Nutritional Inadequacy: Unraveling the Methodological Challenges for the Application of the Probability Approach or the EAR Cut-Point Method—A Pregnancy Perspective
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.1.1. Participants
2.1.2. Exclusion Criteria
2.2. Data Collection
2.2.1. Collection of Demographic/Anthropometric Characteristics and Lifestyle Factors
2.2.2. Collection of Dietary Data
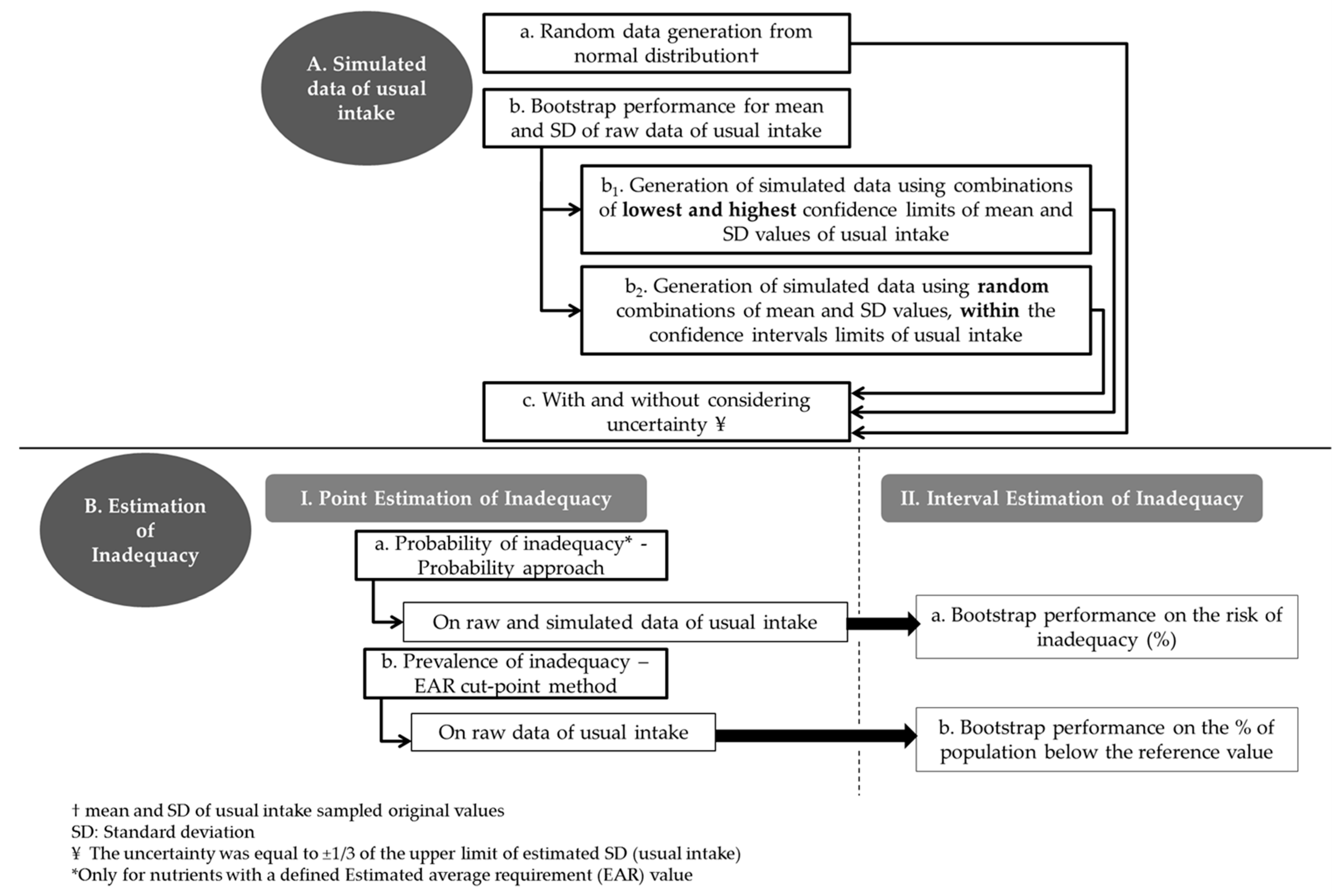
2.3. Schematic Visualization of the Sequence of Steps Followed in the Present Study
2.4. Generation of Simulated Data
- Step a: Random data (n = 608 cases) from Normal Distribution were generated based on the values of mean and standard deviation (SD) of usual intake sampled original values (a).
- Step b: Thirty bias corrected 99% bootstrap confidence intervals (CI) were estimated around the mean and SD of the original data. Each bootstrap run was based on 500 resampling circles (b).
