Vitamin D and the Risks of Depression and Anxiety: An Observational Analysis and Genome-Wide Environment Interaction Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. UK Biobank Cohort
2.2. UK Biobank Phenotypes of Vitamin D
2.3. UK Biobank Phenotypes of Depression and Anxiety
2.4. UK Biobank Genotyping, Imputation and Quality Control
2.5. GWAS Data of Vitamin D
2.6. PRS Analysis of Vitamin D
2.7. Statistical Analysis
2.8. Genome-Wide Environment Interaction Studies (GWEIS)
3. Results
3.1. General Population Characteristics
3.1.1. Characteristics of UK Biobank Subjects with Blood Vitamin D Data
3.1.2. Characteristics of UK Biobank Subjects with Vitamin D PRS Data
3.2. Regression Analysis Result
3.2.1. Associations between Blood Vitamin D and Depression, Anxiety Traits in UK Biobank Cohort
3.2.2. Associations between Vitamin D PRS and Depression, Anxiety Traits in UK Biobank Cohort
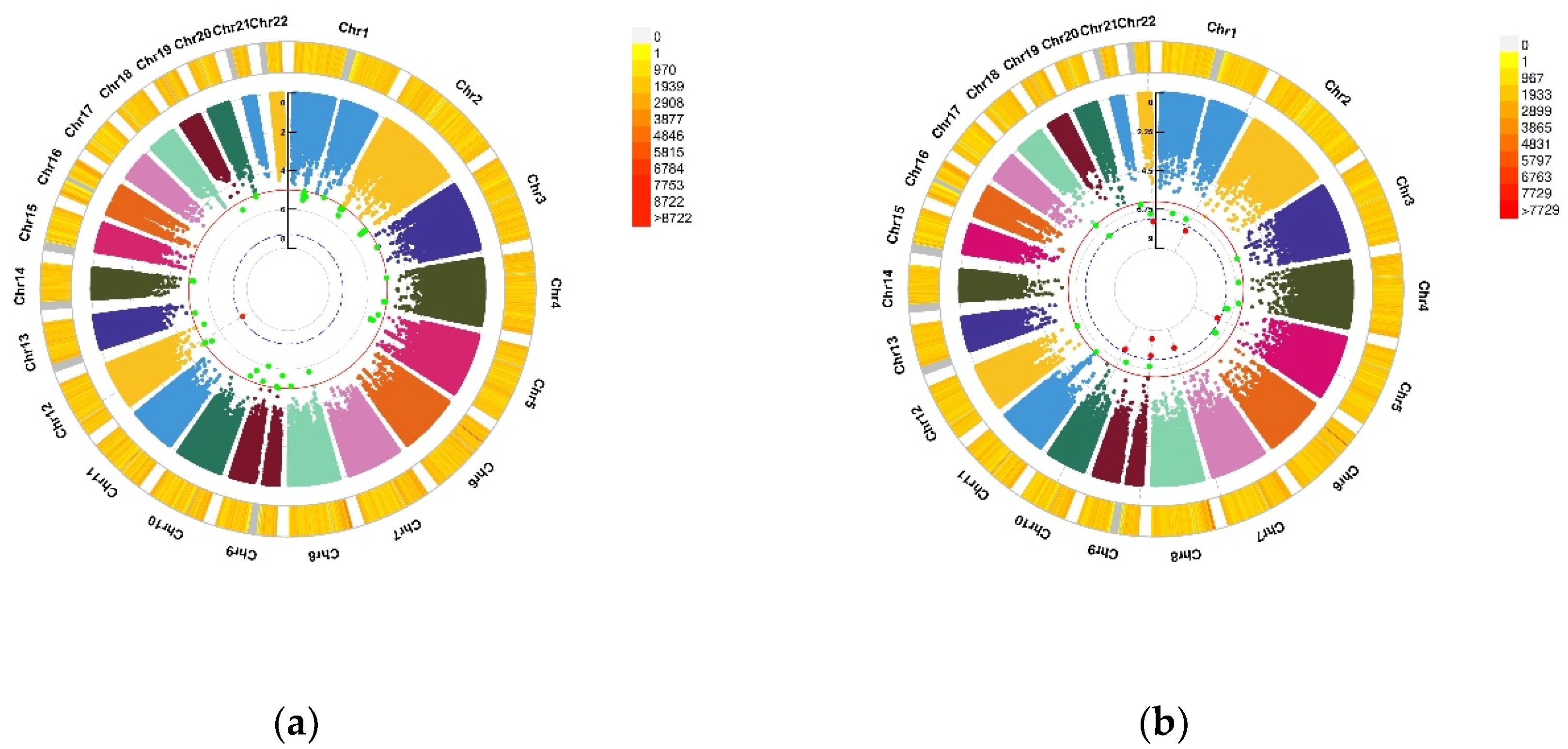
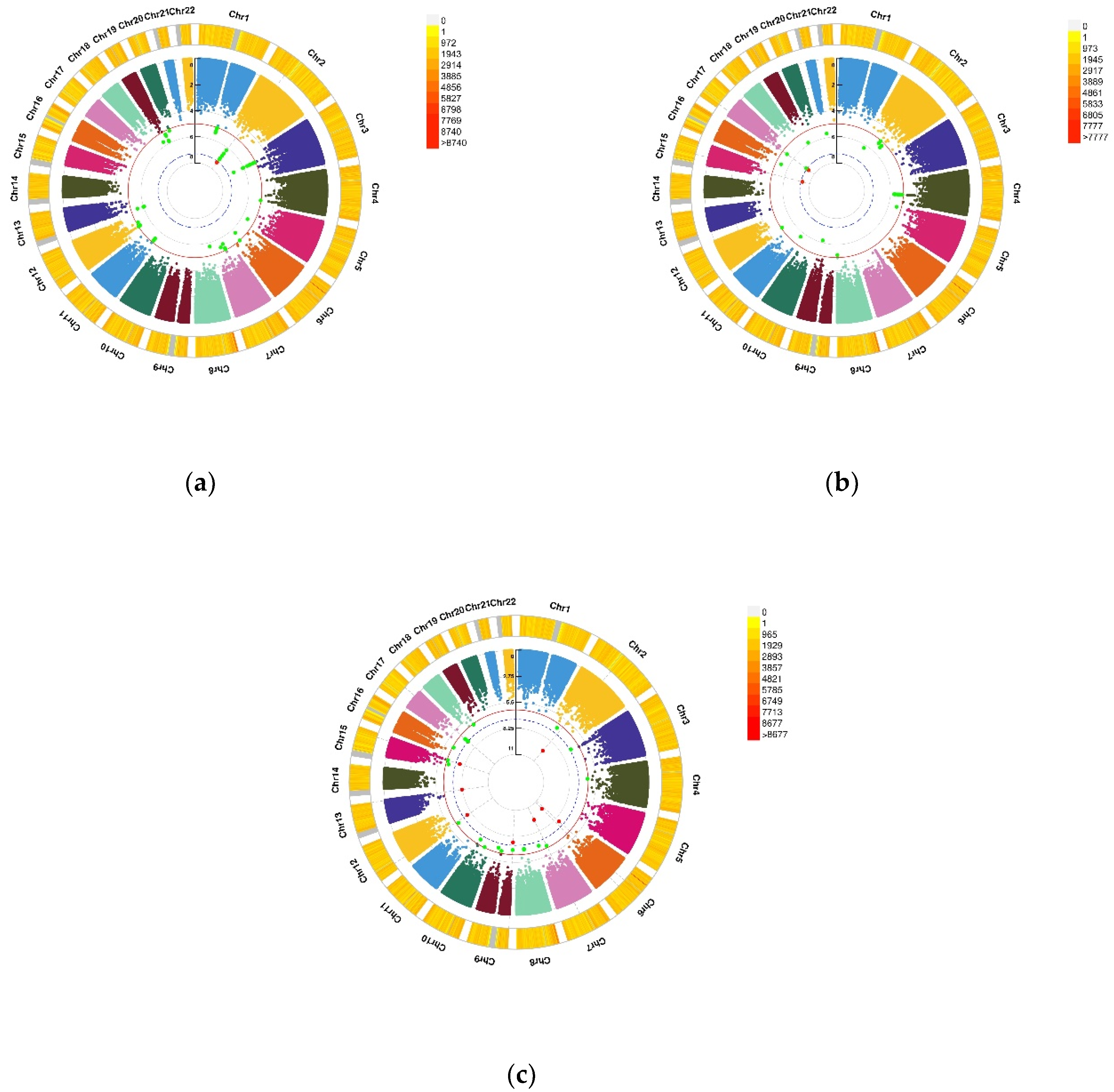
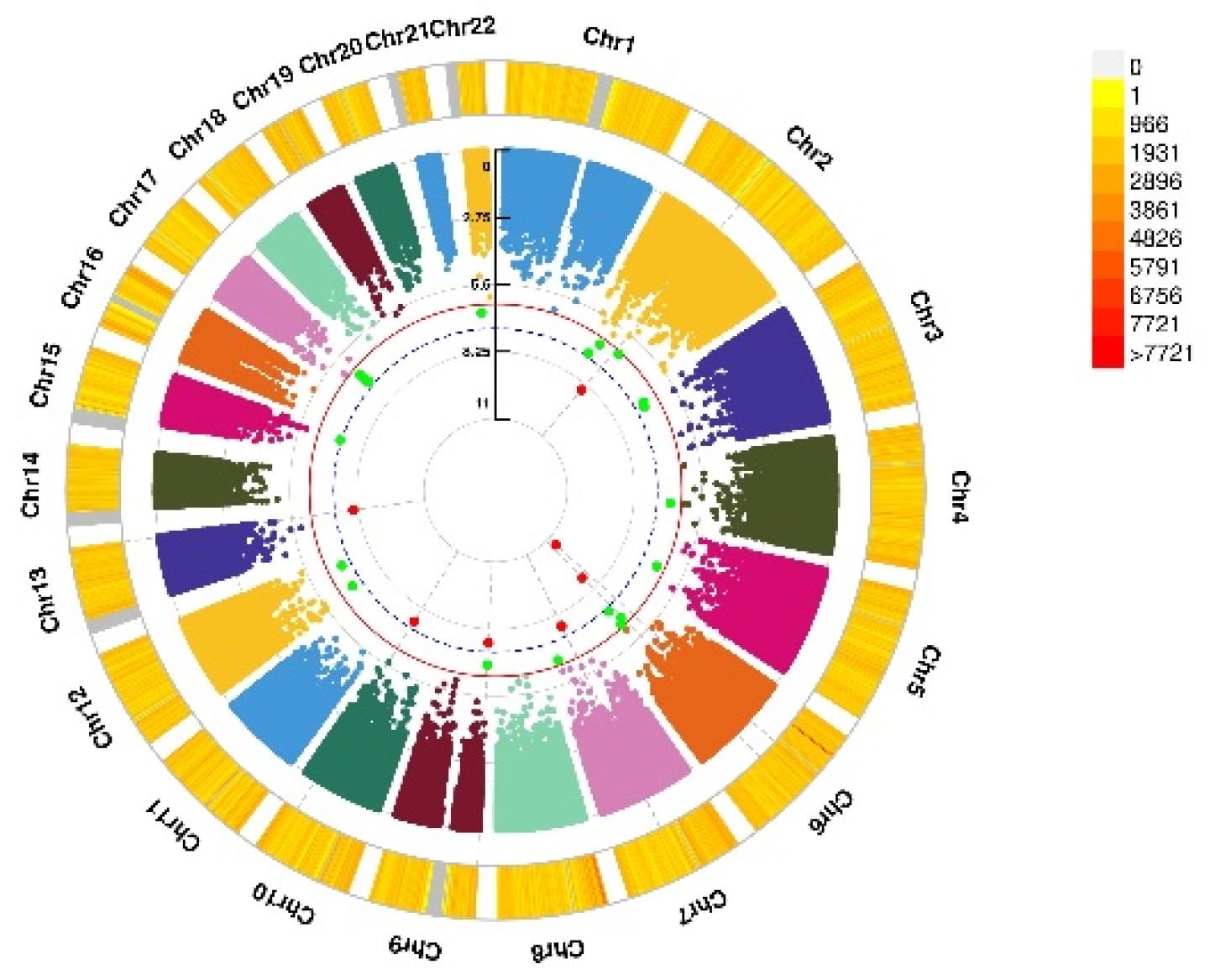
3.3. GWEIS Analysis Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Battle, D.E. Diagnostic and Statistical Manual of Mental Disorders (DSM). Codas 2013, 25, 191–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richter, D.; Wall, A.; Bruen, A.; Whittington, R. Is the global prevalence rate of adult mental illness increasing? Systematic review and meta-analysis. Acta Psychiatr. Scand. 2019, 140, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.J. Depression Is the Leading Cause of Disability Around the World. JAMA 2017, 317, 1517. [Google Scholar] [CrossRef] [PubMed]
- Bekhuis, E.; Boschloo, L.; Rosmalen, J.G.; Schoevers, R.A. Differential associations of specific depressive and anxiety disorders with somatic symptoms. J. Psychosom. Res. 2015, 78, 116–122. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.H.; Li, J.Q.; Shi, J.F.; Que, J.Y.; Liu, J.J.; Lappin, J.M.; Leung, J.; Ravindran, A.V.; Chen, W.Q.; Qiao, Y.L.; et al. Depression and anxiety in relation to cancer incidence and mortality: A systematic review and meta-analysis of cohort studies. Mol. Psychiatry 2020, 25, 1487–1499. [Google Scholar] [CrossRef]
- Groves, N.J.; McGrath, J.J.; Burne, T.H. Vitamin D as a neurosteroid affecting the developing and adult brain. Annu. Rev. Nutr. 2014, 34, 117–141. [Google Scholar] [CrossRef]
- Bahrami, A.; Sadeghnia, H.R.; Tabatabaeizadeh, S.A.; Bahrami-Taghanaki, H.; Behboodi, N.; Esmaeili, H.; Ferns, G.A.; Mobarhan, M.G.; Avan, A. Genetic and epigenetic factors influencing vitamin D status. J. Cell Physiol. 2018, 233, 4033–4043. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Albanes, D.; Berndt, S.I.; Peters, U.; Chatterjee, N.; Freedman, N.D.; Abnet, C.C.; Huang, W.Y.; Kibel, A.S.; Crawford, E.D.; et al. Vitamin D-related genes, serum vitamin D concentrations and prostate cancer risk. Carcinogenesis 2009, 30, 769–776. [Google Scholar] [CrossRef] [Green Version]
- Szpunar, M.J. Association of antepartum vitamin D deficiency with postpartum depression: A clinical perspective. Public Health Nutr. 2020, 23, 1173–1178. [Google Scholar] [CrossRef]
