Low Circulating Concentrations of Very Long Chain Saturated Fatty Acids Are Associated with High Risk of Mortality in Kidney Transplant Recipients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Assessment of Dietary Intake
2.3. Clinical Parameters
2.4. Assessment of Plasma VLSFA
2.5. Study Endpoints
2.6. Statistical Analyses
3. Results
3.1. VLSFA in KTR and Healthy Controls
3.2. Associations between VLSFA and Clinical Baseline Characteristics in KTR
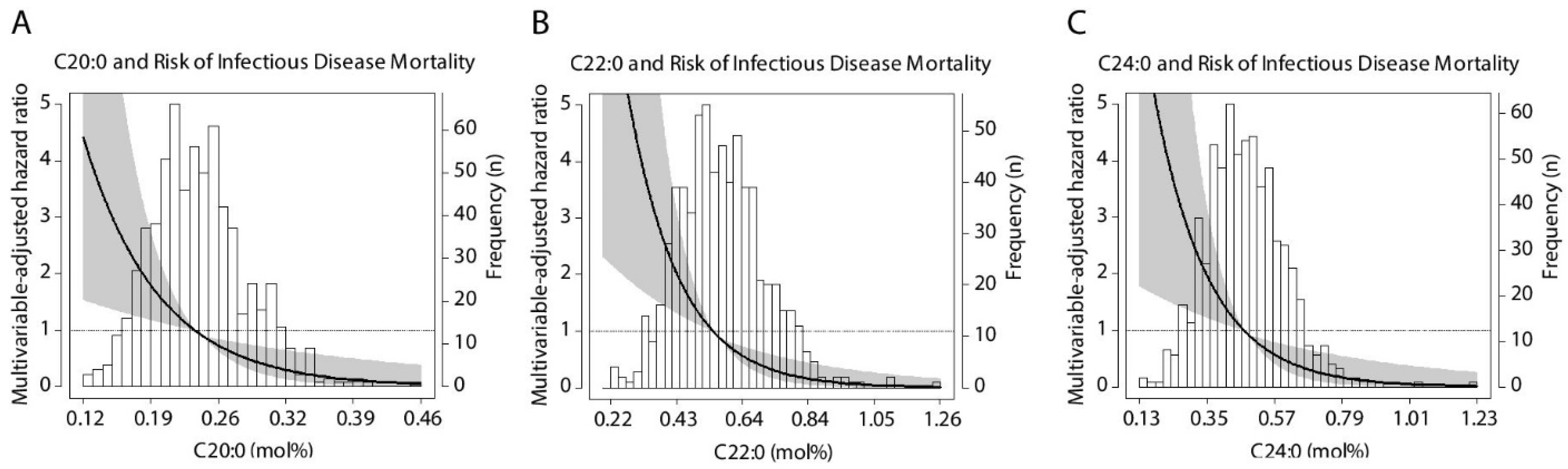
3.3. Prospective Analyses of All-Cause and Cause-Specific Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolfe, R.A.; Ashby, V.B.; Milford, E.L.; Ojo, A.O.; Ettenger, R.E.; Agodoa, L.Y.C.; Held, P.J.; Port, F.K. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N. Engl. J. Med. 1999, 341, 1725–1730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jofré, R.; López-Gómez, J.M.; Moreno, F.; Sanz-Guajardo, D.; Valderrábano, F. Changes in quality of life after renal transplantation. Am. J. Kidney Dis. 1998, 32, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Laupacis, A.; Keown, P.; Pus, N.; Krueger, H.; Ferguson, B.; Wong, C.; Muirhead, N. A study of the quality of life and cost-utility of renal transplantation. Kidney Int. 1996, 50, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briggs, J.D. Causes of death after renal transplantation. Nephrol. Dial. Transplant. 2001, 16, 1545–1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fishman, J.A. Infection in solid-organ transplant recipients. N. Engl. J. Med. 2007, 357, 2601–2614. [Google Scholar] [CrossRef] [Green Version]
- Pagalilauan, G.L.; Limaye, A.P. Infections in Transplant Patients. Med. Clin. N. Am. 2013, 97, 581–600. [Google Scholar] [CrossRef]
- Blazik, M.; Hutchinson, P.; Jose, M.D.; Polkinghorne, K.R.; Holdsworth, S.R.; Atkins, R.C.; Chadban, S.J. Leukocyte phenotype and function predicts infection risk in renal transplant recipients. Nephrol. Dial. Transplant. 2005, 20, 2226–2230. [Google Scholar] [CrossRef] [Green Version]
- Hutchinson, P.; Chadban, S.J.; Atkins, R.C.; Holdsworth, S.R. Laboratory assessment of immune function in renal transplant patients. Nephrol. Dial. Transplant. 2003, 18, 983–989. [Google Scholar] [CrossRef] [Green Version]
- Bicker, H.; Höflich, C.; Wolk, K.; Vogt, K.; Volk, H.D.; Sabat, R. A simple assay to measure phagocytosis of live bacteria. Clin. Chem. 2008, 54, 911–915. [Google Scholar] [CrossRef] [Green Version]
- Tourneur, E.; Ben Mkaddem, S.; Chassin, C.; Bens, M.; Goujon, J.M.; Charles, N.; Pellefigues, C.; Aloulou, M.; Hertig, A.; Monteiro, R.C.; et al. Cyclosporine A Impairs Nucleotide Binding Oligomerization Domain (Nod1)-Mediated Innate Antibacterial Renal Defenses in Mice and Human Transplant Recipients. PLoS Pathog. 2013, 9, e1003152. [Google Scholar] [CrossRef] [Green Version]