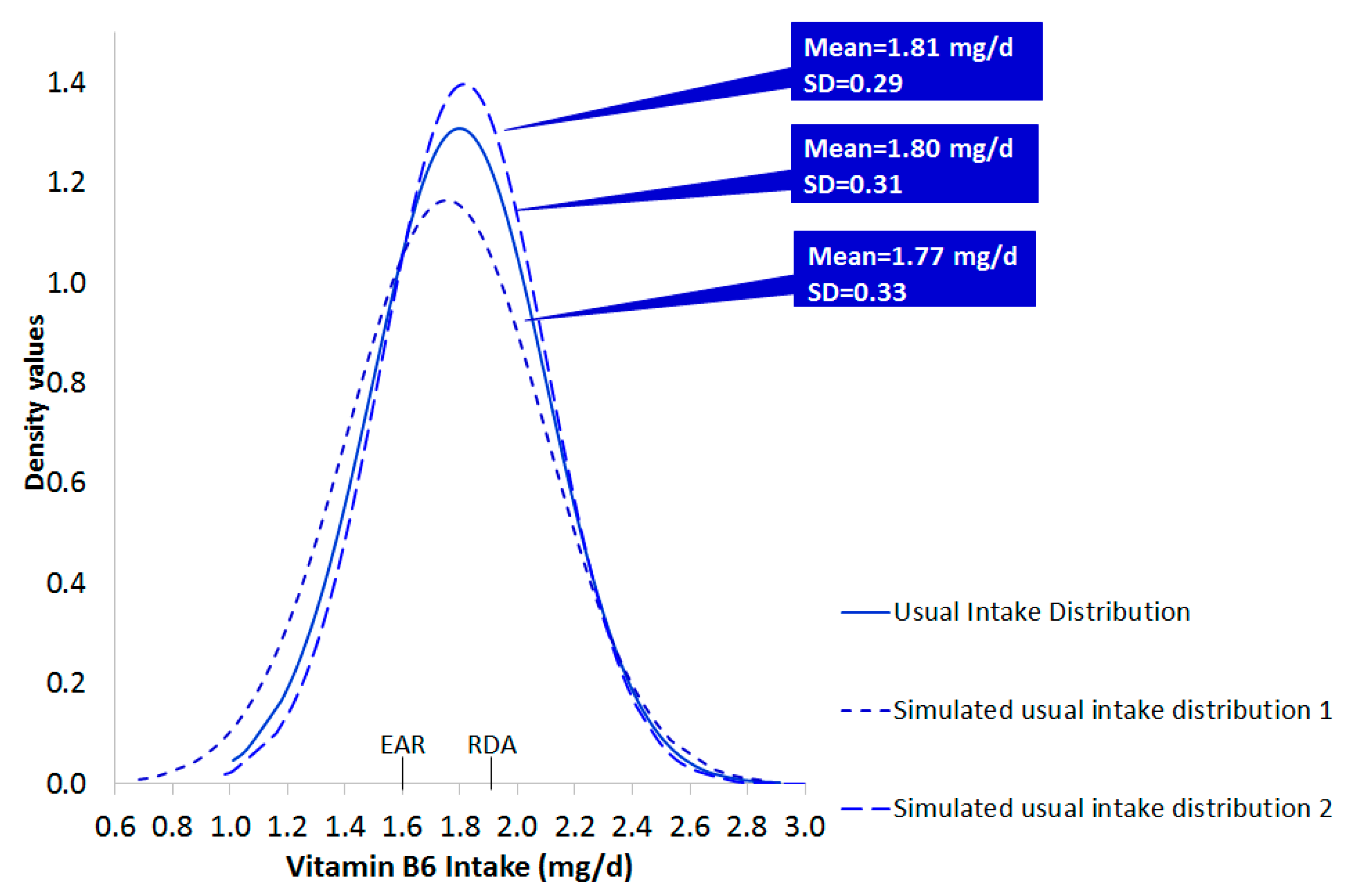
- Step b1–b2: Given the resulted CI from Step b, the lowest (min) and the highest (max) low and upper bounds of the CI, for the mean and the corresponding SD, were selected and used to generate new random normally distributed data sets of usual intake, as in step a. Specifically, one set was based on the combination of the min bound of the mean and the min bound of the corresponding SD. Three other sets were based on the following combinations: min bound of the mean and max bound of the SD, max bound of the mean and min bound of the SD and max bound of mean and max bound of the SD (b1). In addition, 30 new data sets were generated based on random combinations within the lower-upper bounds of the mean and SD values of usual intake (b2). A portion of the results is reported in the manuscript.
- Step c. On each of the previously generated data sets an additional degree of uncertainty was “imposed” by randomly adding or subtracting the 1/3 of the upper limit of estimated SD (usual intake), which is an appropriate measure of uncertainty for normally distributed data (c).
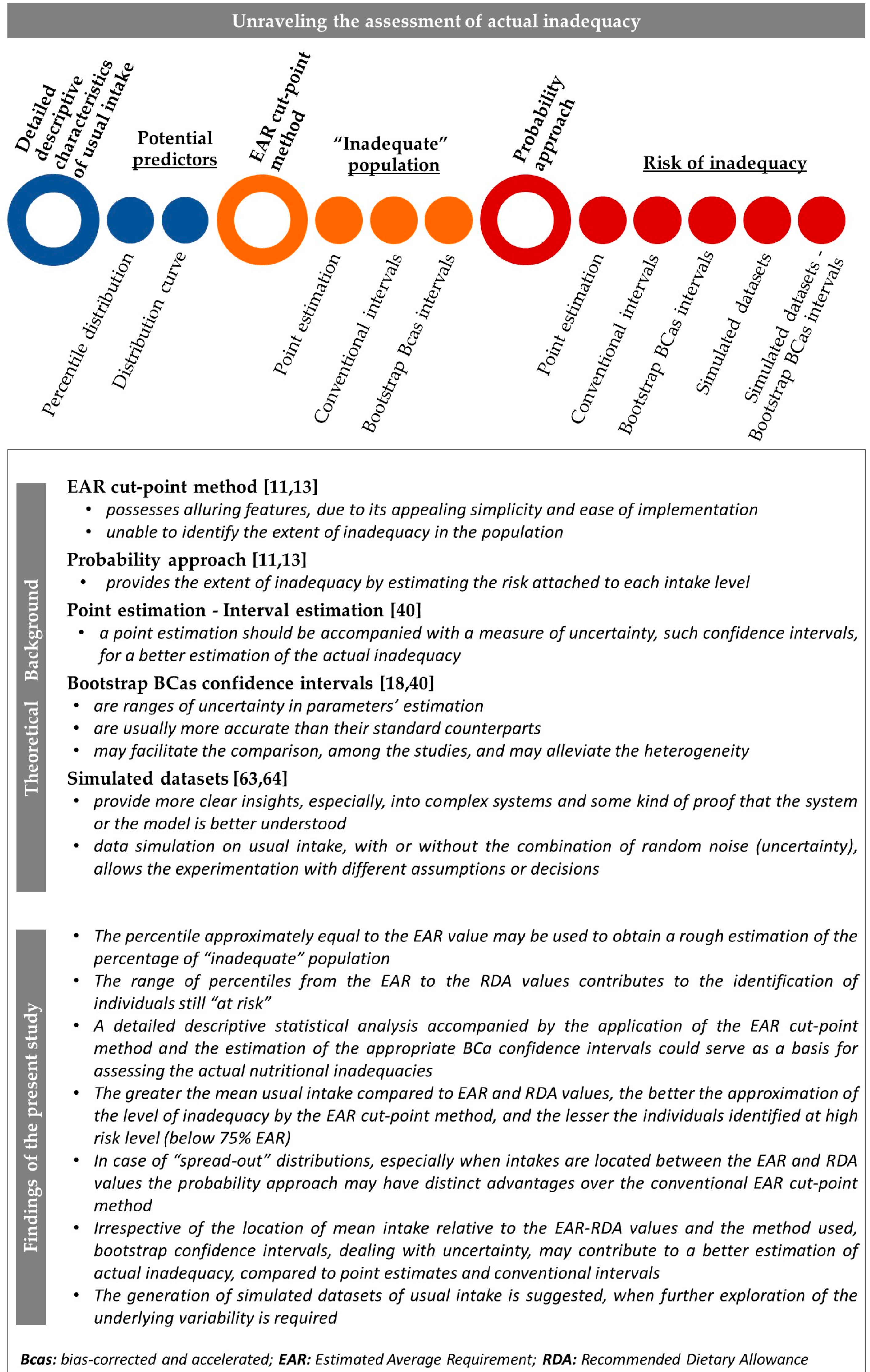
2.5. Assessment of Nutritional Inadequacy
2.5.1. Measures of Nutrient Inadequacy
2.5.2. Methodologies for the Assessment of Inadequate Intake
- i.
- Probability approach
- ii.
- EAR cut-point method
2.6. Statistical Analysis
