Evaluation of an Ileorectostomised Rat Model for Resistant Starch Determination
Abstract
:1. Introduction
2. Results
2.1. Human Study
2.1.1. Compliance
2.1.2. Stoma Digesta Excretion and Starch Digestibility
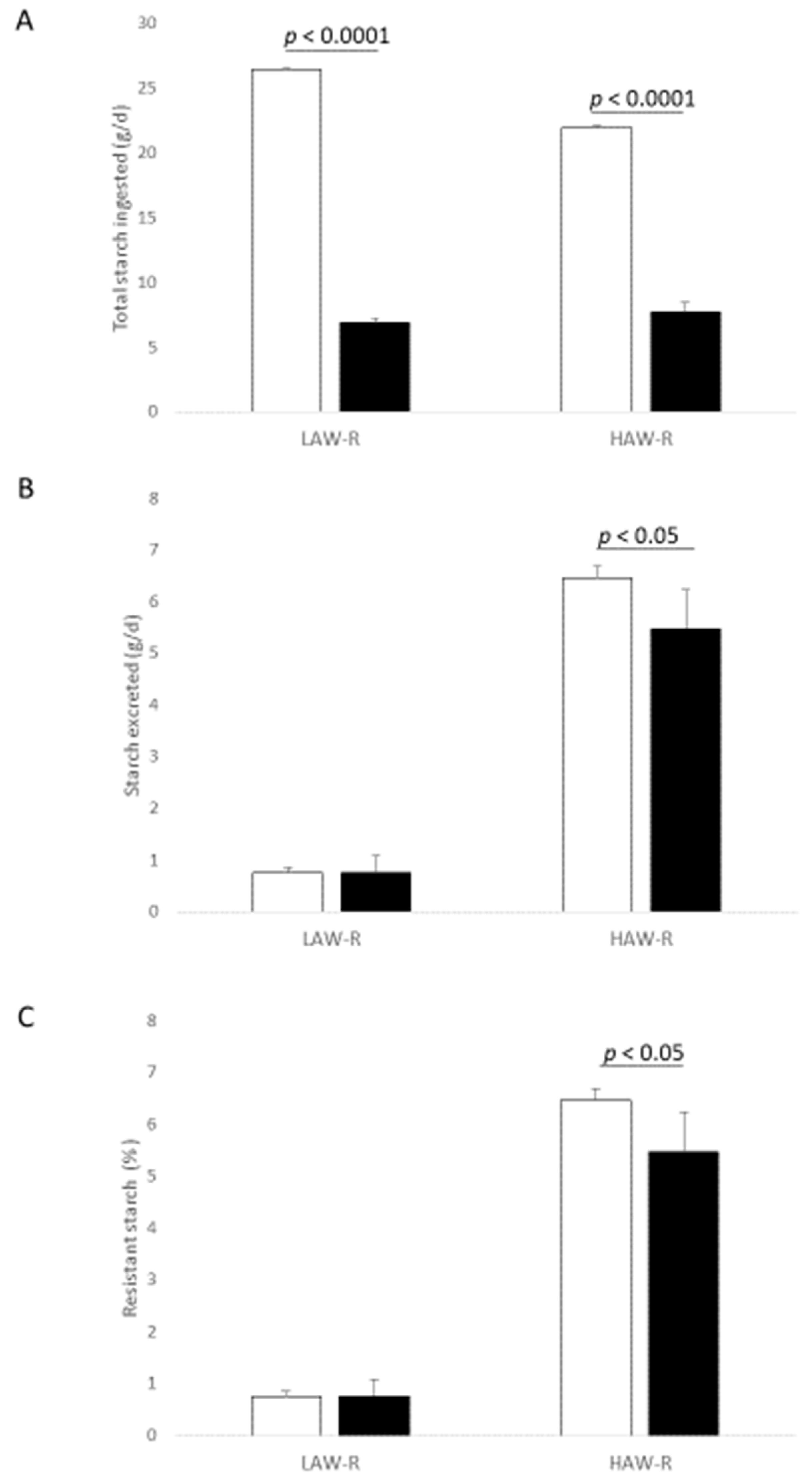
2.2. Rat Ileostomy Study
3. Discussion
4. Materials and Methods
4.1. Human Study
4.1.1. Study Population
4.1.2. Recruitment and Screening
4.1.3. Study Design and Intervention
4.1.4. Stoma Digesta Sample Collection
4.1.5. RS Analysis
4.2. Animal Ileostomy
4.2.1. Rats and Ileorectostomy Surgery
4.2.2. Experimental Protocol
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HAW-R | High amylose wheat-refined |
| HAW-W | High amylose wheat-wholemeal |
| IRM | Ileorectostomised rat model |
| LAW-R | Low amylose wheat-refined |
| LAW-W | Low amylose wheat-wholemeal |
| RS | Resistant starch |
| SCFA | Short-chain fatty acid |
References
- Conlon, M.A.; Bird, A.R. Interactive and individual effects of dietary non-digestible carbohydrates and oils on DNA damage, scfa and bacteria in the large bowel of rats. Br. J. Nutr. 2009, 101, 1171–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.; Qie, Y.; Park, J.; Kim, C.H. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe 2016, 20, 202–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef] [PubMed]
- Brand-Miller, J.; Hayne, S.; Petocz, P.; Colagiuri, S. Low-glycemic index diets in the management of diabetes: A meta-analysis of randomized controlled trials. Diabetes Care 2003, 26, 2261–2267. [Google Scholar] [CrossRef] [Green Version]
- Zafar, M.I.; Mills, K.E.; Zheng, J.; Regmi, A.; Hu, S.Q.; Gou, L.N.; Chen, L.L. Low-glycemic index diets as an intervention for diabetes: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2019, 110, 891–902. [Google Scholar] [CrossRef]
- Livesey, G.; Taylor, R.; Livesey, H.F.; Buyken, A.E.; Jenkins, D.J.A.; Augustin, L.S.A.; Sievenpiper, J.L.; Barclay, A.W.; Liu, S.M.; Wolever, T.M.S.; et al. Dietary glycemic index and load and the risk of type 2 diabetes: A systematic review and updated meta-analyses of prospective cohort studies. Nutrients 2019, 11, 1280. [Google Scholar] [CrossRef] [Green Version]
- Brogna, A.; Ferrara, R.; Bucceri, A.M.; Lanteri, E.; Catalano, F. Influence of aging on gastrointestinal transit time: An ultrasonographic and radiologic study. Investig. Radiol. 1999, 34, 357. [Google Scholar] [CrossRef]
