Androgens in Primary Rat Testicular Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CM | Cordyceps militaris parasitizing Samia cynthia ricini |
| LOH | Late-onset hypogonadism |
| BPH | Benign prostate hyperplasia |
| DHT | Dihydrotestosterone, |
| DMEM/F12 | Dulbecco’s modified eagle’s medium/F12 |
| Db | Dibutyryl-cyclic AMP |
| PBS | Phosphate-buffered saline |
References
- Lunenfeld, B.; Mskhalaya, G.; Zitzmann, M.; Arver, S.; Kalinchenko, S.; Tishova, Y.; Morgentaler, A. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male 2015, 18, 5–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McHenry, J.; Carrier, N.; Hull, E.; Kabbaj, M. Sex differences in anxiety and depression: Role of testosterone. Front. Neuroendocr. 2014, 35, 42–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, F.; Tajar, A.; Beynon, J.M.; Pye, S.R.; Silman, A.J.; Finn, J.D.; O’Neill, T.W.; Bartfai, G.; Casanueva, F.F.; Forti, G.; et al. Identification of Late-Onset Hypogonadism in Middle-Aged and Elderly Men. N. Engl. J. Med. 2010, 363, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Sarma, A.V.; Wei, J.T. Benign Prostatic Hyperplasia and Lower Urinary Tract Symptoms. N. Engl. J. Med. 2012, 367, 248–257. [Google Scholar] [CrossRef]
- Roehrborn, C.G. Pathology of benign prostatic hyperplasia. Int. J. Impot. Res. 2008, 20, S11–S18. [Google Scholar] [CrossRef] [Green Version]
- De Nunzio, C.; Presicce, F.; Tubaro, A. Inflammatory mediators in the development and progression of benign prostatic hyperplasia. Nat. Rev. Urol. 2016, 13, 613–626. [Google Scholar] [CrossRef]
- Oka, M.; Ueda, M.; Oyama, T.; Kyotani, J.; Tanaka, M. Effect of the phytotherapeutic agent Eviprostat on 17beta-estradiol-induced nonbacterial inflammation in the rat prostate. Prostate 2009, 69, 1404–1410. [Google Scholar] [CrossRef]
- Zirkin, B.R.; Papadopoulos, V. Leydig cells: Formation, function, and regulation. Biol. Reprod. 2018, 99, 101–111. [Google Scholar] [CrossRef]
- Schlatt, S.; Ehmcke, J. Regulation of spermatogenesis: An evolutionary biologist’s perspective. Semin. Cell. Dev. Biol. 2014, 29, 2–16. [Google Scholar] [CrossRef]
- Aggarwal, S.; Thareja, S.; Verma, A.; Bhardwaj, T.R.; Kumar, M. An overview on 5α-reductase inhibitors. Steroids 2010, 75, 109–153. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Duan, Y.; Zhang, H.; Ma, H. Advance in Cordyceps militaris (Linn) Link polysaccharides: Isolation, structure, and bioactivities: A review. Int. J. Biol. Macromol. 2019, 132, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Masuda, M.; Sakurai, A.; Sakakibara, M. Medicinal uses of the mushroom Cordyceps militaris: Current state and prospects. Fitoterapia 2010, 81, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, O.J.; Tang, J.; Tola, A.; Auberon, F.; Oluwaniyi, O.; Ouyang, Z. The genus Cordyceps: An extensive review of its traditional uses, phytochemistry and pharmacology. Fitoterapia 2018, 129, 293–316. