Antioxidants Supplementation Reduces Ceramide Synthesis Improving the Cardiac Insulin Transduction Pathway in a Rodent Model of Obesity
Abstract
:1. Introduction
2. Materials and Methods
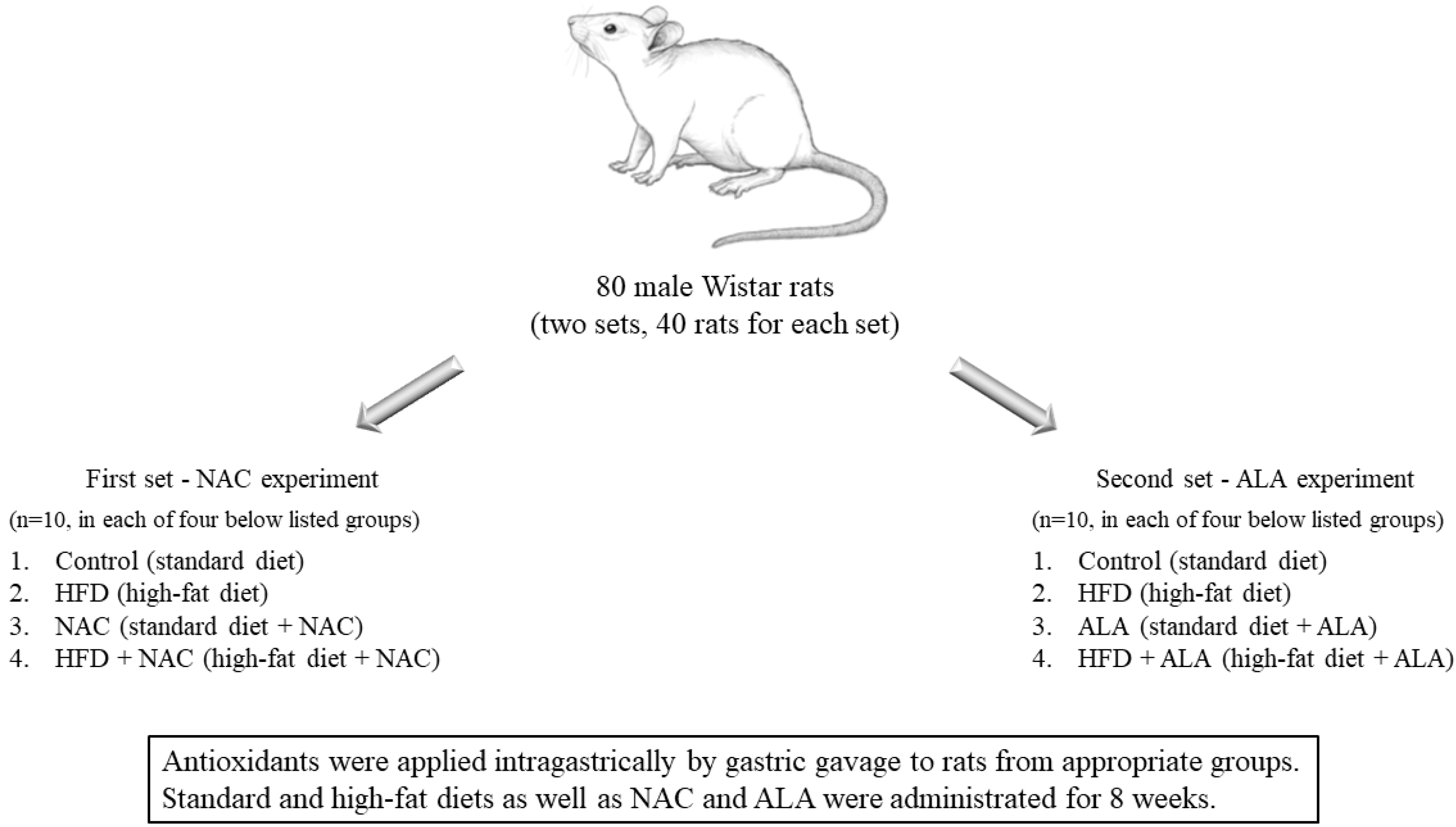
2.1. Animals and Experimental Protocol
2.2. Plasma Measurements
2.3. Analysis of Sphingoid Bases
2.4. Analysis of Ceramide
2.5. Analysis of Proteins Expression
2.6. Statistical Analysis
3. Results
3.1. Effects of Eight-Week NAC Treatment on Body Weight, Plasma Glucose and Insulin Concentrations as Well as HOMA-IR in Rats Subjected to Standard and High-Fat Diets
3.2. Effects of Eight-Week ALA Treatment on Body Weight, Plasma Glucose and Insulin Concentrations as Well as HOMA-IR in Rats Subjected to Standard and High-Fat Diets
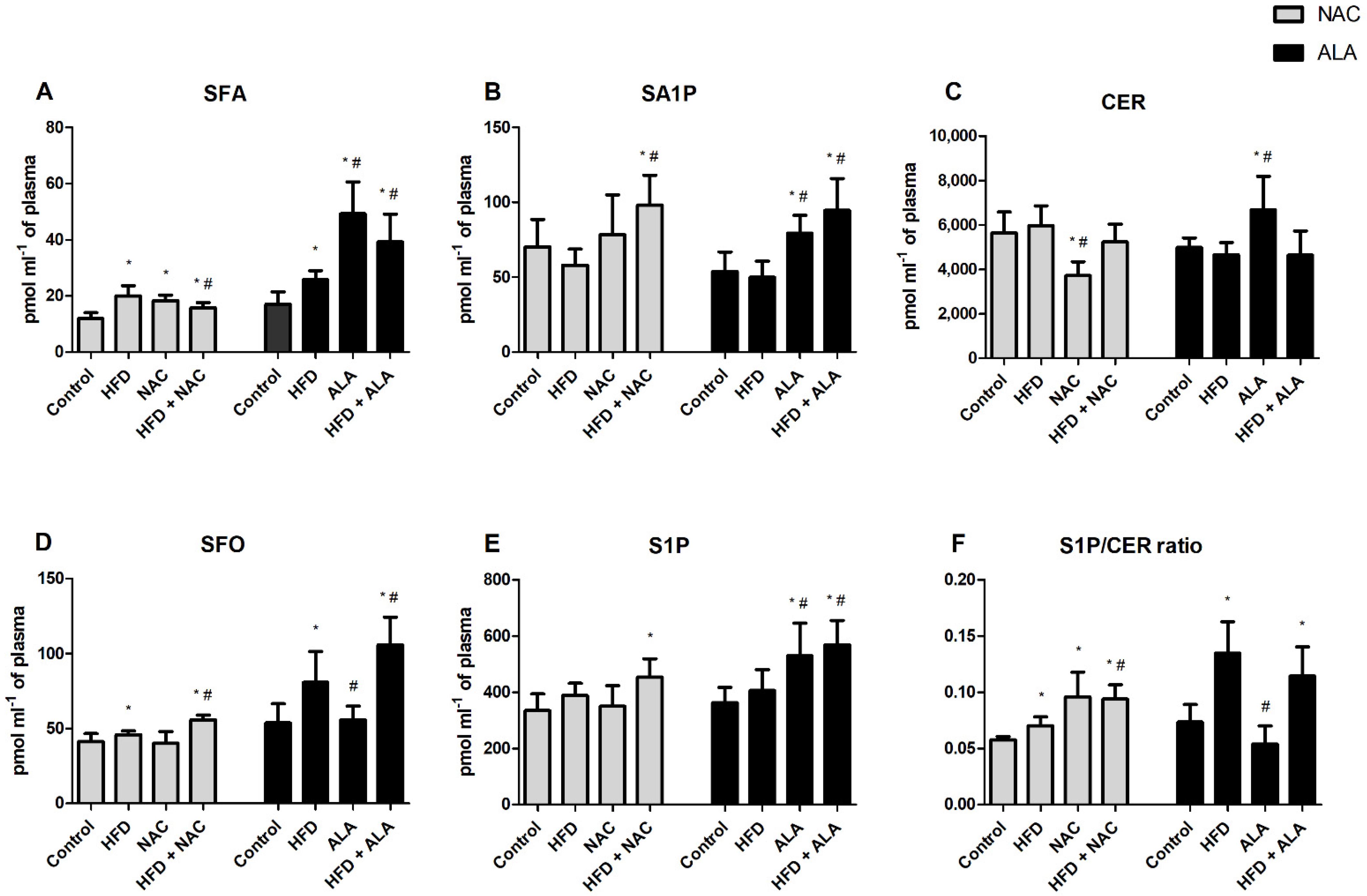
3.3. Effects of Eight-Week NAC Treatment on Sphingolipid Concentrations in Plasma of Rats Subjected to Standard and High-Fat Diets
3.4. Effects of Eight-Week ALA Treatment on Sphingolipid Concentrations in Plasma of Rats Subjected to Standard and High-Fat Diets
3.5. Effects of Eight-Week NAC Treatment on Sphingolipid Concentrations in the Left Ventricle of Rats Subjected to Standard and High-Fat Diets
3.6. Effects of Eight-Week ALA Treatment on Sphingolipid Concentrations in the Left Ventricle of Rats Subjected to Standard and High-Fat Diets
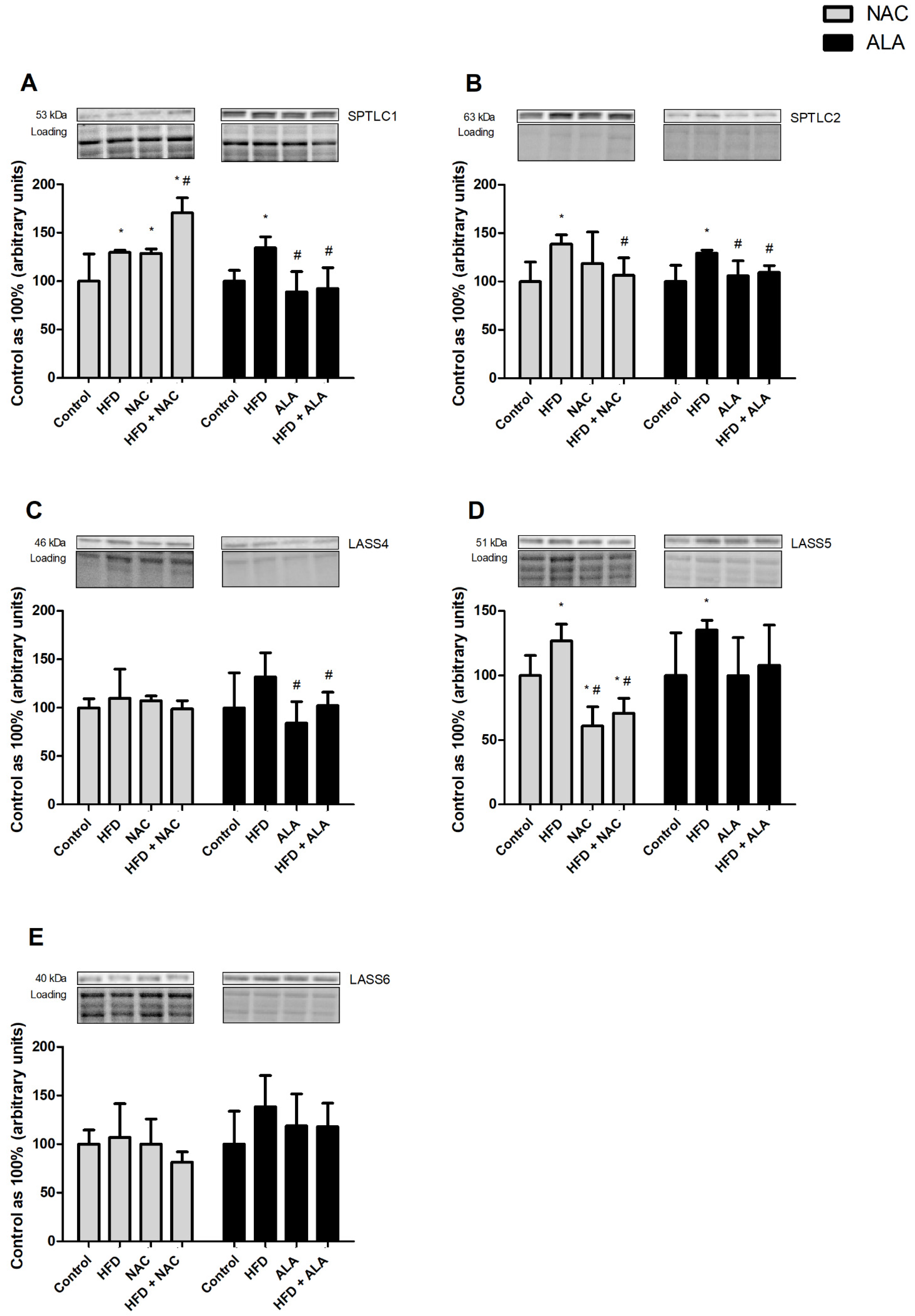
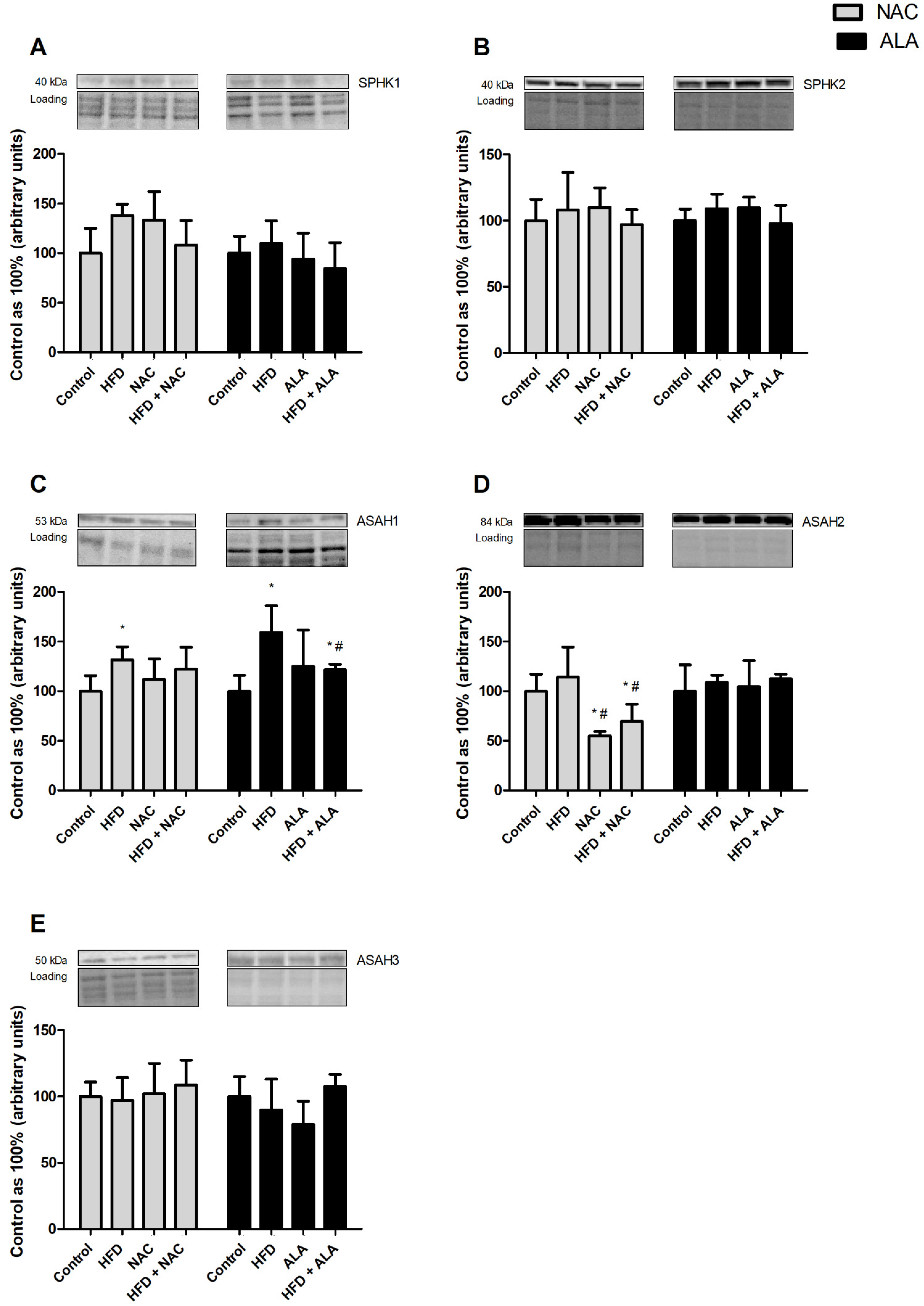
3.7. Effects of Eight-Week NAC and ALA Treatment on the Total Expression of Proteins Involved in the Sphingolipid Pathway in the Left Ventricle of Rats Subjected to Standard and High-Fat Diets