- Ranawana, V.; Monro, J.A.; Mishra, S.; Henry, C.J. Degree of particle size breakdown during mastication may be a possible cause of interindividual glycemic variability. Nutr. Res. 2010, 30, 246–254. [Google Scholar] [CrossRef]
- Ranawana, V.; Clegg, M.E.; Shafat, A.; Henry, C.J. Postmastication digestion factors influence glycemic variability in humans. Nutr. Res. 2011, 31, 452–459. [Google Scholar] [CrossRef]
- Remond, D.; Shahar, D.R.; Gille, D.; Pinto, P.; Kachal, J.; Peyron, M.A.; Dos Santos, C.N.; Walther, B.; Bordoni, A.; Dupont, D.; et al. Understanding the gastrointestinal tract of the elderly to develop dietary solutions that prevent malnutrition. Oncotarget 2015, 6, 13858–13898. [Google Scholar] [CrossRef] [Green Version]
- Langkilde, A.M.; Champ, M.; Andersson, H. Effects of high-resistant-starch banana flour (rs(2)) on in vitro fermentation and the small-bowel excretion of energy, nutrients, and sterols: An ileostomy study. Am. J. Clin. Nutr. 2002, 75, 104–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Read, N.W.; Aljanabi, M.N.; Bates, T.E.; Barber, D.C. Effect of gastrointestinal intubation on the passage of a solid meal through the stomach and small-intestine in humans. Gastroenterology 1983, 84, 1568–1572. [Google Scholar] [CrossRef]
- Morita, T.; Ito, Y.; Brown, I.L.; Ando, R.; Kiriyama, S. In vitro and in vivo digestibility of native maize starch granules varying in amylose contents. J. AOAC Int. 2007, 90, 1628–1634. [Google Scholar] [CrossRef] [Green Version]
- Belobrajdic, D.P.; Hino, S.; Kondo, T.; Jobling, S.A.; Morell, M.K.; Topping, D.L.; Morita, T.; Bird, A.R. High wholegrain barley β-glucan lowers food intake but does not alter small intestinal macronutrient digestibility in ileorectostomised rats. Int. J. Food Sci. Nutr. 2016, 67, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Marlett, J.A.; Longacre, M.J. Comparison of in vitro and in vivo measures of resistant starch in selected grain products. Cereal Chem. 1996, 73, 63–68. [Google Scholar]
- Baghurst, P.A.; Baghurst, K.I.; Record, S.J. Dietary fibre, non-starch polysaccharides and resistant starch-a review. Food Aust. 1996, 48, S3–S35. [Google Scholar]
- Bird, A.R.; Usher, S.; May, B.; Topping, D.L.; Morell, M.K. Resistant starch measurement, intakes, and dietary targets. Diet. Fiber Health 2012, 41–56. [Google Scholar] [CrossRef]