- Bersani, F.S.; Ghezzi, F.; Maraone, A.; Vicinanza, R.; Cavaggioni, G.; Biondi, M.; Pasquini, M. The relationship between Vitamin D and depressive disorders. Riv. Psichiatr. 2019, 54, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Anglin, R.E.; Samaan, Z.; Walter, S.D.; McDonald, S.D. Vitamin D deficiency and depression in adults: Systematic review and meta-analysis. Br. J. Psychiatry 2013, 202, 100–107. [Google Scholar] [CrossRef]
- Hoang, M.T.; Defina, L.F.; Willis, B.L.; Leonard, D.S.; Weiner, M.F.; Brown, E.S. Association between low serum 25-hydroxyvitamin D and depression in a large sample of healthy adults: The Cooper Center longitudinal study. Mayo Clin. Proc. 2011, 86, 1050–1055. [Google Scholar] [CrossRef] [Green Version]
- Fond, G.; Godin, O.; Schürhoff, F.; Berna, F.; Bulzacka, E.; Andrianarisoa, M.; Brunel, L.; Aouizerate, B.; Capdevielle, D.; Chereau, I.; et al. Hypovitaminosis D is associated with depression and anxiety in schizophrenia: Results from the national FACE-SZ cohort. Psychiatry Res. 2018, 270, 104–110. [Google Scholar] [CrossRef]
- Ikonen, H.; Palaniswamy, S.; Nordstrom, T.; Jarvelin, M.R.; Herzig, K.H.; Jaaskelainen, E.; Seppala, J.; Miettunen, J.; Sebert, S. Vitamin D status and correlates of low vitamin D in schizophrenia, other psychoses and non-psychotic depression—The Northern Finland Birth Cohort 1966 study. Psychiatry Res. 2019, 279, 186–194. [Google Scholar] [CrossRef] [Green Version]
- Libuda, L.; Laabs, B.H.; Ludwig, C.; Bühlmeier, J.; Antel, J.; Hinney, A.; Naaresh, R.; Föcker, M.; Hebebrand, J.; König, I.R.; et al. Vitamin D and the Risk of Depression: A Causal Relationship? Findings from a Mendelian Randomization Study. Nutrients 2019, 11, 1085. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; Jeon, S.W.; Lim, W.J.; Oh, K.S.; Shin, D.W.; Cho, S.J.; Park, J.H.; Shin, Y.C. The Relationship between Serum Vitamin D Levels, C-Reactive Protein, and Anxiety Symptoms. Psychiatry Investig. 2020, 17, 312–319. [Google Scholar] [CrossRef]
- Niles, A.N.; Smirnova, M.; Lin, J.; O’Donovan, A. Gender differences in longitudinal relationships between depression and anxiety symptoms and inflammation in the health and retirement study. Psychoneuroendocrinology 2018, 95, 149–157. [Google Scholar] [CrossRef]
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 734. [Google Scholar] [CrossRef] [PubMed]
- Luna, R.A.; Foster, J.A. Gut brain axis: Diet microbiota interactions and implications for modulation of anxiety and depression. Curr. Opin. Biotechnol. 2015, 32, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Bear, T.L.K.; Dalziel, J.E.; Coad, J.; Roy, N.C.; Butts, C.A.; Gopal, P.K. The Role of the Gut Microbiota in Dietary Interventions for Depression and Anxiety. Adv. Nutr. 2020, 11, 890–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, P.F.; Neale, M.C.; Kendler, K.S. Genetic epidemiology of major depression: Review and meta-analysis. Am. J. Psychiatry 2000, 157, 1552–1562. [Google Scholar] [CrossRef]
