Spasmolytic Effects of Aphanizomenon Flos Aquae (AFA) Extract on the Human Colon Contractility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Tissue Specimens and Preparation
2.2. Functional Studies
2.3. TAAR1 Expression Analysis
2.4. Drugs
2.5. Data and Statistical Analysis
3. Results
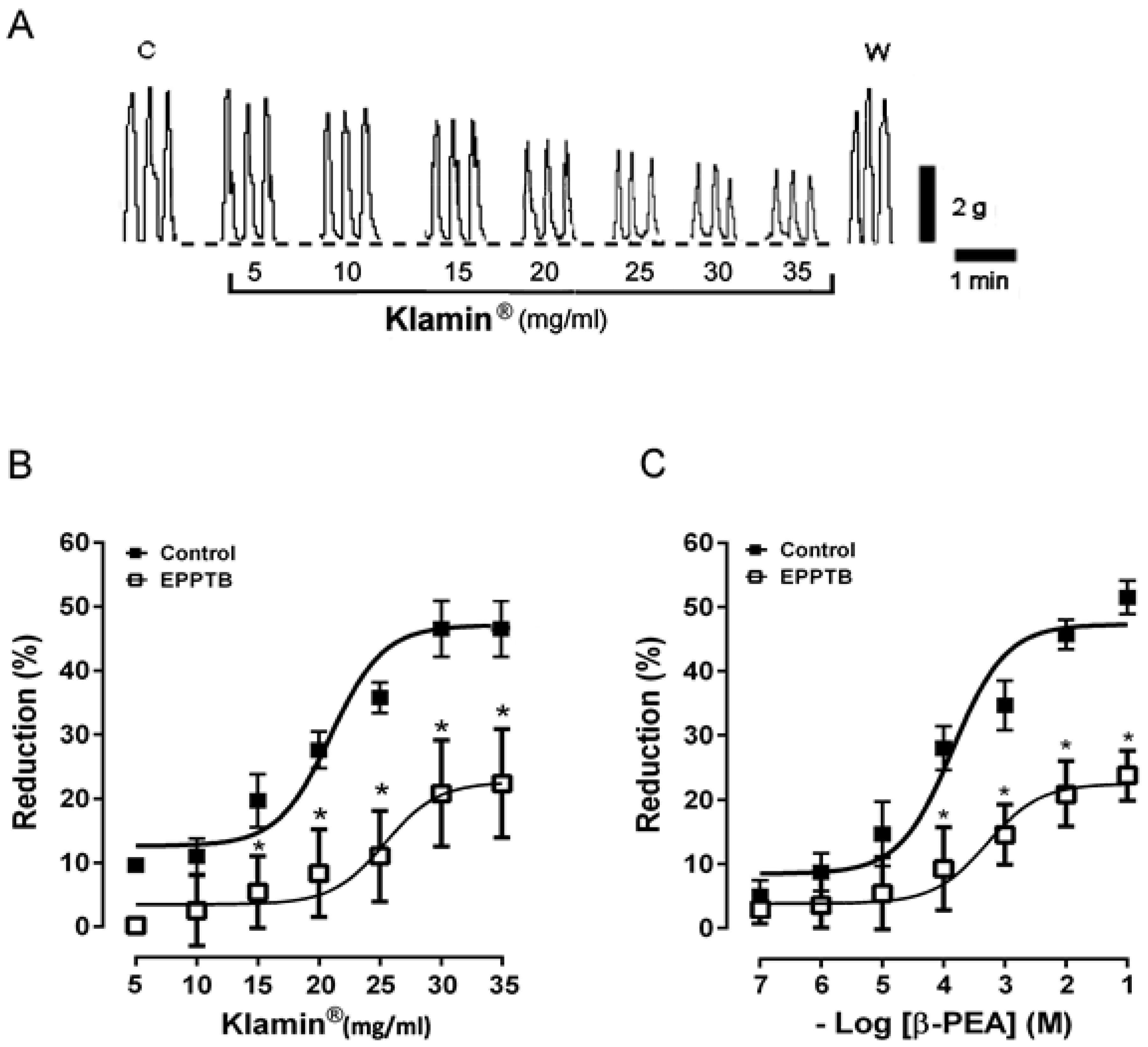
3.1. Functional Study
3.2. TAAR1 Expression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Xue, X.; Lv, Y.; Liu, Q.; Zhang, X.; Zhao, Y.; Zhang, L.; Xu, S. Extracellular polymeric substance from Aphanizomenon flos-aquae induces apoptosis via the mitochondrial pathway in A431 human epidermoid carcinoma cells. Exp. Ther. Med. 2015, 10, 927–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raposo, M.F.D.J.; Morais, R.; Morais, A. Health applications of bioactive compounds from marine microalgae. Life Sci. 2013, 93, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Christaki, E.; Florou-Paneri, P.; Bonos, E. Microalgae: A novel ingredient in nutrition. Int. J. Food Sci. Nutr. 2011, 62, 794–799. [Google Scholar] [CrossRef]
- Romay, C.; Armesto, J.; Remirez, D.; González, R.; Ledon, N.; García, I. Antioxidant and anti-inflammatory properties of C-phycocyanin from blue-green algae. Inflamm. Res. 1998, 47, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Romay, C.; Ledón, N.; González, R. Further studies on anti-inflammatory activity of phycocyanin in some animal models of inflammation. Inflamm. Res. 1998, 47, 334–338. [Google Scholar] [CrossRef]
- Yun, H.; Kim, I.; Kwon, S.-H.; Kang, J.-S.; Om, A.-S. Protective effect of chlorella vulgaris against lead-induced oxidative stress in rat brains. J. Health Sci. 2011, 57, 245–254. [Google Scholar] [CrossRef] [Green Version]
- Guzman, S.; Gato, A.; Calleja, J.M. Antiinflammatory, analgesic and free radical scavenging activities of the marine microalgae Chlorella stigmatophora and Phaeodactylumtricornutum. Phytother. Res. 2001, 15, 224–230. [Google Scholar] [CrossRef]
- Gardeva, E.; Toshkova, R.; Minkova, K.; Gigova, L. Cancer protective action of polysaccharide derived from microalga Porphyridiumcruentum-A biological background. Biotechnol. Equip. 2009, 23, 783–787. [Google Scholar] [CrossRef]
- Benedetti, S.; Benvenuti, F.; Scoglio, S.; Canestrari, F. Oxygen Radical Absorbance Capacity of Phycocyanin and Phycocyanobilin from the Food Supplement Aphanizomenon flos-aquae. J. Med. Food 2010, 13, 223–227. [Google Scholar] [CrossRef]
- Berry, M.D. Mammalian central nervous system trace amines. Pharmacologic amphetamines, physiologic neuromodulators. J. Neurochem. 2004, 90, 257–271. [Google Scholar] [CrossRef]
- Lindemann, L.; Ebeling, M.; Kratochwil, N.A.; Bunzow, J.R.; Grandy, D.K.; Hoener, M.C. Trace amine-associated receptors form structurally and functionally distinct subfamilies of novel G protein-coupled receptors. Genomics 2005, 85, 372–385. [Google Scholar] [CrossRef]
- Burchett, S.A.; Hicks, T.P. The mysterious trace amines: Protean neuromodulators of synaptic transmission in mammalian brain. Prog. Neurobiol. 2006, 79, 223–246. [Google Scholar] [CrossRef] [PubMed]
