The Role of Carrageenan in Inflammatory Bowel Diseases and Allergic Reactions: Where Do We Stand?
Abstract
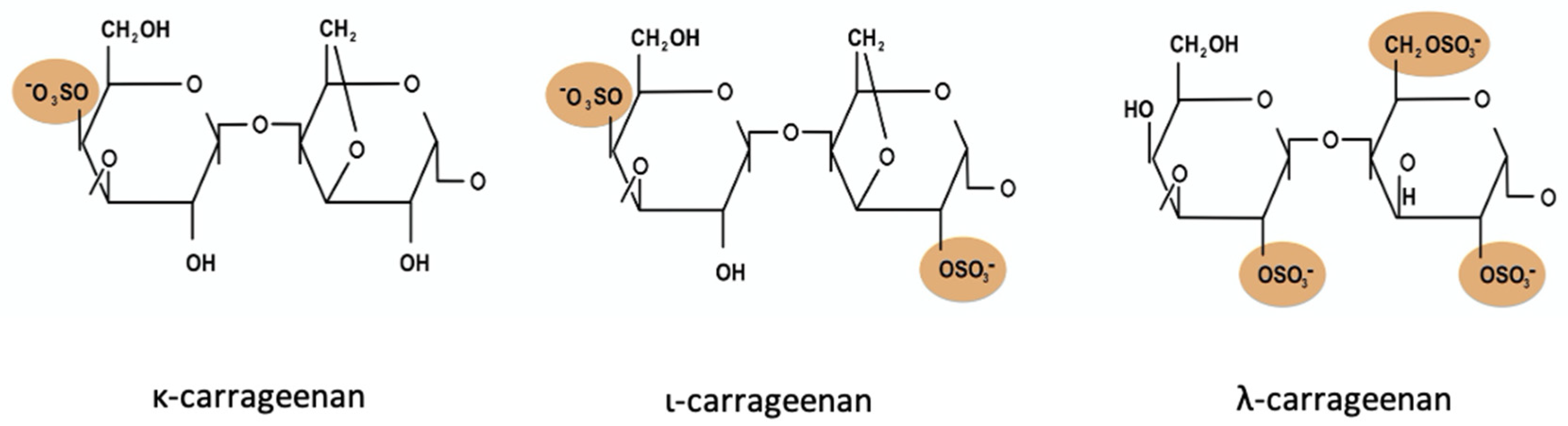
:1. Introduction
2. Dietary Pattern and Inflammation
3. Gut Microbiota and Inflammation
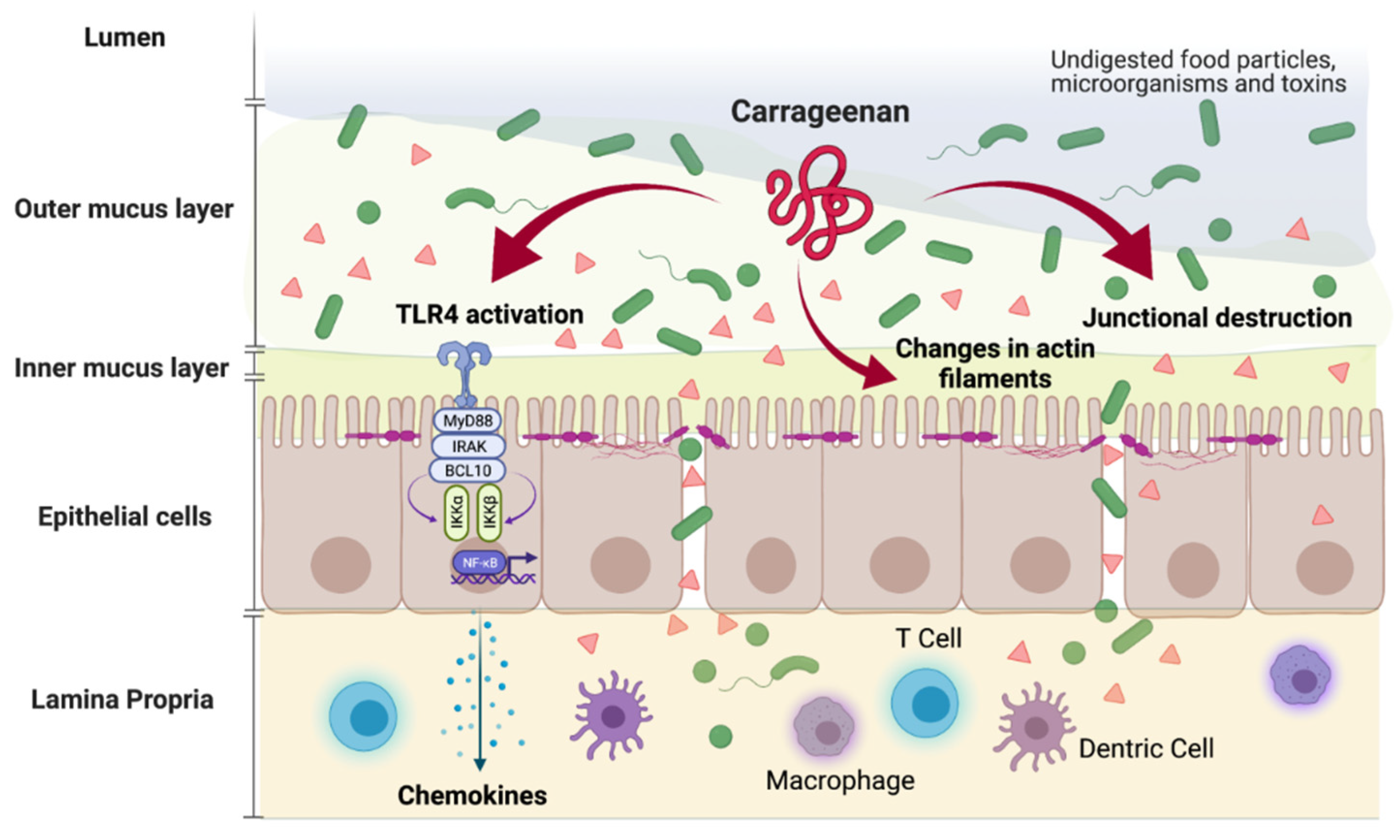
4. In Vitro Evidence
5. Animal Models Evidence
6. Carrageenan and Inflammatory Bowel Diseases
6.1. Carrageenan and Ulcerative Colitis
6.2. Carrageenan and Crohn’s Disease
7. Carrageenan and Allergic Reactions
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Martino, J.V.; Van Limbergen, J.; Cahill, L.E. The role of carrageenan and carboxymethylcellulose in the development of intestinal inflammation. Front. Pediatr. 2017, 5, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.; Swan, C.K.; Suskind, D.; Wahbeh, G.; Vanamala, J.; Baldassano, R.N.; Leonard, M.B.; Lampe, J.W. Children with Crohn’s disease frequently consume select food additives. Dig. Dis. Sci. 2018, 63, 2722–2728. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Shumard, T.; Xie, H.; Dodda, A.; Varady, K.A.; Feferman, L.; Halline, A.G.; Goldstein, J.L.; Hanauer, S.B.; Tobacman, J.K. A randomized trial of the effects of the no-carrageenan diet on ulcerative colitis disease activity. Nutr. Healthy Aging 2017, 4, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Feferman, L.; Bhattacharyya, S.; Oates, E.; Haggerty, N.; Wang, T.; Varady, K.; Tobacman, J.K. Carrageenan-free diet shows improved glucose tolerance and insulin signaling in prediabetes: A randomized, pilot clinical trial. J. Diabetes Res. 2020, 2020, 8267980. [Google Scholar] [CrossRef]