3.7.1. NAC
3.7.2. ALA
3.7.3. NAC
3.7.4. ALA
3.7.5. NAC
3.7.6. ALA
3.8. Effects of Eight-Week NAC and ALA Treatment on the Total Expression of Glucose Transporters and Phosphorylation State of Proteins Involved in Insulin Signaling Pathway in the Left Ventricle of Rats Subjected to Standard and High-Fat Diets
3.8.1. NAC
3.8.2. ALA
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, E984–E1010. [Google Scholar] [CrossRef] [PubMed]
- Harasim, E.; Stȩpek, T.; Konstantynowicz-Nowicka, K.; Baranowski, M.; Górski, J.; Chabowski, A. Myocardial lipid profiling during time course of high fat diet and its relationship to the expression of fatty acid transporters. Cell. Physiol. Biochem. 2015, 37, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shi, X.J.; Bharadwaj, K.G.; Ikeda, S.; Yamashita, H.; Yagyu, H.; Schaffer, J.E.; Yu, Y.H.; Goldberg, I.J. DGAT1 expression increases heart triglyceride content but ameliorates lipotoxicity. J. Biol. Chem. 2009, 284, 36312–36323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovilakath, A.; Jamil, M.; Cowart, L.A. Sphingolipids in the Heart: From Cradle to Grave. Front. Endocrinol. 2020, 11, 652. [Google Scholar] [CrossRef] [PubMed]
- Bruce, C.R.; Risis, S.; Babb, J.R.; Yang, C.; Kowalski, G.M.; Selathurai, A.; Lee-Young, R.S.; Weir, J.M.; Yoshioka, K.; Takuwa, Y.; et al. Overexpression of Sphingosine Kinase 1 Prevents Ceramide Accumulation and Ameliorates Muscle Insulin Resistance in High-Fat Diet-Fed Mice. Diabetes 2012, 61. [Google Scholar] [CrossRef] [Green Version]