- Sabelli, H.; Fink, P.; Fawcett, J.; Tom, C. Sustained antidepressant effect of PEA replacement. J. Neuropsychiatry Clin. Neurosci. 1996, 8, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Borah, A.; Paul, R.; Mazumder, M.K.; Bhattacharjee, N.; Borah, A. Contribution of beta-phenethylamine, a component of chocolate and wine, to dopaminergic neurodegeneration: Implications for the pathogenesis of Parkinson’s disease. Neurosci. Bull. 2013, 29, 655–660. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, M.; Suárez, L.; Andrés, M.T.; Flórez, B.H.; Bordallo, J.; Riestra, S.; Cantabrana, B. Modulatory effect of intestinal polyamines and trace amines on the spontaneous phasic contractions of the isolated ileum and colon rings of mice. Food Nutr. Res. 2017, 61, 1321948. [Google Scholar] [CrossRef]
- Broadley, K.J.; Anwar, M.A.; Herbert, A.A.; Fehler, M.; Jones, E.M.; Davies, W.E.; Kidd, E.; Ford, W. Effects of dietary amines on the gut and its vasculature. Br. J. Nutr. 2008, 101, 1645–1652. [Google Scholar] [CrossRef] [Green Version]
- Batista-Lima, F.J.; Rodrigues, F.M.D.S.; Gadelha, K.K.L.; Oliveira, D.M.N.; Carvalho, E.F.; Oliveira, T.L.; Nóbrega, F.C.; Brito, T.S.; Magalhães, P.J.C. Dual excitatory and smooth muscle-relaxant effect of β-phenylethylamine on gastric fundus strips in rats. Clin. Exp. Pharmacol. Physiol. 2019, 46, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Zucchi, R.; Chiellini, G.; Scanlan, T.S.; Grandy, D.K. Trace Amine-Associated Receptors and Their Ligands. Br. J. Pharmacol. 2006, 149, 967–978. [Google Scholar] [CrossRef] [Green Version]
- Grandy, D.K. Trace amine-associated receptor 1-Family archetype or iconoclast? Pharmacol. Ther. 2007, 116, 355–390. [Google Scholar] [CrossRef] [Green Version]
- Borowsky, B.; Adham, N.; Jones, K.A.; Raddatz, R.; Artymyshyn, R.; Ogozalek, K.L.; Durkin, M.M.; Lakhlani, P.P.; Bonini, J.A.; Pathirana, S.; et al. Trace amines: Identification of a family of mammalian G protein-coupled receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 8966–8971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiellini, G.; Erba, P.; Carnicelli, V.; Manfredi, C.; Frascarelli, S.; Ghelardoni, S.; Mariani, G.; Zucchi, R. Distribution of exogenous [125I]-3-iodothyronamine in mouse in vivo: Relationship with trace amine-associated receptors. J. Endocrinol. 2012, 213, 223–230. [Google Scholar] [CrossRef]
- Ito, J.; Ito, M.; Nambu, H.; Fujikawa, T.; Tanaka, K.; Iwaasa, H.; Tokita, S. Anatomical and histological profiling of orphan G-protein-coupled receptor expression in gastrointestinal tract of C57BL/6J mice. Cell Tissue Res. 2009, 338, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Zizzo, M.G.; Caldara, G.; Bellanca, A.; Nuzzo, D.; Di Carlo, M.; Scoglio, S.; Serio, R. AphaMax®, an Aphanizomenon Flos-Aquae Aqueous Extract, Exerts Intestinal Protective Effects in Experimental Colitis in Rats. Nutrients 2020, 12, 3635. [Google Scholar] [CrossRef] [PubMed]
- Genazzani, A.D.; Chierchia, E.; Lanzoni, C.; Santagni, S.; Veltri, F.; Ricchieri, F.; Rattighieri, E.; Nappi, R.E. Effects of Klamath Algae extract on psychological disorders and depression in menopausal women: A pilot study. Minerva Ginecol. 2010, 62, 381–388. [Google Scholar] [PubMed]