- David, S.; Levi, C.S.; Fahoum, L.; Ungar, Y.; Meyron-Holtz, E.G.; Shpigelman, A.; Lesmes, U. Revisiting the carrageenan controversy: Do we really understand the digestive fate and safety of carrageenan in our foods? Food Funct. 2018, 9, 1344–1352. [Google Scholar] [CrossRef]
- Nicklin, S.; Miller, K. Intestinal uptake and immunological effects of carrageenan—Current concepts. Food Addit. Contam. 1989, 6, 425–436. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panelon Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Re-evaluation of carrageenan (E407) and processed Eucheuma seaweed (E407a) as food additives. EFSA J. 2018, 16, e05238. [Google Scholar] [CrossRef]
- Campo, V.; Kawano, D.; Silva, D.; Ivone, C. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Fahoum, L.; Moscovici, A.; David, S.; Shaoul, R.; Rozen, G.; Meyron-Holtz, E.G.; Lesmes, U. Digestive fate of dietary carrageenan: Evidence of interference with digestive proteolysis and disruption of gut epithelial function. Mol. Nutr. Food Res. 2017, 61, 1600545. [Google Scholar] [CrossRef] [PubMed]
- Shah, Z.C.; Huffman, F.G. Current availability and consumption of carrageenan-containing foods. Ecol. Food Nutr. 2003, 42, 357–371. [Google Scholar] [CrossRef]
- Leet, W.S. California’s Living Marine Resources: A Status Report; University of California, Division of Agriculture and Natural Resources; California Sea Grant: Oakland, CA, USA, 2001.