- Kendler, K.S.; Gatz, M.; Gardner, C.O.; Pedersen, N.L. A Swedish national twin study of lifetime major depression. Am. J. Psychiatry 2006, 163, 109–114. [Google Scholar] [CrossRef]
- Purves, K.L.; Coleman, J.R.I.; Meier, S.M.; Rayner, C.; Davis, K.A.S.; Cheesman, R.; Bækvad-Hansen, M.; Børglum, A.D.; Wan Cho, S.; Jürgen Deckert, J.; et al. A major role for common genetic variation in anxiety disorders. Mol. Psychiatry 2020, 25, 3292–3303. [Google Scholar] [CrossRef] [Green Version]
- Dudbridge, F. Power and predictive accuracy of polygenic risk scores. PLoS Genet. 2013, 9, e1003348. [Google Scholar] [CrossRef]
- Euesden, J.; Lewis, C.M.; O’Reilly, P.F. PRSice: Polygenic Risk Score software. Bioinformatics 2015, 31, 1466–1468. [Google Scholar] [CrossRef] [Green Version]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014, 511, 421–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.P.; Robinson, D.; Yu, J.; Gallego, J.; Fleischhacker, W.W.; Kahn, R.S.; Crespo-Facorro, B.; Vazquez-Bourgon, J.; Kane, J.M.; Malhotra, A.K.; et al. Schizophrenia Polygenic Risk Score as a Predictor of Antipsychotic Efficacy in First-Episode Psychosis. Am. J. Psychiatry 2019, 176, 21–28. [Google Scholar] [CrossRef]
- Revez, J.A.; Lin, T.; Qiao, Z.; Xue, A.; Holtz, Y.; Zhu, Z.; Zeng, J.; Wang, H.; Sidorenko, J.; Kemper, K.E.; et al. Genome-wide association study identifies 143 loci associated with 25 hydroxyvitamin D concentration. Nat. Commun. 2020, 11, 1647. [Google Scholar] [CrossRef] [Green Version]
- Hou, L.; Bergen, S.E.; Akula, N.; Song, J.; Hultman, C.M.; Landén, M.; Adli, M.; Alda, M.; Ardau, R.; Arias, B.; et al. Genome-wide association study of 40,000 individuals identifies two novel loci associated with bipolar disorder. Hum. Mol. Genet. 2016, 25, 3383–3394. [Google Scholar] [CrossRef]
- Rask-Andersen, M.; Karlsson, T.; Ek, W.E.; Johansson, Å. Gene-environment interaction study for BMI reveals interactions between genetic factors and physical activity, alcohol consumption and socioeconomic status. PLoS Genet. 2017, 13, e1006977. [Google Scholar] [CrossRef] [Green Version]
- van Os, J.; Rutten, B.P. Gene-environment-wide interaction studies in psychiatry. Am. J. Psychiatry 2009, 166, 964–966. [Google Scholar] [CrossRef]
- Rivera, N.V.; Patasova, K.; Kullberg, S.; Diaz-Gallo, L.M.; Iseda, T.; Bengtsson, C.; Alfredsson, L.; Eklund, A.; Kockum, I.; Grunewald, J.; et al. A Gene-Environment Interaction Between Smoking and Gene polymorphisms Provides a High Risk of Two Subgroups of Sarcoidosis. Sci. Rep. 2019, 9, 18633. [Google Scholar] [CrossRef]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef] [Green Version]
- Davis, K.A.S.; Cullen, B.; Adams, M.; Brailean, A.; Breen, G.; Coleman, J.R.I.; Dregan, A.; Gaspar, H.A.; Hübel, C.; Lee, W.; et al. Indicators of mental disorders in UK Biobank-A comparison of approaches. Int. J. Methods Psychiatr. Res. 2019, 28, e1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.; Löwe, B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef] [Green Version]