- Bellingeri, P.; Bonucci, M.; Scoglio, S. Complementary treatment of mood disturbances in terminally ill oncological patients with the Aphanizomenon flos-aquae extract Klamin®. BMC Complement. Altern. Med. 2018, 1, 1–5. [Google Scholar]
- Cremonte, M.; Sisti, D.; Maraucci, I. Complementary and alternative supplementation with the Klamath algae extract Klamin® on attention deficit/hyperactivity disorder. J. Med. Food. 2017, 20, 1233–1239. [Google Scholar] [CrossRef]
- Amato, A.; Terzo, S.; Lentini, L.; Marchesa, P.; Mulè, F. TRPM8 Channel Activation reduces the Spontaneous Contractions in Human Distal Colon. Int. J. Mol. Sci. 2020, 21, 5403. [Google Scholar] [CrossRef]
- Amato, A.; Baldassano, S.; Liotta, R.; Serio, R.; Mulè, F. Exogenous glucagon-like peptide 1 reduces contractions in human colon circular muscle. J. Endocrinol. 2014, 221, 29–37. [Google Scholar] [CrossRef]
- Zizzo, M.G.; Bellanca, A.; Amato, A.; Serio, R. Opposite effects of dopamine on the mechanical activity of circular and longitudinal muscle of human colon. Neurogastroenterol. Motil. 2020, 32, e13811. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.H.W.; Chess-Williams, R.; Lohning, A.E. Renal artery responses to trace amines: Multiple and differential mechanisms of action. Life Sci. 2021, 277, 119532. [Google Scholar] [CrossRef] [PubMed]
- Tam, F.-F.; Hillier, K.; Bunce, K. Characterization of the 5-hydroxytryptamine receptor type involved in inhibition of spontaneous activity of human isolated colonic circular muscle. Br. J. Pharmacol. 1994, 113, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Babusyte, A.; Kotthoff, M.; Fiedler, J.; Krautwurst, D. Biogenic amines activate blood leukocytes via trace amine-associated receptors TAAR1 and TAAR2. J. Leukoc. Biol. 2013, 93, 387–394. [Google Scholar] [CrossRef]
- Benedetti, S.; Rinalducci, S.; Benvenuti, F.; Francogli, S.; Pagliarani, S.; Giorgi, L.; Micheloni, M.; D’Amici, G.M.; Zolla, L.; Canestrari, F. Purification and characterization of phycocyanin from the blue-green alga Aphanizomenon flos-aquae. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2006, 833, 12–18. [Google Scholar] [CrossRef]
- Benedetti, S.; Benvenuti, F.; Pagliarani, S.; Francogli, S.; Scoglio, S.; Canestrari, F. Antioxidant properties of a novel phycocyanin extract fromthe blue-green alga Aphanizomenon flos-aquae. Life Sci. 2004, 75, 2353–2362. [Google Scholar] [CrossRef] [PubMed]
- Scoglio, S.; Benedetti, S.; Canino, C.; Santagni, S.; Rattighieri, E.; Chierchia, E.; Canestrari, F.; Genazzani, A.D. Effect of a 2-month treatment with Klamin, a Klamath algae extract, on the general well-being, antioxidant profile and oxidative status of postmenopausal women. Gynecol. Endocrinol. 2009, 25, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Scoglio, S.; Benedetti, Y.; Benvenuti, F.; Battistelli, S.; Canestrari, F.; Benedetti, S. Selective monoamine oxidase B inhibition by an Aphanizomenon flos-aquae extract and by its constitutive active principles phycocyanin and mycosporine-like amino acids. Phytomedicine 2014, 21, 992–997. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, D.; Contardi, M.; Kossyvaki, D.; Picone, P.; Cristaldi, L.; Galizzi, G.; Bosco, G.; Scoglio, S.; Athanassiou, A.; Di Carlo, M. Heat-Resistant Aphanizomenon flos-aquae (AFA) Extract (Klamin) as a Functional Ingredient in Food Strategy for Prevention of Oxidative Stress. Oxidative Med. Cell. Longev. 2019, 2019, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Nuzzo, D.; Presti, G.; Picone, P.; Galizzi, G.; Gulotta, E.; Giuliano, S.; Mannino, C.; Gambino, V.; Scoglio, S.; Di Carlo, M. Effects of the Aphanizomenon flos-aquae Extract (Klamin) on a Neurodegeneration Cellular Model. Oxidative Med. Cell. Longev. 2018, 2018, 9089016. [Google Scholar] [CrossRef]
- Berry, M.D.; Gainetdinov, R.R.; Hoener, M.C.; Shahid, M. Pharmacology of human trace amine-associated receptors: Therapeutic opportuni- ties and challenges. Pharmacol Ther 2017, 180, 161–180. [Google Scholar] [CrossRef]
- Sabelli, H.C.; Mosnaim, A.D. Phenylethylamine hypothesis of affective behaviour. Am. J. Psychiatry 1974, 131, 695–699. [Google Scholar] [CrossRef]
- Hansen, T.R.; Greenberg, J.; Mosnaim, A.D. Direct effect of phenylethylamine upon isolated rat aortic strip. Eur. J. Pharmacol. 1980, 63, 95–101. [Google Scholar] [CrossRef]
- Fehler, M.; Broadley, K.J.; Ford, W.R.; Kidd, E.J. Identification of trace-amine-associated receptors (TAAR) in the rat aorta and their role in vasoconstriction by β-phenylethylamine. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2010, 382, 385–398. [Google Scholar] [CrossRef]
- Broadley, K.J.; Broadley, H.D. Non-adrenergic vasoconstriction and vasodilatation of guinea-pig aorta by β-phenylethylamine and amphetamine—Role of nitric oxide determined with L-NAME and NO scavengers. Eur. J. Pharmacol. 2018, 818, 198–205. [Google Scholar] [CrossRef]
- Stalder, H.; Hoener, M.; Norcross, R.D. Selective antagonists of mouse trace amine-associated receptor 1 (mTAAR1): Discovery of EPPTB (RO5212773). Bioorg. Med. Chem. Lett. 2011, 21, 1227–1231. [Google Scholar] [CrossRef]
- Cichero, E.; Espinoza, S.; Gainetdinov, R.; Brasili, L.; Fossa, P. Insights into the Structure and Pharmacology of the Human Trace Amine-Associated Receptor 1 (hTAAR1): Homology Modelling and Docking Studies. Chem. Biol. Drug Des. 2013, 81, 509–516. [Google Scholar] [CrossRef]
- Anwar, M.; Ford, W.; Broadley, K.; Herbert, A. Vasoconstrictor and vasodilator responses to tryptamine of rat isolated perfused mesentery: Comparison with tyramine and β-phenylethylamine. Br. J. Pharmacol. 2012, 165, 2191–2202. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-Z.; Zhang, X.-L.; Zhou, L.; Wang, T.; Quan, Z.-S.; Zhang, Y.; Li, J.; Li, G.-W.; Zheng, L.-F.; Li, L.-S.; et al. Rasagiline, an inhibitor of MAO-B, decreases colonic motility through elevating colonic dopamine content. Neurogastroenterol. Motil. 2018, 30, e13390. [Google Scholar] [CrossRef]
- Raab, S.; Wang, H.; Uhles, S.; Cole, N.; Alvarez-Sanchez, R.; Künnecke, B.; Ullmer, C.; Matile, H.; Bedoucha, M.; Norcross, R.D.; et al. Incretin-like effects of small molecule trace amine-associated receptor 1 agonists. Mol. Metab. 2015, 5, 47–56. [Google Scholar] [CrossRef]