- Cassidy, A.; Bingham, S.A.; Cummings, J.H. Starch intake and colorectal cancer risk: An international comparison. Br. J. Cancer 1994, 69, 937–942. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.M.; Douglass, J.S.; Birkett, A. Resistant starch intakes in the united states. J. Am. Diet Assoc. 2008, 108, 67–78. [Google Scholar] [CrossRef]
- Roberts, J.; Jones, G.P.; Rutihauser, I.H.E.; Birkett, A.; Gibbons, C. Resistant starch in the australian diet. Nutr. Diet 2004, 61, e104. [Google Scholar]
- Regina, A.; Berbezy, P.; Kosar-Hashemi, B.; Li, S.Z.; Cmiel, M.; Larroque, O.; Bird, A.R.; Swain, S.M.; Cavanagh, C.; Jobling, S.A.; et al. A genetic strategy generating wheat with very high amylose content. Plant Biotechnol. J. 2015, 13, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Newberry, M.; Berbezy, P.; Belobrajdic, D.; Chapron, S.; Tabouillot, P.; Regina, A.; Bird, A. High-amylose wheat foods: A new opportunity to meet dietary fiber targets for health. Cereal Foods World 2018, 63, 188–193. [Google Scholar]
- Belobrajdic, D.; Regina, A.; Klingner, B.; Zajac, I.; Chapron, S.; Berbezy, P.; Bird, A. High-amylose wheat lowers the postprandial glycemic response to bread in healthy adults: A randomized controlled crossover trial. J. Nutr. 2019, 149, 1335–1345. [Google Scholar] [CrossRef] [PubMed]
- Aman, P.; Pettersson, D.; Zhang, J.X.; Tidehag, P.; Hallmans, G. Starch and dietary fiber components are excreted and degraded to variable extents in ileostomy subjects consuming mixed diets with wheat- or oat-bran bread. J. Nutr. 1995, 125, 2341–2347. [Google Scholar] [CrossRef]
- Muir, J.G.; Birkett, A.; Brown, I.; Jones, G.; O’Dea, K. Food processing and maize variety affects amounts of starch escaping digestion in the small intestine. Am. J. Clin. Nutr. 1995, 61, 82–89. [Google Scholar] [CrossRef]
- Zhou, Z.; Topping, D.L.; Morell, M.K.; Bird, A.R. Changes in starch physical characteristics following digestion of foods in the human small intestine. Br. J. Nutr. 2010, 104, 573–581. [Google Scholar] [CrossRef] [Green Version]
- Belobrajdic, D.P.; Wymond, B.; Benassi-Evans, B.; Bird, A.R. Digestive Health Effects of High Amylose Wheat in Healthy Australian Men and Women. Available online: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=375356 (accessed on 1 December 2020).