2.6.1. Descriptive Statistics
2.6.2. Generation of Simulated Data
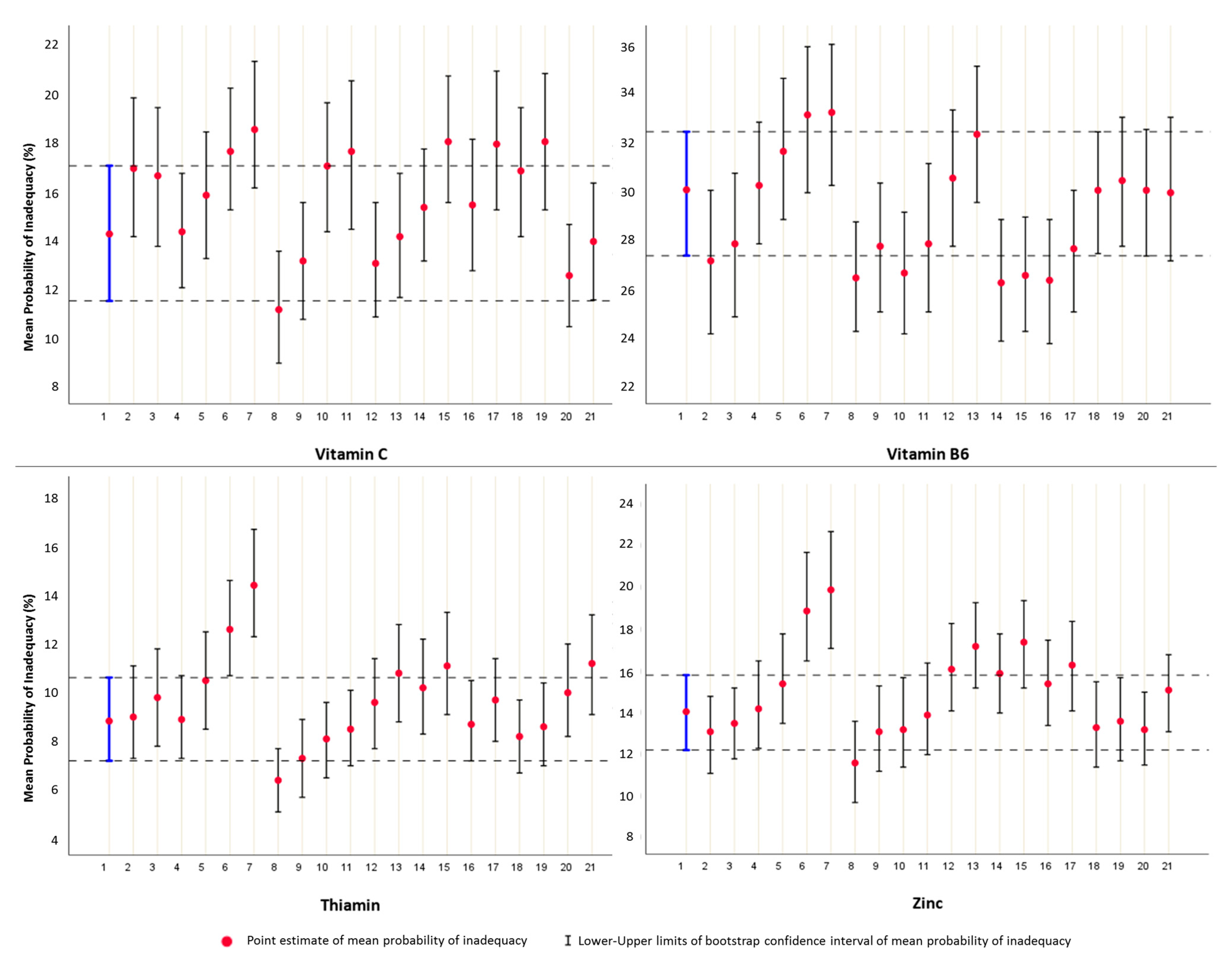
2.6.3. Interval Estimation of Inadequacy
2.6.4. Statistical Tests/Functions and Software Version Used
3. Results and Discussion
3.1. Descriptive Features of the Participants
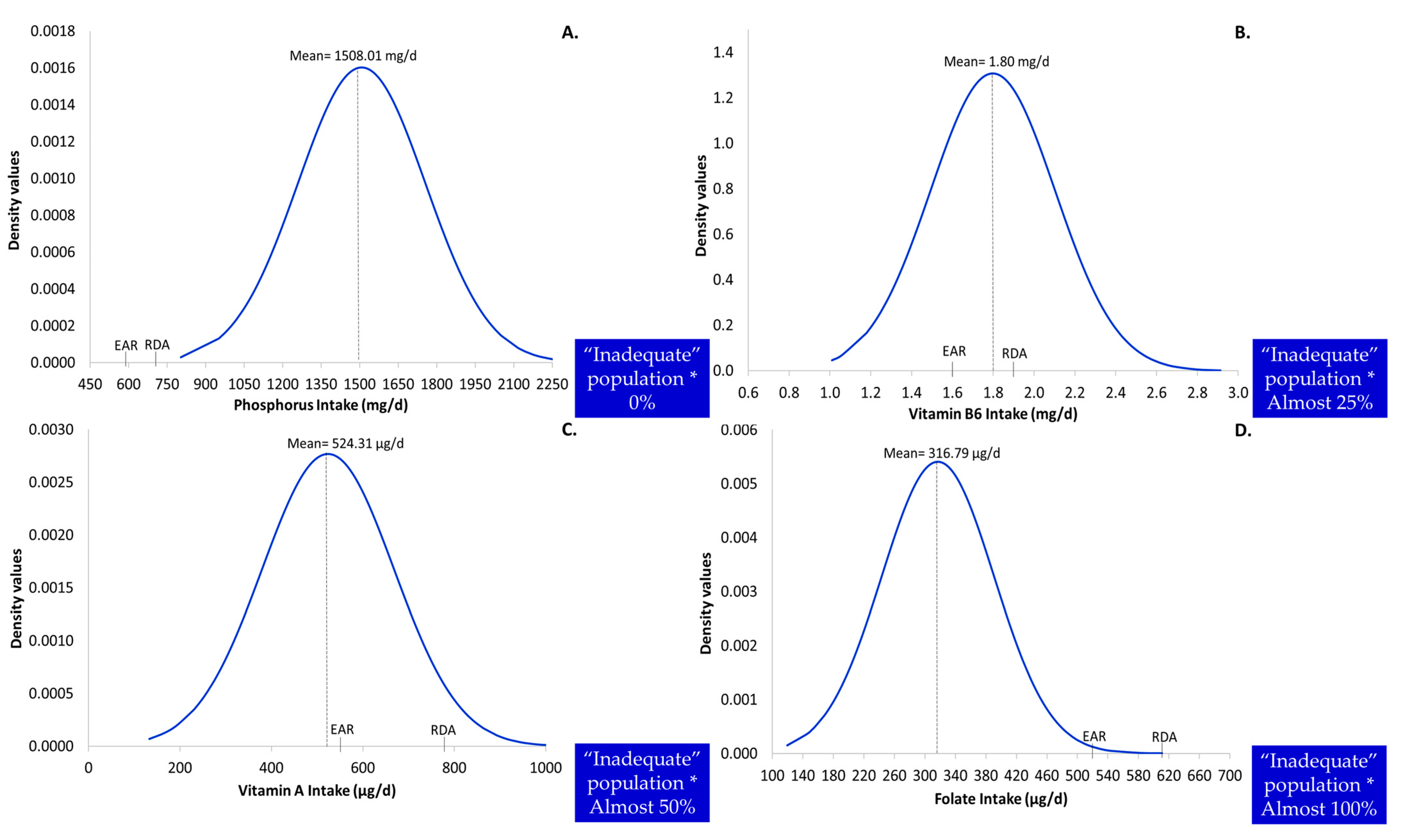
3.2. Detailed Descriptive Characteristics of Usual Intake as Potential Predictors of the Level of Inadequacy
3.3. Documentation for the Generation of Simulated Datasets of Usual Intake
3.4. Profile of Nutritional Inadequacy
3.5. Comparative Analysis Based on the Construction Framework of the Two Approaches
3.6. Commentary on Our Conceptual Design and Findings: A Contextual Point of View
3.7. Strengths and Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
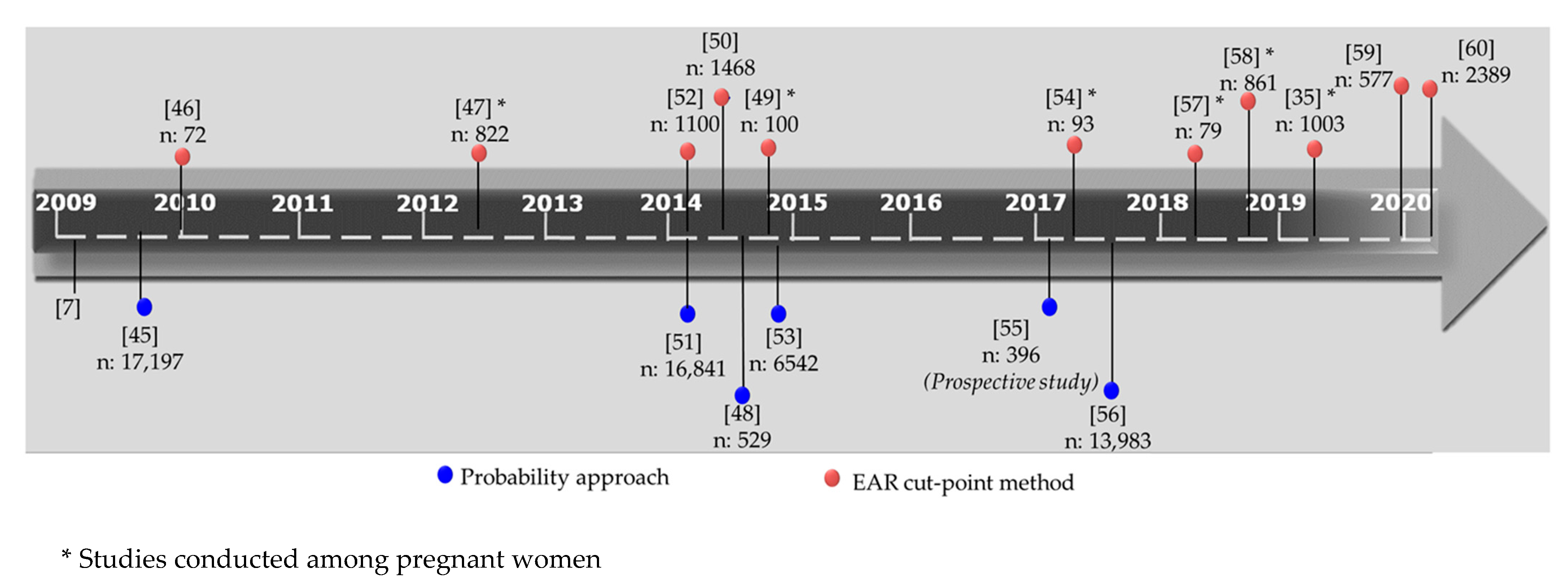
- Blumfield, M.L.; Hure, A.J.; Macdonald-Wicks, L.; Smith, R.; Collins, C.E. Systematic review and meta-analysis of energy and macronutrient intakes during pregnancy in developed countries. Nutr. Rev. 2012, 70, 322–336. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Brands, B.; Chourdakis, M.; Cramer, S.; Grote, V.; Hellmuth, C.; Kirchberg, F.; Prell, C.; Rzehak, P.; Uhl, O.; et al. The Power of Programming and the EarlyNutrition project: Opportunities for health promotion by nutrition during the first thousand days of life and beyond. Ann. Nutr. Metab. 