- Pathak, D.; Mehendale, N.; Singh, S.; Mallik, R.; Kamat, S.S. Lipidomics Suggests a New Role for Ceramide Synthase in Phagocytosis. ACS Chem. Biol. 2018, 13, 2280–2287. [Google Scholar] [CrossRef] [Green Version]
- Haney, M.S.; Bohlen, C.J.; Morgens, D.W.; Ousey, J.A.; Barkal, A.A.; Tsui, C.K.; Ego, B.; Levin, R.; Kamber, R.; Tucker, A.; et al. Identification of phagocytosis regulators using magnetic genome-wide CRISPR screens. Nat. Genet. 2019, 50, 1716–1727. [Google Scholar] [CrossRef]
- Barthelmes, J.; de Bazo, A.M.; Pewzner-Jung, Y.; Schmitz, K.; Mayer, C.A.; Foerch, C.; Eberle, M.; Tafferner, N.; Ferreirós, N.; Henke, M.; et al. Lack of ceramide synthase 2 suppresses the development of experimental autoimmune encephalomyelitis by impairing the migratory capacity of neutrophils. Brain. Behav. Immun. 2015, 46, 280–292. [Google Scholar] [CrossRef]
- Fretts, A.M.; Mozaffarian, D.; Siscovick, D.S.; Djousse, L.; Heckbert, S.R.; King, I.B.; McKnight, B.; Sitlani, C.; Sacks, F.M.; Song, X.; et al. Plasma phospholipid saturated fatty acids and incident atrial fibrillation: The Cardiovascular health study. J. Am. Heart Assoc. 2014, 3, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemaitre, R.N.; Fretts, A.M.; Sitlani, C.M.; Biggs, M.L.; Mukamal, K.; King, I.B.; Song, X.; Djoussé, L.; Siscovick, D.S.; McKnight, B.; et al. Plasma phospholipid very-long-chain saturated fatty acids and incident diabetes in older adults: The cardiovascular health study. Am. J. Clin. Nutr. 2015, 101, 1047–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malik, V.S.; Chiuve, S.E.; Campos, H.; Rimm, E.B.; Mozaffarian, D.; Hu, F.B.; Sun, Q. Circulating Very-Long-Chain Saturated Fatty Acids and Incident Coronary Heart Disease in US Men and Women. Circulation 2015, 176, 139–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, H.T.M.; de Oliveira Otto, M.C.; Lee, Y.; Wu, J.H.Y.; Song, X.; King, I.B.; Psaty, B.M.; Lemaitre, R.N.; McKnight, B.; Siscovick, D.S.; et al. Serial Plasma Phospholipid Fatty Acids in the De Novo Lipogenesis Pathway and Total Mortality, Cause-Specific Mortality, and Cardiovascular Diseases in the Cardiovascular Health Study. J. Am. Heart Assoc. 2019, 8, e012881. [Google Scholar] [CrossRef] [PubMed]
- Kihara, A. Very long-chain fatty acids: Elongation, physiology and related disorders. J. Biochem. 2012, 152, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, R.N.; King, I.B.; Kabagambe, E.K.; Wu, J.H.Y.; McKnight, B.; Manichaikul, A.; Guan, W.; Sun, Q.; Chasman, D.I.; Foy, M.; et al. Genetic loci associated with circulating levels of very long-chain saturated fatty acids. J. Lipid Res. 2015, 56, 176. [Google Scholar] [CrossRef] [Green Version]