- Aburasayn, H.; Al Batran, R.; Ussher, J.R.; Batran, A.R. Targeting ceramide metabolism in obesity. Am. J. Physiol. Endocrinol. Metab. 2016, 311, 423–435. [Google Scholar] [CrossRef]
- Witteles, R.M.; Fowler, M.B.; Stanford, F. Insulin-Resistant Cardiomyopathy Clinical Evidence, Mechanisms, and Treatment Options. J. Am. Coll. Cardiol. 2008. [Google Scholar] [CrossRef]
- Wali, J.A.; Jarzebska, N.; Raubenheimer, D.; Simpson, S.J.; Rodionov, R.N.; O’sullivan, J.F. Cardio-Metabolic Effects of High-Fat Diets and Their Underlying Mechanisms-A Narrative Review. Nutrients 2020, 12, 1505. [Google Scholar] [CrossRef]
- Aroor, A.R.; Mandavia, C.H.; Sowers, J.R. Insulin Resistance and Heart Failure: Molecular Mechanisms. Heart Fail. Clin. 2012, 8, 609–617. [Google Scholar] [CrossRef] [Green Version]
- Santalucía, T.; Boheler, K.R.; Brand, N.J.; Sahye, U.; Fandos, C.; Viñals, F.; Ferré, J.; Testar, X.; Palacín, M.; Zorzano, A. Factors Involved in GLUT-1 Glucose Transporter Gene Transcription in Cardiac Muscle. J. Biol. Chem. 1999, 274, 17626–17634. [Google Scholar] [CrossRef] [Green Version]
- Samuel, V.T.; Shulman, G.I. Nonalcoholic Fatty Liver Disease, Insulin Resistance, and Ceramides. N. Engl. J. Med. 2019, 381, 1866–1869. [Google Scholar] [CrossRef] [PubMed]
- Field, B.C.; Gordillo, R.; Scherer, P.E. The Role of Ceramides in Diabetes and Cardiovascular Disease Regulation of Ceramides by Adipokines. Front. Endocrinol. 2020, 11, 763. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.L.; Kang, C.H.; Wang, S.G.; Lee, H.M. α-lipoic acid regulates lipid metabolism through induction of Sirtuin 1 (SIRT1) and activation of AMP-activated protein kinase. Diabetologia 2012, 55, 1824–1835. [Google Scholar] [CrossRef] [Green Version]