- McKim, J.M. Food additive carrageenan: Part I: A Critical Review of carrageenan in vitro studies, potential pitfalls, and implications for human health and safety. Crit. Rev. Toxicol. 2014, 44, 211–243. [Google Scholar] [CrossRef] [PubMed]
- Joint Expert Committee on Food Additives. Evaluation of Certain Food Additives and Contaminants: Sixty-Eighth Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2007; ISBN 9789241209472. [Google Scholar]
- Weiner, M.L. Food additive carrageenan: Part II: A critical review of carrageenan in vivo safety studies. Crit. Rev. Toxicol. 2014, 44, 244–269. [Google Scholar] [CrossRef] [PubMed]
- Borthakur, A.; Bhattacharyya, S.; Dudeja, P.K.; Tobacman, J.K. Carrageenan induces interleukin-8 production through distinct Bcl10 pathway in normal human colonic epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G829–G838. [Google Scholar] [CrossRef] [PubMed]
- Borthakur, A.; Bhattacharyya, S.; Anbazhagan, A.N.; Kumar, A.; Dudeja, P.K.; Tobacman, J.K. Prolongation of carrageenan-induced inflammation in human colonic epithelial cells by activation of an NFκB-BCL10 loop. Biochim. Biophys. Acta 2012, 1822, 1300–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharyya, S.; Dudeja, P.K.; Tobacman, J.K. Carrageenan-induced NFκB activation depends on distinct pathways mediated by reactive oxygen species and Hsp27 or by Bcl10. Biochim. Biophys. Acta 2008, 1780, 973–982. [Google Scholar] [CrossRef] [Green Version]
- Tobacman, J.K. Review of harmful gastrointestinal effects of carrageenan in animal experiments. Environ. Health Perspect. 2001, 109, 983–994. [Google Scholar] [CrossRef]
- Petryk, N.; Shevchenko, O. Anti-inflammatory activity of mesenchymal stem cells in λ-carrageenan-induced chronic inflammation in rats: Reactions of the blood system, leukocyte-monocyte ratio. Inflammation 2020, 43, 1893–1901. [Google Scholar] [CrossRef]
- Daher, G.C.; Lawson, K.D.; Long, P.H.; Tallmadge, D.H.; Boothe, A.D.; Vanderploeg, P.; Miller, K.W. Comparison of olestra absorption in guinea pigs with normal and compromised gastrointestinal tracts. Fundam. Appl. Toxicol. Off. J. Soc. Toxicol. 1997, 39, 138–147. [Google Scholar] [CrossRef]
- Steffens, S.; Winter, C.; Schloss, M.J.; Hidalgo, A.; Weber, C.; Soehnlein, O. Circadian control of inflammatory processes in atherosclerosis and its complications. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1022–1028. [Google Scholar] [CrossRef]
- Norde, M.M.; Collese, T.S.; Giovannucci, E.; Rogero, M.M. A posteriori dietary patterns and their association with systemic low-grade inflammation in adults: A systematic review and meta-analysis. Nutr. Rev. 2021, 79, 331–350. [Google Scholar] [CrossRef]
- Aleksandrova, K.; Koelman, L.; Rodrigues, C.E. Dietary patterns and biomarkers of oxidative stress and inflammation: A systematic review of observational and intervention studies. Redox Biol. 2021, 42, 101869. [Google Scholar] [CrossRef]
- Christ, A.; Lauterbach, M.; Latz, E. Western diet and the immune system: An inflammatory connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef] [PubMed]