- Gigantesco, A.; Morosini, P. Development, reliability and factor analysis of a self-administered questionnaire which originates from the World Health Organization’s Composite International Diagnostic Interview—Short Form (CIDI-SF) for assessing mental disorders. Clin. Pract. Epidemiol. Ment. Health 2008, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Manea, L.; Gilbody, S.; McMillan, D. Optimal cut-off score for diagnosing depression with the Patient Health Questionnaire (PHQ-9): A meta-analysis. CMAJ 2012, 184, E191–E196. [Google Scholar] [CrossRef] [Green Version]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B.; Löwe, B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: A systematic review. Gen. Hosp. Psychiatry 2010, 32, 345–359. [Google Scholar] [CrossRef]
- Bycroft, C.; Freeman, C.; Petkova, D.; Band, G.; Elliott, L.T.; Sharp, K.; Motyer, A.; Vukcevic, D.; Delaneau, O.; O’Connell, J.; et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018, 562, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Walter, K.; Min, J.L.; Huang, J.; Crooks, L.; Memari, Y.; McCarthy, S.; Perry, J.R.; Xu, C.; Futema, M.; Lawson, D.; et al. The UK10K project identifies rare variants in health and disease. Nature 2015, 526, 82–90. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, S.; Das, S.; Kretzschmar, W.; Delaneau, O.; Wood, A.R.; Teumer, A.; Kang, H.M.; Fuchsberger, C.; Danecek, P.; Sharp, K.; et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat. Genet. 2016, 48, 1279–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Ferreira, T.; Morris, A.P.; Medland, S.E.; Madden, P.A.; Heath, A.C.; Martin, N.G.; Montgomery, G.W.; Weedon, M.N.; Loos, R.J.; et al. Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. Nat. Genet. 2012, 44, s361–s363. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience 2015, 4, 7. [Google Scholar] [CrossRef]
- Clarke, G.M.; Morris, A.P. A comparison of sample size and power in case-only association studies of gene-environment interaction. Am. J. Epidemiol. 2010, 171, 498–505. [Google Scholar] [CrossRef] [Green Version]
- Kimball, S.M.; Mirhosseini, N.; Rucklidge, J. Database Analysis of Depression and Anxiety in a Community Sample-Response to a Micronutrient Intervention. Nutrients 2018, 10, 152. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Zhang, Y.; Wang, T.; Lin, Y.; Yu, J.; Xia, Q.; Zhu, P.; Zhu, D.M. Vitamin D supplementation improves anxiety but not depression symptoms in patients with vitamin D deficiency. Brain Behav. 2020, 10, e01760. [Google Scholar] [CrossRef]
- Zhao, G.; Ford, E.S.; Li, C.; Balluz, L.S. No associations between serum concentrations of 25-hydroxyvitamin D and parathyroid hormone and depression among US adults. Br. J. Nutr. 2010, 104, 1696–1702. [Google Scholar] [CrossRef] [Green Version]
- Nanri, A.; Mizoue, T.; Matsushita, Y.; Poudel-Tandukar, K.; Sato, M.; Ohta, M.; Mishima, N. Association between serum 25-hydroxyvitamin D and depressive symptoms in Japanese: Analysis by survey season. Eur. J. Clin. Nutr. 2009, 63, 1444–1447. [Google Scholar] [CrossRef]