- Garg, M.L.; Blake, R.J.; Wills, R.B.H. Macadamia Nut ConsumptionLowers Plasma Total and LDLCholesterol Levels inHypercholesterolemic Men. J. Nutr. 2003, 1060–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, C.; Wong, D.; Cederbaum, S.; Lim, B.; Qu, Y. Peanut consumption increases levels of plasma very long chain fatty acids in humans. Mol. Genet. Metab. 2012, 107, 620–622. [Google Scholar] [CrossRef]
- de Vries, A.P.J.; Bakker, S.J.L.; van Son, W.J.; van der Heide, J.J.H.; Ploeg, R.J.; The, H.T.; de Jong, P.E.; Gans, R.O.B. Metabolic Syndrome Is Associated with Impaired Long-term Renal Allograft Function; Not All Component criteria Contribute Equally. Am. J. Transplant. 2004, 4, 1675–1683. [Google Scholar] [CrossRef]
- De Vries, A.P.J.; Bakker, S.J.L.; Van Son, W.J.; Homan Van der Heide, J.J.; The, T.H.; De Jong, P.E.; Gans, R.O.B. Insulin resistance as putative cause of chronic renal transplant dysfunction. Am. J. Kidney Dis. 2003, 41, 859–867. [Google Scholar] [CrossRef]
- Gomes-Neto, A.W.; Osté, M.C.J.; Sotomayor, C.G.; Berg, E.V.D.; Geleijnse, J.M.; Gans, R.O.B.; Bakker, S.J.L.; Navis, G.J. Fruit and vegetable intake and risk of post trans plantation diabetes in renal transplant recipients. Diabetes Care 2019, 42, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Feunekes, G.I.; Van Staveren, W.A.; De Vries, J.H.; Burema, J.; Hautvast, J.G. Relative and biomarker-based validity of a food-frequency questionnaire estimating intake of fats and cholesterol. Am. J. Clin. Nutr. 1993, 58, 489–496. [Google Scholar] [CrossRef]
- Stichting, N. Nederlands Voedingsstoffen Bestand: NEVO Tabel 2006, Dutch Nutrient Database; Voorlichtingsbureau Voor Voeding: Hague, The Netherlands, 2006. [Google Scholar]
- Abbasi, A.; Peelen, L.M.; Corpeleijn, E.; Van Der Schouw, Y.T.; Stolk, R.P.; Spijkerman, A.M.W.; Van Der, A.D.L.; Moons, K.G.M.; Navis, G.; Bakker, S.J.L.; et al. Prediction models for risk of developing type 2 diabetes: Systematic literature search and independent external validation study. BMJ 2012, 345. [Google Scholar] [CrossRef] [Green Version]
- Shabir, S.; Jham, S.; Harper, L.; Ball, S.; Borrows, R.; Sharif, A. Validity of glycated haemoglobin to diagnose new onset diabetes after transplantation. Transpl. Int. 2013, 26, 315–321. [Google Scholar] [CrossRef]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Terpos, E.; Christoulas, D.; Kastritis, E.; Katodritou, E.; Pouli, A.; Michalis, E.; Papassotiriou, I.; Dimopoulos, M.A. The chronic kidney disease epidemiology collaboration cystatin C (CKD-EPI-CysC) equation has an independent prognostic value for overall survival in newly diagnosed patients with symptomatic multiple myeloma; is it time to change from MDRD to CKD-EPI-CysC equations? Eur. J. Haematol. 2013, 91, 347–355. [Google Scholar]