- Zinöcker, M.; Lindseth, I. The Western diet–Microbiome-host interaction and its role in metabolic disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiba, M.; Nakane, K.; Komatsu, M. Westernized diet is the most ubiquitous environmental factor in inflammatory bowel disease. Perm. J. 2019, 23, 18–107. [Google Scholar] [CrossRef]
- Al Bander, Z.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The gut microbiota and inflammation: An overview. Int. J. Environ. Res. Public. Health 2020, 17, 7618. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chassaing, B.; Van de Wiele, T.; De Bodt, J.; Marzorati, M.; Gewirtz, A.T. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut 2017, 66, 1414–1427. [Google Scholar] [CrossRef]
- Benard, C.; Cultrone, A.; Michel, C.; Rosales, C.; Segain, J.-P.; Lahaye, M.; Galmiche, J.-P.; Cherbut, C.; Blottière, H.M. Degraded carrageenan causing colitis in rats induces TNF secretion and ICAM-1 upregulation in monocytes through NF-κB activation. PLoS ONE 2010, 5, e8666. [Google Scholar] [CrossRef] [Green Version]
- Naimi, S.; Viennois, E.; Gewirtz, A.T.; Chassaing, B. Direct Impact of commonly used dietary emulsifiers on human gut microbiota. Microbiome 2021, 9, 66. [Google Scholar] [CrossRef]
- Nickerson, K.P.; Chanin, R.; McDonald, C. Deregulation of intestinal anti-microbial defense by the dietary additive, maltodextrin. Gut Microbes 2015, 6, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Madureira, A.R.; Tavares, T.; Gomes, A.M.P.; Pintado, M.E.; Malcata, F.X. Invited review: Physiological properties of bioactive peptides obtained from whey proteins. J. Dairy Sci. 2010, 93, 437–455. [Google Scholar] [CrossRef] [Green Version]
- Udenigwe, C.C.; Aluko, R.E. Food protein-derived bioactive peptides: Production, processing, and potential health benefits. J. Food Sci. 2012, 77, R11–R24. [Google Scholar] [CrossRef]
- Anderson, W.; Baillie, A.J. Carrageenans and the proteolytic activity of human gastric secretion. J. Pharm. Pharmacol. 1967, 19, 720–728. [Google Scholar] [CrossRef]
- Schulzke, J.D.; Ploeger, S.; Amasheh, M.; Fromm, A.; Zeissig, S.; Troeger, H.; Richter, J.; Bojarski, C.; Schumann, M.; Fromm, M. Epithelial tight junctions in intestinal inflammation. Ann. N. Y. Acad. Sci. 2009, 1165, 294–300. [Google Scholar] [CrossRef]
- Khalil, N.A.; Walton, G.E.; Gibson, G.R.; Tuohy, K.M.; Andrews, S.C. In vitro batch cultures of gut microbiota from healthy and ulcerative colitis (UC) subjects suggest that sulphate-reducing bacteria levels are raised in UC and by a protein-rich diet. Int. J. Food Sci. Nutr. 2014, 65, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Lobley, G.E.; Holtrop, G.; Ince, J.; Johnstone, A.M.; Louis, P.; Flint, H.J. Human colonic microbiota associated with diet, obesity and weight loss. Int. J. Obes. 2008, 32, 1720–1724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharyya, S.; Gill, R.; Chen, M.L.; Zhang, F.; Linhardt, R.J.; Dudeja, P.K.; Tobacman, J.K. Toll-like receptor 4 mediates induction of the Bcl10-NFκB-interleukin-8 inflammatory pathway by carrageenan in human intestinal epithelial cells. J. Biol. Chem. 2008, 283, 10550–10558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharyya, S.; Borthakur, A.; Anbazhagan, A.N.; Katyal, S.; Dudeja, P.K.; Tobacman, J.K. Specific effects of BCL10 serine mutations on phosphorylations in canonical and noncanonical pathways of NF-ΚB activation following carrageenan. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G475–G486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, W.; Feng, W.; Xin, G.; Tingting, N.; Zhanghe, Z.; Haimin, C.; Xiaojun, Y. Enhanced effect of κ-carrageenan on TNBS-induced inflammation in mice. Int. Immunopharmacol. 2016, 39, 218–228. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Liu, H.; Zhang, Z.; Jam, M.; Dudeja, P.K.; Michel, G.; Linhardt, R.J.; Tobacman, J.K. Carrageenan-induced innate immune response is modified by enzymes that hydrolyze distinct galactosidic bonds. J. Nutr. Biochem. 2010, 21, 906–913. [Google Scholar] [CrossRef] [Green Version]
- Carrageenan. Available online: https://www.cornucopia.org/carrageenan/ (accessed on 22 June 2021).
- Scaldaferri, F.; Fiocchi, C. Inflammatory bowel disease: Progress and current concepts of etiopathogenesis. J. Dig. Dis. 2007, 8, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54.e42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somogyi, L.P. Food additives. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 1–59. ISBN 9780471238966. [Google Scholar]
- Cohen, S.A.; Gold, B.D.; Oliva, S.; Lewis, J.; Stallworth, A.; Koch, B.; Eshee, L.; Mason, D. Clinical and mucosal improvement with specific carbohydrate diet in pediatric Crohn disease. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Marlow, G.; Ellett, S.; Ferguson, I.R.; Zhu, S.; Karunasinghe, N.; Jesuthasan, A.C.; Han, D.Y.; Fraser, A.G.; Ferguson, L.R. Transcriptomics to study the effect of a mediterranean-inspired diet on inflammation in Crohn’s disease patients. Hum. Genom. 2013, 7, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Day, A.S.; Whitten, K.E.; Sidler, M.; Lemberg, D.A. Systematic review: Nutritional therapy in paediatric Crohn’s disease. Aliment. Pharmacol. Ther. 2008, 27, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.J.; Kabi, A.; Nickerson, K.P.; McDonald, C. Combinatorial effects of diet and genetics on inflammatory bowel disease pathogenesis. Inflamm. Bowel Dis. 2015, 21, 912–922. [Google Scholar] [CrossRef]
- Nickerson, K.P.; McDonald, C. Crohn’s disease-associated adherent-invasive escherichia coli adhesion is enhanced by exposure to the ubiquitous dietary polysaccharide maltodextrin. PLoS ONE 2012, 7, e52132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basson, A.R.; Ahmed, S.; Almutairi, R.; Seo, B.; Cominelli, F. Regulation of intestinal inflammation by soybean and soy-derived compounds. Foods 2021, 10, 774. [Google Scholar] [CrossRef]
- Lock, J.Y.; Taylor, L.C.; Ming Wang, C.; Chen, A.; Carrier, R.L. Acute exposure to commonly ingested emulsifiers alters intestinal mucus structure and transport properties. Sci. Rep. 2018, 8, 10008. [Google Scholar] [CrossRef]
- Sandall, A.M.; Cox, S.R.; Lindsay, J.O.; Gewirtz, A.T.; Chassaing, B.; Rossi, M. Emulsifiers impact colonic length in mice and emulsifier restriction is feasible in people with Crohn’s disease. Nutrients 2020, 12, 2827. [Google Scholar] [CrossRef]
- Limketkai, B.N.; Zipporah, I.E.; Teuta, G.H.; Parian, A.; Matarese, L.E.; Bracewell, K. Dietary interventions for induction and maintenance of remission in inflammatory bowel disease. Cochrane Database Syst. Rev. 2019, 2, CD012839. [Google Scholar] [CrossRef]