- Marušić, M.; Paić, M.; Knobloch, M.; Liberati Pršo, A.M. NAFLD, Insulin Resistance, and Diabetes Mellitus Type 2. Can. J. Gastroenterol. Hepatol. 2021, 2021, 6613827. [Google Scholar] [CrossRef]
- Ma, Y.; Gao, M.; Liu, D. N-acetylcysteine Protects Mice from High Fat Diet-induced Metabolic Disorders. Pharm. Res. 2016. [Google Scholar] [CrossRef] [Green Version]
- Samuhasaneeto, S.; Thong-Ngam, D.; Kulaputana, O.; Patumraj, S.; Klaikeaw, N. Effects of N-acetylcysteine on oxidative stress in rats with non-alcoholic steatohepatitis—PubMed. J. Med. Assoc. Thail. 2007, 90, 788–797. [Google Scholar]
- Namvarpour, Z.; Nasehi, M.; Amini, A.; Zarrindast, M.-R. Protective role of alpha-lipoic acid in impairments of social and stereotyped behaviors induced by early postnatal administration of thimerosal in male rat. Neurotoxicol. Teratol. 2018, 68. [Google Scholar] [CrossRef]
- Ko, C.-Y.; Lo, Y.M.; Xu, J.-H.; Chang, W.-C.; Huang, D.-W.; Wu, J.S.-B.; Yang, C.-H.; Huang, W.-C.; Shen, S.-C. Alpha-lipoic acid alleviates NAFLD and triglyceride accumulation in liver via modulating hepatic NLRP3 inflammasome activation pathway in type 2 diabetic rats. Food Sci. Nutr. 2021. [Google Scholar] [CrossRef] [PubMed]
- Min, J.-K.; Yoo, H.-S.; Lee, E.-Y.; Lee, W.-J.; Lee, Y.-M. Simultaneous Quantitative Analysis of Sphingoid Base 1-Phosphates in Biological Samples by o-Phthalaldehyde Precolumn Derivatization after Dephosphorylation with Alkaline Phosphatase. Anal. Biochem. 2002, 303, 167–175. [Google Scholar] [CrossRef]