- Sokooti, S.; Szili-Torok, T.; Flores-Guerrero, J.L.; Osté, M.C.J.; Gomes-Neto, A.W.; Kootstra-Ros, J.E.; Heerspink, H.J.L.; Connelly, M.A.; Bakker, S.J.L.; Dullaart, R.P.F. High-density lipoprotein particles and their relationship to posttransplantation diabetes mellitus in renal transplant recipients. Biomolecules 2020, 10, 481. [Google Scholar] [CrossRef] [Green Version]
- Ichihara, K.; Fukubayashi, Y. Preparation of fatty acid methyl esters for gas-liquid chromatography. J. Lipid Res. 2010, 51, 635–640. [Google Scholar] [CrossRef] [Green Version]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Gore, E.J.; Gomes-Neto, A.W.; Wang, L.; Bakker, S.J.L.; Niesters, H.G.M.; de Joode, A.A.E.; Verschuuren, E.A.M.; Westra, J.; Leer-Buter, C. Van Torquetenovirus Serum Load and Long-Term Outcomes in Renal Transplant Recipients. J. Clin. Med. 2020, 9, 440. [Google Scholar] [CrossRef] [Green Version]
- Harrell, F.E.; Lee, K.L.; Mark, D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Hilbrands, L.B.; Demacker, P.N.M.; Hoitsma, A.J.; Stalenhoef, A.F.H.; Koene, R.A.P.; Hilbrands, L.B.; Hoitsma, A.J.; Demacker, P.N.M.; Stalenhoef, A.F.H. The Effects of Cyclosporine and Prednisone on Serum Lipid and (Apo)Liproprotein Levels in Renal Transplant Recipients1. J. Am. Soc. Nephrol. 1995, 5, 2073–2081. [Google Scholar] [CrossRef] [PubMed]
- Quehenberger, O.; Armando, A.M.; Brown, A.H.; Milne, S.B.; Myers, D.S.; Merrill, A.H.; Bandyopadhyay, S.; Jones, K.N.; Kelly, S.; Shaner, R.L.; et al. Lipidomics reveals a remarkable diversity of lipids in human plasma1. J. Lipid Res. 2010, 51, 3299–3305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohno, Y.; Suto, S.; Yamanaka, M.; Mizutani, Y.; Mitsutake, S.; Igarashi, Y.; Sassa, T.; Kihara, A. ELOVL1 production of C24 acyl-CoAs is linked to C24 sphingolipid synthesis. Proc. Natl. Acad. Sci. USA 2010, 107, 18439–18444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.R.; Lee, E.J.; Shin, K.O.; Kim, M.H.; Pewzner-Jung, Y.; Lee, Y.M.; Park, J.W.; Futerman, A.H.; Park, W.J. Hepatic triglyceride accumulation via endoplasmic reticulum stress-induced SREBP-1 activation is regulated by ceramide synthases. Exp. Mol. Med. 2019, 51, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Park, W.J.; Kuperman, Y.; Boura-Halfon, S.; Pewzner-Jung, Y.; Futerman, A.H. Ablation of very long acyl chain sphingolipids causes hepatic insulin resistance in mice due to altered detergent-resistant membranes. Hepatology 2013, 57, 525–532. [Google Scholar] [CrossRef]
- Szczuko, M.; Kaczkan, M.; Drozd, A.; Maciejewska, D.; Palma, J.; Owczarzak, A.; Marczuk, N.; Rutkowski, P.; Małgorzewicz, S. Comparison of fatty acid profiles in a group of female patients with chronic kidney diseases (CKD) and metabolic syndrome (MetS)–similar trends of changes, different pathophysiology. Int. J. Mol. Sci. 2019, 20, 1719. [Google Scholar] [CrossRef] [Green Version]