- Barreau, F.; Tisseyre, C.; Ménard, S.; Ferrand, A.; Carriere, M. Titanium dioxide particles from the diet: Involvement in the genesis of inflammatory bowel diseases and colorectal cancer. Part. Fibre Toxicol. 2021, 18, 26. [Google Scholar] [CrossRef]
- Tarlo, S.M.; Dolovich, J.; Listgarten, C. Anaphylaxis to carrageenan: A pseudo-latex allergy. J. Allergy Clin. Immunol. 1995, 95, 933–936. [Google Scholar] [CrossRef]
- Kular, H.; Dean, J.; Cook, V. A case of carrageenan allergy in a pediatric patient. Ann. Allergy Asthma Immunol. 2018, 121, S119. [Google Scholar] [CrossRef]
- Wilson, J.M.; Schuyler, A.J.; Workman, L.; Gupta, M.; James, H.R.; Posthumus, J.; McGowan, E.C.; Commins, S.P.; Platts-Mills, T.A.E. Investigation into the α-gal syndrome: Characteristics of 261 children and adults reporting red meat allergy. J. Allergy Clin. Immunol. Pract. 2019, 7, 2348–2358.e4. [Google Scholar] [CrossRef] [PubMed]
- Steinke, J.W.; Platts-Mills, T.A.E.; Commins, S.P. The alpha-gal story: Lessons learned from connecting the dots. J. Allergy Clin. Immunol. 2015, 135, 589–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Food Additive | Mean Exposure per Day |
|---|---|
| Xanthan Gum | 0.96 ± 0.72 |
| Maltodextrin | 0.95 ± 0.77 |
| Soy Lecithin | 0.90 ± 0.74 |
| Carrageenan | 0.58 ± 0.63 |
| Others (Carboxymethylcellulose, Polysorbate-80, Aluminosilicates, Titanium dioxide) | <0.1 |
| Dairy Products | Ice Cream, chocolate Milk, Sorbets, Milk Desserts, Soymilk, Yogurt, Products Made from Fresh Cheese, Thickened and Sterilized Cream |
| Cured meat | Canned meat, pâtés, frozen food, glazed ham |
| Products with jelling agents | Jams and jellies, candied fruit, icing sugar |
| Powdered products | Instant drink, formulated baby milks, powdered milk desserts, hot milk pudding |
| Soup, sauces | Emulsified sauces (salad dressing, mayonnaise), gravies, soups |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borsani, B.; De Santis, R.; Perico, V.; Penagini, F.; Pendezza, E.; Dilillo, D.; Bosetti, A.; Zuccotti, G.V.; D’Auria, E. The Role of Carrageenan in Inflammatory Bowel Diseases and Allergic Reactions: Where Do We Stand? Nutrients 2021, 13, 3402. https://doi.org/10.3390/nu13103402
Borsani B, De Santis R, Perico V, Penagini F, Pendezza E, Dilillo D, Bosetti A, Zuccotti GV, D’Auria E. The Role of Carrageenan in Inflammatory Bowel Diseases and Allergic Reactions: Where Do We Stand? Nutrients. 2021; 13(10):3402. https://doi.org/10.3390/nu13103402
Chicago/Turabian StyleBorsani, Barbara, Raffaella De Santis, Veronica Perico, Francesca Penagini, Erica Pendezza, Dario Dilillo, Alessandra Bosetti, Gian Vincenzo Zuccotti, and Enza D’Auria. 2021. "The Role of Carrageenan in Inflammatory Bowel Diseases and Allergic Reactions: Where Do We Stand?" Nutrients 13, no. 10: 3402. https://doi.org/10.3390/nu13103402
APA StyleBorsani, B., De Santis, R., Perico, V., Penagini, F., Pendezza, E., Dilillo, D., Bosetti, A., Zuccotti, G. V., & D’Auria, E. (2021). The Role of Carrageenan in Inflammatory Bowel Diseases and Allergic Reactions: Where Do We Stand? Nutrients, 13(10), 3402. https://doi.org/10.3390/nu13103402