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Ran, R.; Yao, J.; Zhang, F.; Xing, M.; Jin, M.; Wang, L.; Zhang, T. Se-Enriched Cordyceps militaris Inhibits Cell Proliferation, Induces Cell Apoptosis, and Causes G2/M Phase Arrest in Human Non-Small Cell Lung Cancer Cells. Onco Targets Ther. 2019, 12, 8751–8763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, L.; Zhao, L.; Yang, F.; Yang, W.; Sun, Y.; Hu, Q. Evaluation of anti-fatigue property of the extruded product of cereal grains mixed with Cordyceps militaris on mice. J. Int. Soc. Sports Nutr. 2017, 14, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.; Jeng, K.-C.; Huang, K.-F.; Lee, Y.-C.; Hou, C.-W.; Chen, K.-H.; Cheng, F.-Y.; Liao, J.-W.; Chen, Y.-S. Effect of Cordyceps Militaris Supplementation on Sperm Production, Sperm Motility and Hormones in Sprague-Dawley Rats. Am. J. Chin. Med. 2008, 36, 849–859. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Chen, C.; Jiang, Z.; Wang, M.; Jiang, H.; Zhang, X. Protective effect of Cordyceps militaris extract against bisphenol A induced reproductive damage. Syst. Biol. Reprod. Med. 2016, 62, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.; Kusama, K.; Ideta, A.; Kimura, K.; Hori, M.; Imakawa, K. Effects of miR-98 in intrauterine extracellular vesicles on maternal immune regulation during the peri-implantation period in cattle. Sci. Rep. 2019, 9, 20330. [Google Scholar] [CrossRef] [Green Version]
- Kusama, K.; Yoshie, M.; Tamura, K.; Nakayama, T.; Nishi, H.; Isaka, K.; Tachikawa, E. The role of exchange protein directly acti-vated by cyclic AMP 2-mediated calreticulin expression in the decidualization of human endometrial stromal cells. Endocrinology 2014, 155, 240–248. [Google Scholar] [CrossRef] [Green Version]
- Lin, B.; Ferguson, C.; White, J.T.; Wang, S.; Vessella, R.; True, L.D.; Hood, L.; Nelson, P.S. Prostate-localized and androgen-regulated expression of the membrane-bound serine protease TMPRSS2. Cancer Res. 1999, 59, 4180–4184. [Google Scholar]
- Magee, J.A.; Chang, L.-W.; Stormo, G.D.; Milbrandt, J. Direct, Androgen Receptor-Mediated Regulation of the FKBP5 Gene via a Distal Enhancer Element. Endocrinology 2006, 147, 590–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, C.-Y.; Kim, G.-Y.; Choi, Y.H. Induction of apoptosis by aqueous extract of Cordyceps militaris through activation of caspases and inactivation of Akt in human breast cancer MDA-MB-231 Cells. J. Microbiol. Biotechnol. 2008, 18, 1997–2003. [Google Scholar] [PubMed]
- Jo, E.; Jang, H.J.; Yang, K.E.; Jang, M.S.; Huh, Y.H.; Yoo, H.S.; Park, J.S.; Jang, I.S.; Park, S.J. Cordyceps militaris induces apoptosis in ovarian cancer cells through TNF-α/TNFR1-mediated inhibition of NF-κB phosphorylation. BMC Complement. BMC Complement Med. Ther. 2020, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Xu, X.; Wei, X.; Feng, W.; Huang, H.; Liu, H.; Xu, R.; Lin, J.; Han, L.; Zhang, D. Natural medicines for the treatment of fatigue: Bioactive components, pharmacology, and mechanisms. Pharmacol. Res. 2019, 148, 104409. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Song, J.; Kim, M.; Han, D.-W.; Park, H.-J.; Song, M. Cordyceps militaris Grown on Germinated Soybean Suppresses KRAS-Driven Colorectal Cancer by Inhibiting the RAS/ERK Pathway. Nutrients 2018, 11, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.C.; Chen, Y.H.; Pan, B.S.; Chang, M.M.; Huang, B.M. Functional study of Cordyceps sinensis and cordycepin in male re-production: A review. J. Food Drug. Anal. 2017, 25, 197–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, M.-W.; Gao, X.-S.; Yu, H.-L.; Qi, X.; Sun, S.-Q.; Wang, D. Cordyceps sinensis Promotes the Growth of Prostate Cancer Cells. Nutr. Cancer 2018, 70, 1166–1172. [Google Scholar] [CrossRef]