- Pan, A.; Lu, L.; Franco, O.H.; Yu, Z.; Li, H.; Lin, X. Association between depressive symptoms and 25-hydroxyvitamin D in middle-aged and elderly Chinese. J. Affect. Disord. 2009, 118, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Milaneschi, Y.; Hoogendijk, W.; Lips, P.; Heijboer, A.C.; Schoevers, R.; van Hemert, A.M.; Beekman, A.T.; Smit, J.H.; Penninx, B.W. The association between low vitamin D and depressive disorders. Mol. Psychiatry 2014, 19, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson, A.; Arias de la Torre, J.; Gaughran, F.; Bakolis, I.; Hatch, S.L.; Hotopf, M.; Dregan, A. Prospective associations between vitamin D and depression in middle-aged adults: Findings from the UK Biobank cohort. Psychol. Med. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Laurén, J.; Airaksinen, M.S.; Saarma, M.; Timmusk, T. A novel gene family encoding leucine-rich repeat transmembrane proteins differentially expressed in the nervous system. Genomics 2003, 81, 411–421. [Google Scholar] [CrossRef]
- Reichman, R.D.; Gaynor, S.C.; Monson, E.T.; Gaine, M.E.; Parsons, M.G.; Zandi, P.P.; Potash, J.B.; Willour, V.L. Targeted sequencing of the LRRTM gene family in suicide attempters with bipolar disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2020, 183, 128–139. [Google Scholar] [CrossRef]
- Marini, F.; Bartoccini, E.; Cascianelli, G.; Voccoli, V.; Baviglia, M.G.; Magni, M.V.; Garcia-Gil, M.; Albi, E. Effect of 1alpha,25-dihydroxyvitamin D3 in embryonic hippocampal cells. Hippocampus 2010, 20, 696–705. [Google Scholar] [CrossRef]
- Eyles, D.; Almeras, L.; Benech, P.; Patatian, A.; Mackay-Sim, A.; McGrath, J.; Féron, F. Developmental vitamin D deficiency alters the expression of genes encoding mitochondrial, cytoskeletal and synaptic proteins in the adult rat brain. J. Steroid Biochem. Mol. Biol. 2007, 103, 538–545. [Google Scholar] [CrossRef]
- Shamseldin, H.E.; Masuho, I.; Alenizi, A.; Alyamani, S.; Patil, D.N.; Ibrahim, N.; Martemyanov, K.A.; Alkuraya, F.S. GNB5 mutation causes a novel neuropsychiatric disorder featuring attention deficit hyperactivity disorder, severely impaired language development and normal cognition. Genome Biol. 2016, 17, 195. [Google Scholar] [CrossRef] [Green Version]
- Gezen-Ak, D.; Dursun, E.; Yilmazer, S. The effects of vitamin D receptor silencing on the expression of LVSCC-A1C and LVSCC-A1D and the release of NGF in cortical neurons. PLoS ONE 2011, 6, e17553. [Google Scholar] [CrossRef]
- Grimm, M.O.W.; Lauer, A.A.; Grösgen, S.; Thiel, A.; Lehmann, J.; Winkler, J.; Janitschke, D.; Herr, C.; Beisswenger, C.; Bals, R.; et al. Profiling of Alzheimer’s disease related genes in mild to moderate vitamin D hypovitaminosis. J. Nutr. Biochem. 2019, 67, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Mai, J.H.; Ou, Z.H.; Chen, L.; Duan, J.; Liao, J.X.; Han, C.X. Intellectual developmental disorder with cardiac arrhythmia syndrome in a family caused by GNB5 variation and literature review. Chin. J. Pediatrics 2020, 58, 833–837. [Google Scholar] [CrossRef]
- Sun, W.; Maffie, J.K.; Lin, L.; Petralia, R.S.; Rudy, B.; Hoffman, D.A. DPP6 establishes the A-type K(+) current gradient critical for the regulation of dendritic excitability in CA1 hippocampal neurons. Neuron 2011, 71, 1102–1115. [Google Scholar] [CrossRef] [Green Version]