- MacEyka, M.; Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Saroha, A.; Pewzner-jung, Y.; Futerman, A.H. LPS-mediated septic shock is augmented in ceramide synthase 2 null mice due to elevated activity of TNF a -converting enzyme. FEBS Lett. 2015, 589, 2213–2217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seitz, A.P.; Grassmé, H.; Edwards, M.J.; Pewzner-Jung, Y.; Gulbins, E. Ceramide and sphingosine in pulmonary infections. Biol. Chem. 2015, 396, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Prakash, H. Sphingolipids are dual specific drug targets for the management of pulmonary infections: Perspective. Front. Immunol. 2017, 8, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fretts, A.M.; Mozaffarian, D.; Siscovick, D.S.; King, I.B.; McKnight, B.; Psaty, B.M.; Rimm, E.B.; Sitlani, C.; Sacks, F.M.; Song, X.; et al. Associations of Plasma Phospholipid SFAs with Total and Cause-Specific Mortality in Older Adults Differ According to SFA Chain Length. J. Nutr. 2016, 146, 298–305. [Google Scholar] [CrossRef] [Green Version]
- Aune, D.; Keum, N.N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Nut consumption and risk of cardiovascular disease, total cancer, all-cause and cause-specific mortality: A systematic review and dose-response meta-analysis of prospective studies. BMC Med. 2016, 14, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Schumann, J. It is all about fluidity: Fatty acids and macrophage phagocytosis. Eur. J. Pharmacol. 2016, 785, 18–23. [Google Scholar] [CrossRef]
| Baseline Characteristics | KTR n = 680 | Healthy Control n = 193 | p |
|---|---|---|---|
| Demographics | |||
| Age, years | 54.7 (44.6–63.0) | 54.0 (45.6–63.1) | 0.76 |
| Male gender, n (%) | 401 (56.8) | 111 (57.5) | 0.87 |
| Body mass index, kg/m2 | 25.9 (23.2–29.4) | 25.3 (23.5–27.8) | 0.10 |
| Waist circumference, cm | 98 ± 15 | 91 ± 10 | <0.001 |
| Metabolic syndrome (yes), n (%) | 436 (64.1) | 42 (21.8) | <0.001 |
| Fatty liver index, arbitrary units | 56 (31–80) | 32 (17–58) | <0.001 |
| Circulating VLSFA | |||
| C20:0, mol% | 0.24 ± 0.05 | 0.25 ± 0.05 | <0.001 |
| C22:0, mol% | 0.57 ± 0.14 | 0.64 ± 0.14 | <0.001 |
| C24:0, mol% | 0.48 ± 0.13 | 0.55 ± 0.13 | <0.001 |
| Renal function parameters | |||
| Creatinine, umol/L | 156 (112–181) | 82 (72–93) | <0.001 |
| eGFR, mL/min/1.73 m2 | 41.0 ± 18.6 | 93.1 ± 16.1 | <0.001 |
| Proteinuria 0.5 g/day, n (%) | 152 (22.4) | 1 (0.5) | <0.001 |
| Glucose homeostasis | |||
| Glucose, mmol/L | 5.3 (4.9–6.0) | 5.3 (5.0–5.7) | 0.58 |
| HbA1C, % | 5.8 (5.5–6.2) | 5.6 (5.4–5.8) | <0.001 |
| Diabetes, n (%) | 162 (23.8) | 11 (5.7) | <0.001 |
| Lipids | |||
| Total cholesterol, mmol/L | 5.1 ± 1.1 | 5.3 ± 1.1 | 0.04 |
| Triglycerides, mmol/L | 1.7 (1.3–2.3) | 1.2 (0.8–1.6) | <0.001 |
| HDL cholesterol, mmol/L | 1.3 (1.1–1.6) | 1.4 (1.2–1.7) | <0.001 |
| Statin use, n (%) | 359 (52.8) | 7 (3.6) | <0.001 |