- Lu, Y.; Zhi, Y.; Miyakawa, T.; Tanokura, M. Metabolic profiling of natural and cultured Cordyceps by NMR spectroscopy. Sci. Rep. 2019, 9, 7735. [Google Scholar] [CrossRef]





| Name (Accession No.) | Sequence | Product Length (bp) |
|---|---|---|
| Gapdh (NM_017008.4) | F: 5′- AAAGCTGTGGCGTGATGG -3′ | 96 |
| R: 5′- TTCAGCTCTGGGATGACCTT -3′ | ||
| Actb (NM_031144.3) | F: 5′- GGAGATTACTGCCCTGGCTCCTA -3′ | 150 |
| R: 5′- GACTCATCGTACTCCTGCTTGCTG -3′ | ||
| Srd5a1 (NM_017070.3) | F: 5′- GGTCTCCTCTCAAAACCTCAGG -3′ | 106 |
| R: 5′- GGAGGTCAAGTTCACAGCAAAC -3′ | ||
| Srd5a2 (NM_022711.4) | F: 5′- TATACTCCTTTCTGCCCAGGGA -3′ | 171 |
| R: 5′- GTTTTGACTGCAGAACTTCCCC -3′ | ||
| Srd5a3 (NM_001013990.1) | F: 5′- CTTTGGGCTTTGCTCAGAACTC -3′ | 137 |
| R: 5′- AGACTATGGACCCAGAGGAACA -3′ | ||
| Hsd3b (NM_001007719.3) | F: 5′- CCAGTGTGCCAGCCTTCATCTAC -3′ | 104 |
| R: 5′- GCTTTCATGATGCTCTTCCTCATG -3′ | ||
| Cyp11a1 (NM_017286.3) | F: 5′- AGTTCAGATGCCTGGAGGAAAG -3′ | 165 |
| R: 5′- GTCCCCTGAGAACTTTCCAGAG -3′ | ||
| Tmprss2 (NM_130424.3) | F: 5′- ACAACTCAAGCCTCAACACC -3′ | 150 |
| R: 5′- CTTCCAAAGCAAGCCAGCAG -3′ | ||
| Fkbp5 (NM_001012174.1) | F: 5′- TTCCAGTCGTGACAGAAACG -3′ | 98 |
| R: 5′- CGCCTTTCTTCATGGTAGACAC -3′ | ||
| TMPRSS (NM_001135099.1) | F: 5′- CCTCTAACTGGTGTGATGGCGT -3′ | 121 |
| R: 5′- TGCCAGGACTTCCTCTGAGATG -3′ | ||
| FKBP5 (NM_004117.4) | F: 5′- CTGCAGAGATGTGGCATTCACT-3′ | 75 |
| R: 5′- TCCAGAGCTTTGTCAATTCCAA -3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusama, K.; Miyagawa, M.; Ota, K.; Kuwabara, N.; Saeki, K.; Ohnishi, Y.; Kumaki, Y.; Aizawa, T.; Nakasone, T.; Okamatsu, S.; et al. Cordyceps militaris Fruit Body Extract Decreases Testosterone Catabolism and Testosterone-Stimulated Prostate Hypertrophy. Nutrients 2021, 13, 50. https://doi.org/10.3390/nu13010050
Kusama K, Miyagawa M, Ota K, Kuwabara N, Saeki K, Ohnishi Y, Kumaki Y, Aizawa T, Nakasone T, Okamatsu S, et al. Cordyceps militaris Fruit Body Extract Decreases Testosterone Catabolism and Testosterone-Stimulated Prostate Hypertrophy. Nutrients. 2021; 13(1):50. https://doi.org/10.3390/nu13010050
Chicago/Turabian StyleKusama, Kazuya, Mayuko Miyagawa, Koichiro Ota, Naoko Kuwabara, Kaori Saeki, Yuki Ohnishi, Yasuhiro Kumaki, Tomoyasu Aizawa, Toyokazu Nakasone, Shigemi Okamatsu, and et al. 2021. "Cordyceps militaris Fruit Body Extract Decreases Testosterone Catabolism and Testosterone-Stimulated Prostate Hypertrophy" Nutrients 13, no. 1: 50. https://doi.org/10.3390/nu13010050
APA StyleKusama, K., Miyagawa, M., Ota, K., Kuwabara, N., Saeki, K., Ohnishi, Y., Kumaki, Y., Aizawa, T., Nakasone, T., Okamatsu, S., Miyaoka, H., & Tamura, K. (2021). Cordyceps militaris Fruit Body Extract Decreases Testosterone Catabolism and Testosterone-Stimulated Prostate Hypertrophy. Nutrients, 13(1), 50. https://doi.org/10.3390/nu13010050