- Cacace, R.; Heeman, B.; Van Mossevelde, S.; De Roeck, A.; Hoogmartens, J.; De Rijk, P.; Gossye, H.; De Vos, K.; De Coster, W.; Strazisar, M.; et al. Loss of DPP6 in neurodegenerative dementia: A genetic player in the dysfunction of neuronal excitability. Acta Neuropathol. 2019, 137, 901–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.; Murphy, J.G.; Karlsson, R.M.; Petralia, R.S.; Gutzmann, J.J.; Abebe, D.; Wang, Y.X.; Cameron, H.A.; Hoffman, D.A. DPP6 Loss Impacts Hippocampal Synaptic Development and Induces Behavioral Impairments in Recognition, Learning and Memory. Front. Cell Neurosci. 2018, 12, 84. [Google Scholar] [CrossRef] [Green Version]
- Tang, B.L. Vesicle transport through interaction with t-SNAREs 1a (Vti1a)’s roles in neurons. Heliyon 2020, 6, e04600. [Google Scholar] [CrossRef]
- Oh, W.J.; Gu, C. The role and mechanism-of-action of Sema3E and Plexin-D1 in vascular and neural development. Semin Cell Dev. Biol. 2013, 24, 156–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casale, M.; Borriello, A.; Scianguetta, S.; Roberti, D.; Caiazza, M.; Bencivenga, D.; Tartaglione, I.; Ladogana, S.; Maruzzi, M.; Della Ragione, F.; et al. Hereditary hypochromic microcytic anemia associated with loss-of-function DMT1 gene mutations and absence of liver iron overload. Am. J. Hematol. 2018, 93, E58–E60. [Google Scholar] [CrossRef] [Green Version]
- Bastian, T.W.; von Hohenberg, W.C.; Mickelson, D.J.; Lanier, L.M.; Georgieff, M.K. Iron Deficiency Impairs Developing Hippocampal Neuron Gene Expression, Energy Metabolism, and Dendrite Complexity. Dev. Neurosci. 2016, 38, 264–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saadat, S.M.; Değirmenci, İ.; Özkan, S.; Saydam, F.; Özdemir Köroğlu, Z.; Çolak, E.; Güneş, H.V. Is the 1254T > C polymorphism in the DMT1 gene associated with Parkinson’s disease? Neurosci. Lett. 2015, 594, 51–54. [Google Scholar] [CrossRef] [PubMed]
- López, L.; Zuluaga, M.J.; Lagos, P.; Agrati, D.; Bedó, G. The Expression of Hypoxia-Induced Gene 1 (Higd1a) in the Central Nervous System of Male and Female Rats Differs According to Age. J. Mol. Neurosci. 2018, 66, 462–473. [Google Scholar] [CrossRef]
- Cui, X.; McGrath, J.J.; Burne, T.H.; Mackay-Sim, A.; Eyles, D.W. Maternal vitamin D depletion alters neurogenesis in the developing rat brain. Int. J. Dev. Neurosci. 2007, 25, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Brewer, L.D.; Thibault, V.; Chen, K.C.; Langub, M.C.; Landfield, P.W.; Porter, N.M. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J. Neurosci. 2001, 21, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Cross-Disorder Group of the Psychiatric Genomics Consortium. Genomic Relationships, Novel Loci, and Pleiotropic Mechanisms across Eight Psychiatric Disorders. Cell 2019, 179, 1469–1482.e1411. [Google Scholar] [CrossRef] [Green Version]
- Garber, J.; Brunwasser, S.M.; Zerr, A.A.; Schwartz, K.T.; Sova, K.; Weersing, V.R. Treatment and Prevention of Depression and Anxiety in Youth: Test of Cross-Over Effects. Depress. Anxiety 2016, 33, 939–959. [Google Scholar] [CrossRef]