| Liver parameter | |||
| Gamma GT, U/L | 21 (15–33) | 26 (19–41) | <0.001 |
| Health lifestyle | |||
| Current smoker, n (%) | 81 (11.9) | 39 (20.2) | 0.004 |
| Total energy intake, kJ/day | 8713 (7172–10607) | 9055 (7458–10635) | 0.38 |
| Peanuts, g/day | 0.6 (0.0–3.4) | 1.3 (0.0–4.8) | 0.09 |
| Peanut butter, g/day | 0.0 (0.0–4.0) | 0.0 (0.0–4.3) | 0.77 |
| Tree nuts, g/day | 0.0 (0.0–3.9) | 0.8 (0.0–3.5) | 0.35 |
| Baseline Characteristics | Total Population | C20:0 (mol%) | C22:0 (mol%) | C24:0 (mol%) |
|---|---|---|---|---|
| Std. β | Std. β | Std. β | ||
| Demographics | ||||
| Age, years | 54.7 (44.6–63.0) | 0.02 | −0.11 ** | −0.09 * |
| Male gender, n (%) | 401 (56.8) | 0.13 *** | 0.04 | −0.03 |
| BMI, kg/m2 | 25.9 (23.2–29.4) | −0.10 * | −0.12 ** | −0.17 *** |
| Waist circumference, cm | 98 ± 15 | −0.20 *** | −0.19 *** | −0.21 *** |
| Caucasian, n (%) | 677 (99.6) | 0.04 | −0.01 | −0.02 |
| Circulating VLSFA | ||||
| C20:0, mol% | 0.24 ± 0.05 | – | 0.74 *** | 0.63 *** |
| C22:0, mol% | 0.57 ± 0.14 | 0.74 *** | – | 0.94 *** |
| C24:0, mol% | 0.48 ± 0.13 | 0.63 *** | 0.94 *** | – |
| Primary kidney disease | ||||
| Glomerulonephritis, n (%) | 175 (25.7) | −0.01 | −0.01 | 0.01 |
| Interstitial nephritis, n (%) | 87 (12.8) | 0.05 | 0.06 | 0.03 |
| Cystic kidney disease, n (%) | 139 (20.4) | −0.02 | −0.02 | −0.03 |
| Other congenital and hereditary kidney disease, n (%) | 37 (5.4) | −0.03 | −0.04 | −0.05 |
| Renal vascular disease, excluding vasculitis, n (%) | 51 (7.5) | −0.02 | −0.01 | 0.002 |
| Diabetes mellitus, n (%) | 34 (5.0) | 0.02 | −0.03 | −0.02 |
| Other multisystem disease, n (%) | 32 (4.7) | 0.01 | 0.01 | 0.02 |
| Other, n (%) | 18 (2.6) | −0.01 | 0.02 | 0.01 |
| Unknown, n (%) | 107 (15.7) | −0.01 | 0.01 | 0.01 |
| Kidney transplant | ||||
| Pre-emptive transplantation, n (%) | 105 (15.5) | 0.03 | 0.08 | 0.06 |
| Time between transplantation and baseline measurement, years | 5.4 (1.9–12.0) | 0.08 * | 0.02 | 0.01 |
| Male donor, n (%) | 343 (50.4) | −0.07 | −0.06 | 0.04 |
| Donor age, years | 46.0 (32.0–54.0) | −0.09 * | −0.04 | 0.00 |
| Postmortal donor, n (%) | 446 (65.6) | −0.03 | 0.07 | 0.08 * |
| Immunosuppressive therapy | ||||
| Prednisolone, % | 673 (99.0) | −0.01 | 0.01 | 0.02 |
| Prednisolone dose, mg | 10.0 (7.5–10.0) | −0.04 | −0.001 | 0.02 |
| Tacrolimus, % | 121 (17.8) | 0.00 | 0.02 | 0.01 |
| Cyclosporine, n (%) | 269 (39.6) | −0.12 ** | −0.12 ** | −0.09 * |
| Azathioprine, n (%) | 120 (17.6) | 0.07 | 0.00 | 0.00 |
| Mycophenolic acid, n (%) | 446 (65.6) | −0.02 | 0.05 | 0.04 |
| Everolimus/Sirolimus | 13 (1.9) | −0.05 | −0.02 | 0.01 |
| Clinical variables | ||||
| Systolic blood pressure, mmHg | 136 ± 17 | −0.08 * | −0.08 * | −0.07 |
| Diastolic blood pressure, mmHg | 83 ± 11 | −0.07 | −0.01 | −0.003 |
| Heart rate, beats per minute | 69 ± 12 | −0.06 | −0.06 | −0.11 ** |