- Conlon, M.A.; Kerr, C.A.; McSweeney, C.S.; Dunne, R.A.; Shaw, J.M.; Kang, S.; Bird, A.R.; Morell, M.K.; Lockett, T.J.; Molloy, P.L.; et al. Resistant starches protect against colonic DNA damage and alter microbiota and gene expression in rats fed a western diet. J. Nutr. 2012, 142, 832–840. [Google Scholar] [CrossRef] [Green Version]
- Regina, A.; Bird, A.; Topping, D.; Bowden, S.; Freeman, J.; Barsby, T.; Kosar-Hashemi, B.; Li, Z.; Rahman, S.; Morell, M. High-amylose wheat generated by rna interference improves indices of large-bowel health in rats. Proc. Natl. Acad. Sci. USA 2006, 103, 3546–3551. [Google Scholar] [CrossRef] [Green Version]
- Aberg, S.; Mann, J.; Neumann, S.; Ross, A.B.; Reynolds, A.N. Whole-grain processing and glycemic control in type 2 diabetes: A randomized crossover trial. Diabetes Care 2020, 43, 1717–1723. [Google Scholar] [CrossRef]
- Yusof, B.N.M.; Talib, R.A.; Karim, N.A.; Kamarudin, N.A.; Arshad, F. Glycaemic index of four commercially available breads in malaysia. Int. J. Food Sci. Nutr. 2009, 60, 487–496. [Google Scholar] [CrossRef]
- Hettiaratchi, U.P.K.; Ekanayake, S.; Welihinda, J. Glycaemic indices of three sri lankan wheat bread varieties and a bread-lentil meal. Int. J. Food Sci. Nutr. 2009, 60, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Granfeldt, Y.E.; Drews, A.W.; Bjorck, I.M.E. Starch bioavailability in arepas made from ordinary or high amylose corn-concentration and gastrointestinal fate of resistant starch in rats. J. Nutr. 1993, 123, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Aldaz, D.G.; Guice, J.L.; Page, R.C.; Raggio, A.M.; Martin, R.J.; Husseneder, C.; Durham, H.A.; Geaghan, J.; Janes, M.; Gauthier, T.; et al. Simultaneous delivery of antibiotics neomycin and ampicillin in drinking water inhibits fermentation of resistant starch in rats. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Roe, M.; Brown, J.; Faulks, R.; Livesey, G. Is the rat a suitable model for humans on studies of cereal digestion? Eur. J. Clin. Nutr. 1996, 50, 710–712. [Google Scholar] [PubMed]
- Coles, L.T.; Moughan, P.J.; Awati, A.; Darragh, A.J.; Zou, M.L. Predicted apparent digestion of energy-yielding nutrients differs between the upper and lower digestive tracts in rats and humans. J. Nutr. 2010, 140, 469–476. [Google Scholar] [CrossRef]
- Perera, A.; Meda, V.; Tyler, R.T. Resistant starch a review of analytical protocols for determining resistant starch and of factors affecting the resistant starch content of foods. Food Res. Int. 2010, 43, 1959–1974. [Google Scholar] [CrossRef]
- Siljestrom, M.; Asp, N.G. Resistant starch formation during baking-effect of baking time and temperature and variations in the recipe. Z Lebensm Unters For. 1985, 181, 4–8. [Google Scholar] [CrossRef]
- Liljeberg, H.; Akerberg, A.; Bjorck, I. Resistant starch formation in bread as influenced by choice of ingredients or baking conditions. Food Chem. 1996, 56, 389–394. [Google Scholar] [CrossRef]
- Keenan, M.J.; Zhou, J.; McCutcheon, K.L.; Raggio, A.M.; Bateman, H.G.; Todd, E.; Jones, C.K.; Tulley, R.T.; Melton, S.; Martin, R.J.; et al. Effects of resistant starch, a non-digestible fermentable fiber, on reducing body fat. Obesity 2006, 14, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- Belobrajdic, D.P.; King, R.A.; Christophersen, C.T.; Bird, A.R. Dietary resistant starch dose-dependently reduces adiposity in obesity-prone and obesity-resistant male rats. Nutr. Metab. 2012, 9, 93. [Google Scholar] [CrossRef] [Green Version]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. Ain-93 purified diets for laboratory rodents: Final report of the american institute of nutrition ad hoc writing committee on the reformulation of the ain-76a rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.B. Determination of starch, including maltooligosaccharides, in animal feeds: Comparison of methods and a method recommended for aoac collaborative study. J. AOAC Int. 2009, 92. [Google Scholar] [CrossRef] [Green Version]