- Balogh, L.; Tanaka, M.; Török, N.; Vécsei, L.; Taguchi, S. Crosstalk between Existential Phenomenological Psychotherapy and Neurological Sciences in Mood and Anxiety Disorders. Biomedicines 2021, 9, 340. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. Editorial of Special Issue “Crosstalk between Depression, Anxiety, and Dementia: Comorbidity in Behavioral Neurology and Neuropsychiatry”. Biomedicines 2021, 9, 517. [Google Scholar] [CrossRef] [PubMed]
- Middeldorp, C.M.; Cath, D.C.; Van Dyck, R.; Boomsma, D.I. The co-morbidity of anxiety and depression in the perspective of genetic epidemiology. A review of twin and family studies. Psychol. Med. 2005, 35, 611–624. [Google Scholar] [CrossRef]
- Thorp, J.G.; Campos, A.I.; Grotzinger, A.D.; Gerring, Z.F.; An, J.; Ong, J.S.; Wang, W.; Shringarpure, S.; Byrne, E.M.; MacGregor, S.; et al. Symptom-level modelling unravels the shared genetic architecture of anxiety and depression. Nat. Hum. Behav. 2021, 1–11. [Google Scholar] [CrossRef]
- Gonda, X.; Petschner, P.; Eszlari, N.; Sutori, S.; Gal, Z.; Koncz, S.; Anderson, I.M.; Deakin, B.; Juhasz, G.; Bagdy, G. Effects of Different Stressors Are Modulated by Different Neurobiological Systems: The Role of GABA-A Versus CB1 Receptor Gene Variants in Anxiety and Depression. Front. Cell. Neurosci. 2019, 13, 138. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.A.; Cuthbert, B.; Lewis-Fernández, R.; Narrow, W.E.; Reed, G.M. Three Approaches to Understanding and Classifying Mental Disorder: ICD-11, DSM-5, and the National Institute of Mental Health’s Research Domain Criteria (RDoC). Psychol. Sci. Public Interes. 2017, 18, 72–145. [Google Scholar] [CrossRef] [Green Version]
| Outcome Variable | Independent Variable | Number/ (Case/Control) | Sex (Female) | Age ± SD |
|---|---|---|---|---|
| Depression status | Blood VD | 52,766/57,978 | 61,458 (55.50%) | 56.40 ±7.68 |
| VDPRS After COJO | 58,349/63,336 | 68,365 (56.18%) | 56.47 ± 7.65 | |
| VDPRS Before COJO | 58,349/63,336 | 68,365 (56.18%) | 56.47 ± 7.65 | |
| Anxiety status | Blood VD | 19,759/79,025 | 53,541 (54.20%) | 56.42 ± 7.60 |
| VDPRS After COJO | 21,807/86,502 | 59,453 (54.89%) | 56.50 ± 7.57 | |
| VDPRS Before COJO | 21,807/86,502 | 59,453 (54.89%) | 56.50 ± 7.57 | |
| Depression (PHQ score) | Blood VD | 109,543 | 60,377 (55.12%) | 56.16 ± 7.65 |
| VDPRS After COJO | 120,033 | 66,934 (55.76%) | 56.24 ± 7.62 | |
| VDPRS Before COJO | 120,033 | 66,934 (55.76%) | 56.24 ± 7.62 | |
| Anxiety (GAD score) | Blood VD | 110,023 | 60,629 (55.11%) | 56.15 ± 7.65 |
| VDPRS After COJO VDPRS Before COJO | 120,590 120,590 | 67,235 (55.76%) 67,235 (55.76%) | 56.23 ± 7.61 56.23 ± 7.61 |
| Outcome Variable | Independent Variable | Beta | SE | T | p−Value | OR |
|---|---|---|---|---|---|---|
| Depression status | Blood VD | −0.12 | 0.01 | −18.57 | 5.92 × 10−77 | 0.89 |
| VDPRS After COJO | −0.014 | 0.006 | −2.36 | 1.84 × 10−2 | 0.99 | |
| VDPRS Before COJO | −0.012 | 0.006 | −2.07 | 3.82 × 10−2 | 0.99 | |