| Antihypertensives, n (%) | 598 (87.9) | −0.12 ** | −0.12 ** | −0.09 * |
| Renal function parameters | ||||
| Creatinine, umol/L | 156 (112–181) | −0.13 *** | −0.12 ** | −0.08 * |
| Cystatin C, mg/L | 1.7 (1.3–2.2) | −0.17 *** | −0.23 *** | −0.20 *** |
| eGFR, mL/min/1.73 m2 | 41.0 ± 18.6 | 0.07 | 0.12 ** | 0.09 * |
| Proteinuria 0.5 g/day, n (%) | 152 (22.4) | −0.05 | −0.06 | −0.07 |
| Glucose homeostasis | ||||
| Glucose, mmol/L | 5.3 (4.9–6.0) | −0.10 ** | −0.13 *** | −0.15 *** |
| HbA1C, % | 5.8 (5.5–6.2) | −0.07 | −0.12 ** | −0.14 *** |
| Diabetes mellitus, n (%) | 162 (23.8) | −0.12 ** | −0.15 *** | −0.18 *** |
| Antidiabetic medication, n (%) | 105 (15.4) | −0.09 * | −0.09 * | −0.12 ** |
| Serum parameters | ||||
| Albumin, g/L | 43.0 ± 3.0 | 0.04 | 0.13 *** | 0.16 *** |
| hs-CRP, mg/L | 1.6 (0.7–4.6) | −0.04 | −0.03 | −0.09 * |
| Procalcitonin, ug/L | 0.06 ± 0.06 | −0.11 ** | −0.15 *** | −0.12 ** |
| Lipids | ||||
| Total cholesterol, mmol/L | 5.1 ± 1.1 | −0.09 * | 0.04 | 0.06 |
| LDL cholesterol, mmol/L | 3.0 ± 0.9 | −0.05 | 0.14 *** | 0.15 *** |
| HDL cholesterol, mmol/L | 1.3 (1.1–1.6) | 0.35 *** | 0.34 *** | 0.37 *** |
| Triglycerides, mmol/L | 1.7 (1.3–2.3) | −0.58 *** | −0.60 *** | −0.59 *** |
| Statin use, n (%) | 359 (52.8) | 0.05 | −0.13 *** | −0.10 * |
| Liver parameters | ||||
| Total bilirubin, umol/L | 10 (7–13) | 0.03 | 0.07 | 0.12 ** |
| ASAT, U/L | 22 (18–27) | 0.09 * | 0.03 | 0.04 |
| ALAT, U/L | 19 (14–25) | 0.001 | −0.05 | −0.04 |
| Total protein, g/L | 71.23 ± 5.1 | −0.03 | 0.02 | 0.04 |
| Gamma-GT, U/L | 26 (19–41) | −0.03 | −0.09 * | −0.11 ** |
| Healthy lifestyle | ||||
| Current smoker, n (%) | 81 (11.9) | −0.12 ** | −0.08 * | −0.05 |
| Alcohol intake, g/day | 2.6 (0.0–11.1) | −0.07 | −0.03 | 0.07 |
| Physical activity, intensity x hours | 5590 (3060–8415) | −0.01 | 0.07 | 0.09 * |
| Total energy intake, kJ/day | 8713 (7172–10607) | −0.09 | 0.05 | 0.10 * |
| Metabolic syndrome (yes), n (%) | 436 (64.1) | −0.19 *** | −0.28 *** | −0.31 *** |
| Fatty liver index, arbitrary units | 56 (31–80) | −0.24 *** | −0.26 *** | 0.29 *** |
| Dietary intake | ||||
| Peanuts, g/day | 0.6 (0.0–3.4) | 0.04 | 0.19 *** | 0.23 *** |
| Peanut butter, g/day | 0.0 (0.0–4.0) | 0.01 | 0.13 ** | 0.13 ** |
| Tree nuts, g/day | 0.0 (0.0–3.9) | 0.13 *** | 0.18 *** | 0.19 *** |
| Models | C20:0, per 1-SD Relative Increment | C22:0, per 1-SD Relative Increment | C24:0, per 1-SD Relative Increment | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Model 1 | 0.78 (0.66–0.93) | 0.001 | 0.65 (0.54–0.79) | <0.001 | 0.65 (0.54–0.79) | <0.001 |
| Model 2 | 0.79 (0.67–0.95) | 0.01 | 0.69 (0.57–0.84) | <0.001 | 0.68 (0.56–0.82) | <0.001 |
| Model 3 | 0.80 (0.67–0.95) | 0.01 | 0.71 (0.59–0.85) | <0.001 | 0.71 (0.59–0.86) | <0.001 |