2014, 64, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Parisi, F.; Laoreti, A.; Cetin, I. Multiple micronutrient needs in pregnancy in industrialized countries. Ann. Nutr. Metab. 2014, 65, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Dodo, K.A. Food Policy: Understanding Food Insecurity Risk Factors and the Food Environment on an HBCU Campus. Doctoral Dissertation, Morgan State University, Baltimore, MD, USA, May 2018. [Google Scholar]
- Hanson, M.A.; Bardsley, A.; De-Regil, L.M.; Moore, S.E.; Oken, E.; Poston, L.; Ma, R.C.; McAuliffe, F.M.; Maleta, K.; Chittaranjan, N.; et al. The International Federation of Gynecology and Obstetrics (FIGO) recommendations on adolescent, preconception, and maternal nutrition:“Think Nutrition First”. Int. J. Gynaecol. Obstet. 2015, 131, S213–S253. [Google Scholar] [CrossRef] [Green Version]
- Dhonukshe-Rutten, R.A.; Bouwman, J.; Brown, K.A.; Cavelaars, A.E.; Collings, R.; Grammatikaki, E.; de Groot, L.C.; Gurinovic, M.; Harvey, L.J.; Hermoso, M.; et al. EURRECA—Evidence-based methodology for deriving micronutrient recommendations. Crit. Rev. Food Sci. Nutr. 2013, 53, 999–1040. [Google Scholar] [CrossRef] [PubMed]
- Tabacchi, G.; Wijnhoven, T.M.; Branca, F.; Román-Vinas, B.; Ribas-Barba, L.; Ngo, J.; Garcia-Alvarez, A.; Serra-Majem, L. How is the adequacy of micronutrient intake assessed across Europe? A systematic literature review. Br. J. Nutr. 2009, 101 (Suppl. 2), S29–S36. [Google Scholar] [CrossRef]