| Anxiety status | Blood VD | −0.080 | 0.01 | −9.77 | 1.46 × 10−22 | 0.92 |
| VDPRS After COJO | 0.00 | 0.01 | −0.29 | 7.71 × 10−1 | 1.00 | |
| VDPRS Before COJO | 0.00 | 0.01 | −0.32 | 7.47 × 10−1 | 1.00 | |
| Depression (PHQ score) | Blood VD | −0.062 | 0.003 | −20.81 | 5.95 × 10−96 | – |
| VDPRS After COJO | −0.007 | 0.003 | −2.61 | 9.15 × 10−3 | – | |
| VDPRS Before COJO | −0.006 | 0.003 | −2.14 | 3.25 × 10−2 | – | |
| Anxiety (GAD score) | Blood VD | −0.030 | 0.00 | −9.56 | 1.21 × 10−21 | – |
| VDPRS After COJO VDPRS | −0.010 | 0.00 | −2.57 | 1.02 × 10−2 | – | |
| Before COJO | −0.010 | 0.00 | −2.02 | 4.36 × 10−2 | – |
| CHR | SNP | Model | Beta | SE | Gene | p−Value | |
|---|---|---|---|---|---|---|---|
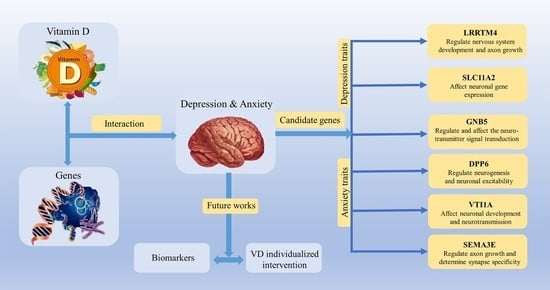
| Depression status | 2 | rs114086183 | ADD ×VD PRS afterCOJO | 0.16 | 0.029 | LRRTM4 | 4.11 × 10−8 |
| Anxiety status | 15 | rs149760119 | ADD × VD PRS afterCOJO | −0.22 | 0.04 | GNB5 | 3.88 × 10−8 |
| Depression (PHQ score) | 12 | rs117102029 | ADD × VD blood | 0.01 | 0.002 | SLC11A2, HIGD1C | 4.02 × 10−8 |
| Anxiety (GAD score) | 1 | rs142593645 | ADD × VD blood | 1.52 | 0.27 | SMYD3 | 2.51 × 10−8 |
| 7 | rs13228257 | ADD × VD blood | −1.25 | 0.22 | DPP6 | 1.45 × 10−8 | |
| 7 | rs76440131 | ADD × VD PRS afterCOJO | −2.64 | 0.42 | SEMA3E | 2.80 × 10−10 | |
| 9 | rs78029983 | ADD × VD PRS afterCOJO | −1.08 | 0.19 | DOCK8 | 2.43 × 10−10 | |
| 13 | rs76004204 | ADD × VD PRS afterCOJO | 2.17 | 0.37 | TMCO3 | 6.38 × 10−9 | |
| 7 | rs76440131 | ADD × VD PRS beforeCOJO | −2.16 | 0.38 | SEMA3E | 1.76 × 10−8 | |
| 9 | rs78029983 | ADD × VD PRS beforeCOJO | −1.10 | 0.20 | DOCK8 | 2.10 × 10−8 | |
| 10 | rs17266687 | ADD × VD PRS beforeCOJO | −1.20 | 0.21 | VTI1A | 2.48 × 10−8 | |
| 13 | rs76004204 | ADD × VD PRS beforeCOJO | 1.97 | 0.34 | TMCO3 | 8.89 × 10−9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Yang, X.; Jia, Y.; Wen, Y.; Cheng, S.; Meng, P.; Li, C.; Zhang, H.; Pan, C.; Zhang, J.; et al. Vitamin D and the Risks of Depression and Anxiety: An Observational Analysis and Genome-Wide Environment Interaction Study. Nutrients 2021, 13, 3343. https://doi.org/10.3390/nu13103343
Zhang Z, Yang X, Jia Y, Wen Y, Cheng S, Meng P, Li C, Zhang H, Pan C, Zhang J, et al. Vitamin D and the Risks of Depression and Anxiety: An Observational Analysis and Genome-Wide Environment Interaction Study. Nutrients. 2021; 13(10):3343. https://doi.org/10.3390/nu13103343
Chicago/Turabian StyleZhang, Zhen, Xuena Yang, Yumeng Jia, Yan Wen, Shiqiang Cheng, Peilin Meng, Chun’e Li, Huijie Zhang, Chuyu Pan, Jingxi Zhang, and et al. 2021. "Vitamin D and the Risks of Depression and Anxiety: An Observational Analysis and Genome-Wide Environment Interaction Study" Nutrients 13, no. 10: 3343. https://doi.org/10.3390/nu13103343
APA StyleZhang, Z., Yang, X., Jia, Y., Wen, Y., Cheng, S., Meng, P., Li, C., Zhang, H., Pan, C., Zhang, J., Chen, Y., & Zhang, F. (2021). Vitamin D and the Risks of Depression and Anxiety: An Observational Analysis and Genome-Wide Environment Interaction Study. Nutrients, 13(10), 3343. https://doi.org/10.3390/nu13103343