| Model 4 | 0.78 (0.64–0.94) | 0.01 | 0.73 (0.60–0.89) | 0.002 | 0.75 (0.61–0.92) | 0.01 |
| Model 5 | 0.77 (0.62–0.96) | 0.02 | 0.70 (0.55–0.90) | 0.01 | 0.73 (0.57–0.93) | 0.01 |
| Model 6 | 0.77 (0.63–0.93) | 0.01 | 0.72 (0.59–0.89) | 0.003 | 0.73 (0.60–0.90) | 0.003 |
| Model 7 | 0.85 (0.69–1.05) | 0.14 | 0.79 (0.64–0.99) | 0.04 | 0.80 (0.64–1.01) | 0.06 |
| Models | C20:0, per 1-SD Relative Increment | C22:0, per 1-SD Relative Increment | C24:0, per 1-SD Relative Increment | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Model 1 | 0.58 (0.41–0.82) | 0.002 | 0.48 (0.33–0.69) | <0.001 | 0.51 (0.35–0.73) | <0.001 |
| Model 2 | 0.57 (0.41–0.80) | 0.001 | 0.50 (0.35–0.72) | <0.001 | 0.53 (0.37–0.75) | <0.001 |
| Model 3 | 0.55 (0.38–0.77) | <0.001 | 0.51 (0.35–0.73) | <0.001 | 0.55 (0.39–0.78) | <0.001 |
| Model 4 | 0.57 (0.39–0.82) | 0.002 | 0.52 (0.35–0.77) | 0.001 | 0.57 (0.39–0.83) | 0.004 |
| Model 5 | 0.51 (0.32–0.81) | 0.004 | 0.43 (0.26–0.72) | 0.001 | 0.50 (0.31–0.82) | 0.005 |
| Model 6 | 0.58 (0.40–0.84) | 0.004 | 0.52 (0.35–0.78) | 0.001 | 0.57 (0.38–0.83) | 0.004 |
| Model 7 | 0.53 (0.35–0.82) | 0.004 | 0.48 (0.30–0.75) | 0.001 | 0.51 (0.33–0.80) | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vogelpohl, F.A.; Gomes-Neto, A.W.; Martini, I.A.; Sotomayor, C.G.; Groothof, D.; Osté, M.C.J.; Heiner-Fokkema, M.R.; Muskiet, F.A.J.; Berger, S.P.; Navis, G.; et al. Low Circulating Concentrations of Very Long Chain Saturated Fatty Acids Are Associated with High Risk of Mortality in Kidney Transplant Recipients. Nutrients 2021, 13, 3383. https://doi.org/10.3390/nu13103383
Vogelpohl FA, Gomes-Neto AW, Martini IA, Sotomayor CG, Groothof D, Osté MCJ, Heiner-Fokkema MR, Muskiet FAJ, Berger SP, Navis G, et al. Low Circulating Concentrations of Very Long Chain Saturated Fatty Acids Are Associated with High Risk of Mortality in Kidney Transplant Recipients. Nutrients. 2021; 13(10):3383. https://doi.org/10.3390/nu13103383
Chicago/Turabian StyleVogelpohl, Fabian A., António W. Gomes-Neto, Ingrid A. Martini, Camilo G. Sotomayor, Dion Groothof, Maryse C. J. Osté, Margaretha Rebecca Heiner-Fokkema, Frits A. J. Muskiet, Stefan P. Berger, Gerjan Navis, and et al. 2021. "Low Circulating Concentrations of Very Long Chain Saturated Fatty Acids Are Associated with High Risk of Mortality in Kidney Transplant Recipients" Nutrients 13, no. 10: 3383. https://doi.org/10.3390/nu13103383
APA StyleVogelpohl, F. A., Gomes-Neto, A. W., Martini, I. A., Sotomayor, C. G., Groothof, D., Osté, M. C. J., Heiner-Fokkema, M. R., Muskiet, F. A. J., Berger, S. P., Navis, G., Kema, I. P., & Bakker, S. J. L. (2021). Low Circulating Concentrations of Very Long Chain Saturated Fatty Acids Are Associated with High Risk of Mortality in Kidney Transplant Recipients. Nutrients, 13(10), 3383. https://doi.org/10.3390/nu13103383