- Gwilt, K.B.; Olliffe, N.; Hoffing, R.A.; Miller, G.M. Trace amine associated receptor 1 (TAAR1) expression and modulation of inflammatory cytokine production in mouse bone marrow-derived macrophages: A novel mechanism for inflammation in ulcerative colitis. Immunopharmacol. Immunotoxicol. 2019, 41, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Gwilt, K.B.; González, D.P.; Olliffe, N.; Oller, H.; Hoffing, R.; Puzan, M.; El Aidy, S.; Miller, G.M. Actions of Trace Amines in the Brain-Gut-Microbiome Axis via Trace Amine-Associated Receptor-1 (TAAR1). Cell. Mol. Neurobiol. 2019, 40, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Gallego, D.; Gil, V.; Aleu, J.; Aulí, M.; Clavé, P.; Jimenez, M. Purinergic and nitrergic junction potential in the human colon. Am. J. Physiol. Liver Physiol. 2008, 295, G522–G533. [Google Scholar] [CrossRef] [Green Version]
- Bunzow, J.R.; Sonders, M.S.; Arttamangkul, S. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethyl-amide, and metabolites of the catecholamine neurotransmit- ters are agonists of a rat trace amine receptor. Mol. Pharmacol. 2001, 60, 1181. [Google Scholar] [CrossRef] [PubMed]
- Gwynne, R.M.; Clarke, A.J.; Furness, J.B.; Bornstein, J. Both exogenous 5-HT and endogenous 5-HT, released by fluoxetine, enhance distension evoked propulsion in guinea-pig ileum in vitro. Front. Neurosci. 2014, 8, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borman, R.A.; Tilford, N.S.; Harmer, D.W.; Day, N.; Ellis, E.S.; Sheldrick, R.L.G.; Carey, J.; Coleman, R.A.; Baxter, G.S. 5-HT2B receptors play a key role in mediating the excitatory effects of 5-HT in human colon in vitro. Br. J. Pharmacol. 2002, 135, 1144–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.S. 5-Hydroxytryptamine4 receptor agonists and colonic motility. J. Smooth Muscle Res. 2009, 45, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Borman, R.A.; Burleigh, D.E. Heterogeneity of 5-HT receptors in human sigmoid colon. Br. J. Pharmacol. 1994, 112, 558. [Google Scholar]
- Prins, N.H.; Briejer, M.R.; Van Bergen, P.J.; Akkermans, L.M.; Schuurkes, J.A. Evidence for 5-HT7 receptors mediating relaxation of human colonic circular smooth muscle. Br. J. Pharmacol. 1999, 128, 849–852. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amato, A.; Terzo, S.; Marchesa, P.; Maffongelli, A.; Martorana, M.; Scoglio, S.; Mulè, F. Spasmolytic Effects of Aphanizomenon Flos Aquae (AFA) Extract on the Human Colon Contractility. Nutrients 2021, 13, 3445. https://doi.org/10.3390/nu13103445
Amato A, Terzo S, Marchesa P, Maffongelli A, Martorana M, Scoglio S, Mulè F. Spasmolytic Effects of Aphanizomenon Flos Aquae (AFA) Extract on the Human Colon Contractility. Nutrients. 2021; 13(10):3445. https://doi.org/10.3390/nu13103445
Chicago/Turabian StyleAmato, Antonella, Simona Terzo, Pierenrico Marchesa, Angela Maffongelli, Martina Martorana, Stefano Scoglio, and Flavia Mulè. 2021. "Spasmolytic Effects of Aphanizomenon Flos Aquae (AFA) Extract on the Human Colon Contractility" Nutrients 13, no. 10: 3445. https://doi.org/10.3390/nu13103445
APA StyleAmato, A., Terzo, S., Marchesa, P., Maffongelli, A., Martorana, M., Scoglio, S., & Mulè, F. (2021). Spasmolytic Effects of Aphanizomenon Flos Aquae (AFA) Extract on the Human Colon Contractility. Nutrients, 13(10), 3445. https://doi.org/10.3390/nu13103445