- Baranowski, M.; Zabielski, P.; Blachnio, A.; Gorski, J. Effect of exercise duration on ceramide metabolism in the rat heart. Acta Physiol. 2008, 192, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Konstantynowicz-Nowicka, K.; Harasim, E.; Baranowski, M.; Chabowski, A. New evidence for the role of ceramide in the development of hepatic insulin resistance. PLoS ONE 2015, 10, e0116858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novelli, L.E.B.; Santos, P.P.; Assalin, H.B.; Souza, G.; Rocha, K.; Ebaid, G.X.; Rodrigues, F.; Seiva, F.; Mani, F.; Fernandes, A.A. N-acetylcysteine in high-sucrose diet-induced obesity: Energy expenditure and metabolic shifting for cardiac health. Pharmacol. Res. 2009, 59, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Wende, A.R.; Abel, E.D. Lipotoxicity in the heart. Biochim. Biophys. Acta 2010, 1801, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Barouch, L.A. Leptin Signaling and Obesity. Circ. Res. 2007, 101, 545–559. [Google Scholar] [CrossRef]
- Henique, C.; Mansouri, A.; Fumey, G.; Lenoir, V.; Girard, J.; Bouillaud, F.; Prip-Buus, C.; Cohen, I. Increased Mitochondrial Fatty Acid Oxidation Is Sufficient to Protect Skeletal Muscle Cells from Palmitate-induced Apoptosis. J. Biol. Chem. 2010, 285, 36818–36827. [Google Scholar] [CrossRef] [Green Version]
- Chabowski, A.; Górski, J.; Glatz, J.F.; Luiken, J.J.F.P.; Bonen, A. Protein-mediated Fatty Acid Uptake in the Heart. Curr. Cardiol. Rev. 2008, 4, 12. [Google Scholar] [CrossRef] [Green Version]
- Paumen, M.B.; Ishida, Y.; Muramatsu, M.; Yamamoto, M.; Honjo, T. Inhibition of Carnitine Palmitoyltransferase I Augments Sphingolipid Synthesis and Palmitate-induced Apoptosis*. J. Biol. Chem. 1997. [Google Scholar] [CrossRef] [Green Version]