- Brown, J.E.; Buzzard, I.M.; Jacobs, D.R., Jr.; Hannan, P.J.; Kushi, L.H.; Barosso, G.M.; Schmid, L.A. A food frequency questionnaire can detect pregnancy-related changes in diet. J. Acad. Nutr. Diet. 1996, 96, 262–266. [Google Scholar] [CrossRef]
- Doets, E.L.; de Wit, L.S.; Dhonukshe-Rutten, R.A.; Cavelaars, A.E.; Raats, M.M.; Timotijevic, L.; Brzozowska, A.; Wijnhoven, T.M.A.; Pavlovic, M.; Totland, T.H.; et al. Current micronutrient recommendations in Europe: Towards understanding their differences and similarities. Eur. J. Nutr. 2008, 47, 17–40. [Google Scholar] [CrossRef]
- Blumfield, M.L.; Hure, A.J.; Macdonald-Wicks, L.; Smith, R.; Collins, C.E. Micronutrient intakes during pregnancy in developed countries: Systematic review and meta-analysis. Nutr. Rev. 2013, 71, 118–132. [Google Scholar] [CrossRef]
- IOM 2000 Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary reference intakes: Applications in dietary assessment.; National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- Herrick, K.A.; Rossen, L.; Parsons, R.; Dodd, K. Estimating Usual Dietary Intake from National Health and Nutrition Examination Survey Data Using the National Cancer Institute Method; U.S. Department of Health and Human Services: Hyattsville, MD, USA, 2018.