| LAW-R | HAW-R | LAW-W | HAW-W | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Digesta wet weight (g/day) | 548 | 129 | 542 | 97 | 629 | 254 | 693 | 174 |
| Digesta moisture (%) | 91.9 | 0.9 | 91.3 | 1.0 | 91.7 | 1.1 | 91.7 | 0.86 |
| Digesta dry weight (g/day) | 44 | 9 | 46 | 4 | 51 | 15 | 57 † | 11 |
| Total starch ingested (g/day) | 26.4 | 0.7 | 21.9 * | 0.6 | 20.9 | 0.6 | 17.0 † | 0.34 |
| Starch excreted (g/day) | 0.8 | 0.2 | 4.5 * | 0.2 | 0.65 | 0.2 | 3.9 † | 0.4 |
| Undigested Starch (% of starch intake) | 2.0 | 0.6 | 19.6 † | 1.0 | 2.0 | 0.5 | 21.1 † | 2.5 |
| Resistant Starch (g per 100 g bread) | 0.75 | 0.10 | 6.47 * | 0.32 | 0.61 | 0.18 | 5.09 † | 0.61 |
| Bread Diet | Flour Diet | |||||||
|---|---|---|---|---|---|---|---|---|
| LAW-R | HAW-R | LAW-R | HAW-R | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Digesta wet weight (g/day) | 3.0 | 0.7 | 7.3 †† | 0.5 | 3.4 | 0.8 | 6.0 * | 1.6 |
| Digesta moisture (%) | 53.2 | 1.0 | 49.0 | 4.4 | 52.9 | 5.8 | 49.7 | 3.6 |
| Digesta dry weight (g/day) | 1.4 | 0.3 | 3.7 † | 0.5 | 1.7 | 0.5 | 3.0 * | 0.7 |
| Total starch ingested (g/day) | 6.9 | 0.3 | 7.7 | 0.5 | 8.0 | 1.1 | 8.1 | 0.4 |
| Starch excreted (g/day) | 0.12 | 0.03 | 1.24 †† | 0.15 | 0.03 | 0.01 | 0.83 ** | 0.23 |
| Undigested Starch (% of starch intake) | 1.8 | 0.5 | 15.6 †† | 2.0 | 0.8 | 0.4 | 11.2 ** | 1.6 |
| Resistant Starch (g per 100 g) | 0.76 | 0.22 | 5.48 | 0.76 | 0.4 | 0.1 | 10.3 *** | 1.6 |
| LAW-R | HAW-R | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Test diets | ||||
| Phase 1, 11 day (n = 4) | ||||
| Body weight gain, g/day | 6.2 | 1.7 | 4.0 * | 1.6 |
| Feed intake, g/day | 21.2 | 1.0 | 22.1 | 3.7 |
| Feed conversion efficiency | 0.25 | 0.01 | 0.16 | 0.1 |
| Phase 2, 5 day (n = 4) | ||||
| Body weight gain, g/day | 2.1 | 0.6 | 1.8 | 1.1 |
| Feed intake, g/day | 20.9 | 2.2 | 22.1 | 1.1 |
| Feed conversion efficiency | 0.08 | 0.01 | 0.10 | 0.02 |
| Bread | ||||
| Phase 3, 7 day (n = 3) | ||||
| Body weight gain, g/day | 2.5 | 0.3 | 2.8 | 0.7 |
| Feed intake, g/day | 18.9 | 0.9 | 21.8 | 1.3 |
| Feed conversion efficiency | 0.13 | 0.01 | 0.13 | 0.05 |
| Flour | Bread | |||
|---|---|---|---|---|
| LAW | HAW | LAW | HAW | |
| Ingredients, g/kg | ||||
| LAW refined flour | 55.45 | |||
| HAW refined flour | 55.45 | |||
| LAW refined flour bread | 55.45 | |||
| HAW refined flour bread | 55.45 | |||
| Casein | 19 | 19 | 19 | 19 |
| Sucrose | 10 | 10 | 10 | 10 |
| Sunflower oil | 7 | 7 | 7 | 7 |
| Vitamin mix | 1 | 1 | 1 | 1 |
| Mineral mix | 3.5 | 3.5 | 3.5 | 3.5 |
| L-Cysteine | 0.3 | 0.3 | 0.3 | 0.3 |
| Choline | 0.25 | 0.25 | 0.25 | 0.25 |
| Alpha-cellulose | 3.5 | 3.5 | 3.5 | 3.5 |
| Total | 100 | 100 | 100 | 100 |
| Composition of diets | ||||
| Total starch | 34.8 | 32.0 | 38.1 | 33.7 |
| Protein | 21.7 | 24.5 | 17.0 | 17.0 |
| fat | 9.4 | 9.5 | 7.8 | 8.0 |
| Total dietary fibre | 5.7 | 10.1 | 4.8 | 6.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belobrajdic, D.P.; Bird, A.R. Evaluation of an Ileorectostomised Rat Model for Resistant Starch Determination. Nutrients 2021, 13, 91. https://doi.org/10.3390/nu13010091
Belobrajdic DP, Bird AR. Evaluation of an Ileorectostomised Rat Model for Resistant Starch Determination. Nutrients. 2021; 13(1):91. https://doi.org/10.3390/nu13010091
Chicago/Turabian StyleBelobrajdic, Damien P., and Anthony R. Bird. 2021. "Evaluation of an Ileorectostomised Rat Model for Resistant Starch Determination" Nutrients 13, no. 1: 91. https://doi.org/10.3390/nu13010091