- Charron, M.J.; Williams, L.; Seki, Y.; Du, X.Q.; Chaurasia, B.; Saghatelian, A.; Summers, S.A.; Katz, E.B.; Vuguin, P.M.; Reznik, S.E. Antioxidant Effects of N-Acetylcysteine Prevent Programmed Metabolic Disease in Mice. Diabetes 2020, 69, 1650–1661. [Google Scholar] [CrossRef]
- Korou, L.-M.; Agrogiannis, G.; Pantopoulou, A.; Vlachos, I.S.; Iliopoulos, D.; Karatzas, T.; Perrea, D.N. Comparative antilipidemic effect of N-acetylcysteine and sesame oil administration in diet-induced hypercholesterolemic mice. Lipids Health Dis. 2010, 9, 23. [Google Scholar] [CrossRef] [Green Version]
- Mardinoglu, A.; Ural, D.; Zeybel, M.; Yuksel, H.H.; Uhlén, M.; Borén, J. The Potential Use of Metabolic Cofactors in Treatment of NAFLD. Nutrients 2019, 11, 1578. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.R.; Visioli, F.; Frei, B.; Hagen, T.M. Lipoic acid significantly restores, in rats, the age-related decline in vasomotion. Br. J. Pharmacol. 2008, 153, 1615. [Google Scholar] [CrossRef] [PubMed]
- Monette, J.S.; Gómez, L.A.; Moreau, R.F.; Dunn, K.C.; Butler, J.A.; Finlay, L.A.; Michels, A.J.; Shay, K.P.; Smith, E.J.; Hagen, T.M. (R)-Lipoic acid treatment restores ceramide balance in aging rat cardiac mitochondria. Pharmacol. Res. 2011, 63, 23–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tardif, J.-C.; Rhéaume, E. Lipoic acid supplementation and endothelial function. Br. J. Pharmacol. 2008, 153, 1587–1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.C.; Jun, D.W.; Jang, E.C.; Kim, S.H.; Kim, E.K.; Lee, S.P.; Lee, K.N.; Lee, H.L.; Lee, O.Y.; Yoon, B.C.; et al. Lipoic Acid Prevents the Changes of Intracellular Lipid Partitioning by Free Fatty Acid. Gut Liver 2013, 7, 221. [Google Scholar] [CrossRef] [Green Version]