- Otten, J.J.; Pitzi Hellwig, J.; Meyers, L.D. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; The National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Murphy, S.P.; Poos, M.I. Dietary reference intakes: Summary of applications in dietary assessment. Public Health Nutr. 2002, 5, 843–849. [Google Scholar] [CrossRef] [Green Version]
- Murphy, S.P.; Vorster, H.H. Methods for using nutrient intake values (NIVs) to assess or plan nutrient intakes. Food Nutr. Bull. 2007, 28 (Suppl. 1), S51–S60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Román-Vinas, B.; Serra-Majem, L.; Ribas-Barba, L.; Ngo, J.; García-Álvarez, A.; Wijnhoven, T.M.; Tabacchi, G.; Branca, F.; de Vries, L.; De Groot, L.C. Overview of methods used to evaluate the adequacy of nutrient intakes for individuals and populations. Br. J. Nutr. 2009, 101 (Suppl. 2), S6–S11. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). EFSA Panel on Plant Protection Products and their Residues (PPR). Guidance on the use of probabilistic methodology for modelling dietary exposure to pesticide residues. EFSA J. 2012, 10, 2839. [Google Scholar] [CrossRef]
- Efron, B. Better bootstrap confidence intervals. JASA 1987, 82, 171–185. [Google Scholar] [CrossRef]
- Mooney, C.F.; Mooney, C.Z.; Mooney, C.L.; Duval, R.D.; Duvall, R. Bootstrapping: A Nonparametric Approach to Statistical Inference (No. 95); Sage University Paper series on Quantitative Applications in the Social Sciences; SAGE Publications: Newbury Park, UK, 1993. [Google Scholar]
- Hansen, C.M.; Evans, M.A.; Shultz, T.D. Application of the bootstrap procedure provides an alternative to standard statistical procedures in the estimation of the vitamin B-6 requirement. J. Nutr. 1999, 129, 1915–1919. [Google Scholar] [CrossRef] [Green Version]
- Fotiou, M.; Fotakis, C.; Tsakoumaki, F.; Athanasiadou, E.; Kyrkou, C.; Dimitropoulou, A.; Tsiaka, T.; Chatziioannou, A.C.; Sarafidis, K.; Menexes, G.; et al. H NMR-based metabolomics reveals the effect of maternal habitual dietary patterns on human amniotic fluid profile. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Barker, D.J. Developmental origins of chronic disease. Public Health 2012, 126, 185–189. [Google Scholar] [CrossRef]
- Koletzko, B.; Brands, B.; Poston, L.; Godfrey, K.; Demmelmair, H. Early Nutrition Project. Early nutrition programming of long-term health. Proc. Nutr. Soc. 2012, 71, 371–378. [Google Scholar] [CrossRef] [Green Version]
- Cetin, I.; Bühling, K.; Demir, C.; Kortam, A.; Prescott, S.L.; Yamashiro, Y.; Yarmolinskaya, M.; Koletzko, B. Impact of micronutrient status during pregnancy on early nutrition programming. Ann. Nutr. Metab. 2019, 74, 269–278. [Google Scholar] [CrossRef]
- Wu, G.; Imhoff-Kunsch, B.; Girard, A.W. Biological mechanisms for nutritional regulation of maternal health and fetal development. Paediatr. Perinat. Epidemiol. 2012, 26, 4–26. [Google Scholar] [CrossRef]
- Grieger, J.A.; Clifton, V.L. A review of the impact of dietary intakes in human pregnancy on infant birthweight. Nutrients 2015, 7, 153–178. [Google Scholar] [CrossRef] [Green Version]
- Bjørke-Monsen, A.L.; Ulvik, A.; Nilsen, R.M.; Midttun, Ø.; Roth, C.; Magnus, P.; Stoltenberg, C.; Vollset, E.S.; Reichborn-Kjennerud, T.; Ueland, P.M. Impact of pre-pregnancy BMI on B vitamin and inflammatory status in early pregnancy: An observational cohort study. Nutrients 2016, 8, 776. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C. Nutrition Epidemiology, 2nd ed.; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- European Food Safety Authority (EFSA). Panel on dietetic products, nutrition and allergies (NDA), scientific opinion on dietary reference values for energy. EFSA J. 2013, 11, 3005. [Google Scholar] [CrossRef] [Green Version]
- Athanasiadou, E.; Kyrkou, C.; Fotiou, M.; Tsakoumaki, F.; Dimitropoulou, A.; Polychroniadou, E.; Menexes, G.; Athanasiadis, A.P.; Biliaderis, C.G.; Michaelidou, A.M. Development and validation of a Mediterranean oriented culture-specific semi-quantitative food frequency questionnaire. Nutrients 2016, 8, 522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organisation (WHO). 2019 Europe, WHO Body Mass Index-BMI. Available online: http://www.euro.who.int/en/health-topics/diseaseprevention/nutrition/a-healthylifestyle/body-mass-index-bmi (accessed on 16 April 2019).
- International Physical Activity Questionnaire (IPAQ). The IPAQ Home Page. Available online: https://sites.google.com/site/theipaq/home (accessed on 15 May 2010).