- Timmers, S.; de Vogel-van den Bosch, J.; Towler, M.C.; Schaart, G.; Moonen-Kornips, E.; Mensink, R.P.; Hesselink, M.K.; Hardie, D.G.; Schrauwen, P. Prevention of high-fat diet-induced muscular lipid accumulation in rats by α lipoic acid is not mediated by AMPK activation. J. Lipid Res. 2010, 51, 352. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, M.S.; Pieri, B.L.d.S.; Silveira, G.d.B.; Zaccaron, R.P.; Venturini, L.M.; Comin, V.H.; Luiz, K.D.; Silveira, P.C.L. Reduction of oxidative stress improves insulin signaling in cardiac tissue of obese mice. Einstein 2020, 18, eAO5022. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.; Sangweni, N.F.; Mabhida, S.E.; Dludla, P.V.; Mabasa, L.; Riedel, S.; Chapman, C.; Mosa, R.A.; Kappo, A.P.; Louw, J.; et al. An In Vitro Study on the Combination Effect of Metformin and N-Acetyl Cysteine against Hyperglycaemia-Induced Cardiac Damage. Nutrients 2019, 11, 2850. [Google Scholar] [CrossRef] [Green Version]
- Asrih, M.; Lerch, R.; Papageorgiou, I.; Pellieux, C.; Montessui, C. Differential regulation of stimulated glucose transport by free fatty acids and PPARα or -δ agonists in cardiac myocytes. Am. J. Physiol.—Endocrinol. Metab. 2012, 302, 872–884. [Google Scholar] [CrossRef]
- Gupte, A.A.; Bomhoff, G.L.; Morris, J.K.; Gorres, B.K.; Geiger, P.C. Lipoic acid increases heat shock protein expression and inhibits stress kinase activation to improve insulin signaling in skeletal muscle from high-fat-fed rats. J. Appl. Physiol. 2009, 106, 1425–1434. [Google Scholar] [CrossRef]
- Gomes, M.B.; Negrato, C.A. Alpha-lipoic acid as a pleiotropic compound with potential therapeutic use in diabetes and other chronic diseases. Diabetol. Metab. Syndr. 2014, 6. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.R.; Shenvi, S.V.; Widlansky, M.; Suh, J.H.; Hagen, T.M. Lipoic acid as a potential therapy for chronic diseases associated with oxidative stress. Curr. Med. Chem. 2004, 11, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Moini, H.; Tirosh, O.; Park, Y.C.; Cho, K.J.; Packer, L. R-α-Lipoic Acid Action on Cell Redox Status, the Insulin Receptor, and Glucose Uptake in 3T3-L1 Adipocytes. Arch. Biochem. Biophys. 2002, 397, 384–391. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hodun, K.; Sztolsztener, K.; Chabowski, A. Antioxidants Supplementation Reduces Ceramide Synthesis Improving the Cardiac Insulin Transduction Pathway in a Rodent Model of Obesity. Nutrients 2021, 13, 3413. https://doi.org/10.3390/nu13103413
Hodun K, Sztolsztener K, Chabowski A. Antioxidants Supplementation Reduces Ceramide Synthesis Improving the Cardiac Insulin Transduction Pathway in a Rodent Model of Obesity. Nutrients. 2021; 13(10):3413. https://doi.org/10.3390/nu13103413
Chicago/Turabian StyleHodun, Katarzyna, Klaudia Sztolsztener, and Adrian Chabowski. 2021. "Antioxidants Supplementation Reduces Ceramide Synthesis Improving the Cardiac Insulin Transduction Pathway in a Rodent Model of Obesity" Nutrients 13, no. 10: 3413. https://doi.org/10.3390/nu13103413