- Beaton, G.H. Uses and limits of the use of the Recommended Dietary Allowances for evaluating dietary intake data. Am. J. Clin. Nutr. 1985, 41, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Parrott, M.S.; Bodnar, L.M.; Simhan, H.N.; Harger, G.; Markovic, N.; Roberts, J.M. Maternal cereal consumption and adequacy of micronutrient intake in the periconceptional period. Public Health Nutr. 2009, 12, 1276–1283. [Google Scholar] [CrossRef] [Green Version]
- Bailey, R.L.; Pac, S.G.; Fulgoni, V.L.; Reidy, K.C.; Catalano, P.M. Estimation of total usual dietary intakes of pregnant women in the United States. JAMA Netw. Open 2019, 2, e195967. [Google Scholar] [CrossRef] [Green Version]
- Carriquiry, A.L. Assessing the prevalence of nutrient inadequacy. Public Health Nutr. 1998, 2, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Ribas-Barba, L.; Serra-Majem, L.; Román-Vinas, B.; Ngo, J.; Garcia-Alvarez, A. Effects of dietary assessment methods on assessing risk of nutrient intake adequacy at the population level: From theory to practice. Br. J. Nutr. 2009, 101 (Suppl. 2), S64–S72. [Google Scholar] [CrossRef] [Green Version]
- Anderson, G.H.; Peterson, R.D.; Beaton, G.H. Estimating nutrient deficiencies in a population from dietary records: The use of probability analyses. Nutr. Res. 1982, 2, 409–415. [Google Scholar] [CrossRef]
- Dodd, K.W.; Guenther, P.M.; Freedman, L.S.; Subar, A.F.; Kipnis, V.; Midthune, D.; Tooze, J.A.; Krebs-Smith, S.M. Statistical methods for estimating usual intake of nutrients and foods: A review of the theory. J. Am. Diet. Assoc. 2006, 106, 1640–1650. [Google Scholar] [CrossRef] [PubMed]
- Efron, B. Second Thoughts on the Bootstrap. Stat. Sci. 2003, 18, 135–140. [Google Scholar] [CrossRef]
- Gernand, A.D.; Schulze, K.J.; Stewart, C.P.; West, K.P., Jr.; Christian, P. Micronutrient deficiencies in pregnancy worldwide: Health effects and prevention. Nat. Rev. Endocrinol. 2016, 12, 274–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mensink, G.B.M.; Fletcher, R.; Gurinovic, M.; Huybrechts, I.; Lafay, L.; Serra-Majem, L.; Szponar, L.; Tetens, I.; Verkaik-Kloosterman, J.; Baka, A.; et al. Mapping low intake of micronutrients across Europe. Br. J. Nutr. 2013, 110, 755–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashwell, M.; Lambert, J.P.; Alles, M.S.; Branca, F.; Bucchini, L.; Brzozowska, A.; de Groot, L.C.P.G.M.; Dhonukshe-Rutten, R.A.M.; Dwyer, J.T.; Fairweather-Tait, S.; et al. How we will produce the evidence-based EURRECA toolkit to support nutrition and food policy. Eur. J. Nutr. 2008, 47, 2–16. [Google Scholar] [CrossRef]
- Murphy, S.P. Using DRIs for dietary assessment. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. 1), 299–301. [Google Scholar] [CrossRef] [Green Version]
- Serra-Majem, L.; Bes-Rastrollo, M.; Román-Vinas, B.; Pfrimer, K.; Sánchez-Villegas, A.; Martínez-González, M.A. Dietary patterns and nutritional adequacy in a Mediterranean country. Br. J. Nutr. 2009, 101 (Suppl. 2), S21–S28. [Google Scholar] [CrossRef] [Green Version]
- Heaney, S.; O’Connor, H.; Gifford, J.; Naughton, G. Comparison of strategies for assessing nutritional adequacy in elite female athletes’ dietary intake. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 245–256. [Google Scholar] [CrossRef]
- Rodriguez-Bernal, C.L.; Ramón, R.; Quiles, J.; Murcia, M.; Navarrete-Munoz, E.M.; Vioque, J.; Ballester, F.; Rebagliato, M. Dietary intake in pregnant women in a Spanish Mediterranean area: As good as it is supposed to be? Public Health Nutr. 2013, 16, 1379–1389. [Google Scholar] [CrossRef] [Green Version]
- Gewa, C.A.; Murphy, S.P.; Weiss, R.E.; Neumann, C.G. Determining minimum food intake amounts for diet diversity scores to maximize associations with nutrient adequacy: An analysis of schoolchildren’s diets in rural Kenya. Public Health Nutr. 2014, 17, 2667–2673. [Google Scholar] [CrossRef] [Green Version]
- Hatzopoulou, K.; Filis, V.; Grammatikopoulou, M.G.; Kotzamanidis, C.; Tsigga, M. Greek pregnant women demonstrate inadequate micronutrient intake despite supplement use. J. Diet. Suppl. 2014, 11, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Manios, Y.; Moschonis, G.; Mavrogianni, C.; Bos, R.; Singh-Povel, C. Micronutrient intakes among children and adults in Greece: The role of age, sex and socio-economic status. Nutrients 2014, 6, 4073–4092. [Google Scholar] [CrossRef] [PubMed]
- Zazpe, I.; Sanchez-Tainta, A.; Santiago, S.; de la Fuente-Arrillaga, C.; Bes-Rastrollo, M.; Martínez, J.A.; Martínez-González, M.Á. Association between dietary carbohydrate intake quality and micronutrient intake adequacy in a Mediterranean cohort: The SUN (Seguimiento Universidad de Navarra) Project. Br. J. Nutr. 2014, 111, 2000–2009. [Google Scholar] [CrossRef] [Green Version]
- Manios, Y.; Moschonis, G.; Grammatikaki, E.; Mavrogianni, C.; van den Heuvel, E.G.H.M.; Bos, R.; Singh-Povel, C. Food group and micronutrient intake adequacy among children, adults and elderly women in greece. Nutrients 2015, 7, 1841–1858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Tainta, A.; Zazpe, I.; Bes-Rastrollo, M.; Salas-Salvado, J.; Bullo, M.; Sorli, J.V.; Corella, D.; Covas, M.I.; Arós, F.; Gutierrez-Bedmar, M.; et al. Nutritional adequacy according to carbohydrates and fat quality. Eur. J. Nutr. 2016, 55, 93–106. [Google Scholar] [CrossRef]
- Groth, S.W.; Stewart, P.A.; Ossip, D.J.; Block, R.C.; Wixom, N.; Fernandez, I.D. Micronutrient intake is inadequate for a sample of pregnant African-American women. J. Acad. Nutr. Diet. 2017, 117, 589–598. [Google Scholar] [CrossRef] [Green Version]
- Zaragoza-Jordana, M.; Closa-Monasterolo, R.; Luque, V.; Ferré, N.; Grote, V.; Koletzko, B.; Pawellek, I.; Verduci, E.; ReDionigi, A.; Socha, J.; et al. Micronutrient intake adequacy in children from birth to 8 years. Data from the childhood obesity project. Clin. Nutr. 2018, 37, 630–637. [Google Scholar] [CrossRef]
- Sánchez-Villegas, A.; Pérez-Cornago, A.; Zazpe, I.; Santiago, S.; Lahortiga, F.; Martínez-González, M.A. Micronutrient intake adequacy and depression risk in the SUN cohort study. Eur. J. Nutr. 2018, 57, 2409–2419. [Google Scholar] [CrossRef]
- Savard, C.; Lemieux, S.; Weisnagel, S.J.; Fontaine-Bisson, B.; Gagnon, C.; Robitaille, J.; Morisset, A.S. Trimester-specific dietary intakes in a sample of French-Canadian pregnant women in comparison with national nutritional guidelines. Nutrients 2019, 10, 768. [Google Scholar] [CrossRef] [Green Version]
- Dubois, L.; Diasparra, M.; Bédard, B.; Colapinto, C.K.; Fontaine-Bisson, B.; Tremblay, R.E.; Fraser, W.D. Adequacy of nutritional intake during pregnancy in relation to prepregnancy BMI: Results from the 3D Cohort Study. Br. J. Nutr. 2018, 120, 335–344. [Google Scholar] [CrossRef] [Green Version]
- Mitsopoulou, A.V.; Magriplis, E.; Dimakopoulos, I.; Karageorgou, D.; Bakogianni, I.; Micha, R.; Michas, G.; Chourdakis, M.; Ntouroupi, T.; Tsaniklidou, S.M.; et al. Micronutrient intakes and their food sources among Greek children and adolescents. Public Health Nutr. 2020, 23, 2314–2326. [Google Scholar] [CrossRef] [PubMed]
- Mitsopoulou, A.V.; Magriplis, E.; Michas, G.; Micha, R.; Chourdakis, M.; Chrousos, G.P.; Roma, E.; Panagiotakos, D.P.; Zampelas, A.; Karageorgou, D.; et al. Micronutrient dietary intakes and their food sources in adults: The Hellenic National Nutrition and Health Survey (HNNHS). J. Hum. Nutr. Diet. 2021, 34, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Roman-Viñas, B.; Ortiz-Andrellucchi, A.; Mendez, M.; Sánchez-Villegas, A.; Quintana, L.P.; Aznar, L.A.M.; Hermoso, M.; Serra-Majem, L. Is the food frequency questionnaire suitable to assess micronutrient intake adequacy for infants, children and adolescents? Matern. Child. Nutr. 2010, 6, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Cade, J.; Thompson, R.; Burley, V.; Warm, D. Development validation and utilization of food-frequency questionnaires—A review. Public Health Nutr. 2002, 5, 567–587. [Google Scholar] [CrossRef] [Green Version]
- Kéry, M.; Royle, J.A. Applied Hierarchical Modeling in Ecology: Analysis of Distribution, Abundance and Species Richness in R and BUGS, 1st ed.; Academic Press: Amsterdam, The Netherlands, 2016; pp. 123–143. [Google Scholar]
- Mooney, C.Z. Monte Carlo Simulation; SAGE Publications: Newbury Park, UK, 1997; Volume 116. [Google Scholar]
- Murphy, S.P.; Guenther, P.M.; Kretsch, M.J. Using the dietary reference intakes to assess intakes of groups: Pitfalls to avoid. J. Acad. Nutr. Diet. 2006, 106, 1550–1553. [Google Scholar] [CrossRef]
- Hellenic Statistical Authority (ELSTAT) 2011 Population-Housing Census. Available online: www.statistics.gr/en/2011-census-pop-hous (accessed on 21 August 2021).
- Hellenic Statistical Authority (ELSTAT) 2015 Vital Statistics. Available online: www.statistics.gr/en/statistics/-/publication/SPO03/2015 (accessed on 21 August 2021).
- Aparicio, E.; Jardí, C.; Bedmar, C.; Pallejà, M.; Basora, J.; Arija, V.; ECLIPSES Study Group. Nutrient Intake during Pregnancy and Post-Partum: ECLIPSES Study. Nutrients 2020, 12, 1325. [Google Scholar] [CrossRef]
| Probability Approach | EAR Cut-Point Method | Comments | ||
|---|---|---|---|---|
| 1 | Protein | + | + | |
| 2 | Carbohydrate | + | + | |
| 3 | Fiber | + | AI | |
| 4 | Thiamin | + | + | |
| 5 | Riboflavin | + | + | |
| 6 | Niacin | + | + | |
| 7 | Vitamin B6 | + | + | |
| 8 | Folate | + | + | |
| 9 | Vitamin B12 | + | + | |
| 10 | Vitamin C | + | + | |
| 11 | Vitamin A | + | + | |
| 12 | Vitamin E | + | Skewed distribution | |
| 13 | Calcium | + | AI | |
| 14 | Phosphorus | + | + | |
| 15 | Magnesium | + | + | |
| 16 | Potassium | + | AI | |
| 17 | Sodium | + | AI | |
| 18 | Zinc | + | + | |
| 19 | Copper | + | + | |
| 20 | Selenium | + | + | |
| 21 | Iron | + | Not established SD |
| Demographic/Anthropometric Characteristics | Mean (SD) |
|---|---|
| Maternal age (year) | 36.50 (3.77) |
| n (%) | |
| Pre-pregnancy BMI | |
| Underweight (BMI < 18.5 kg/m2) | 26 (4.3) |
| Normal (BMI 18.5–24.9 kg/m2) | 399 (65.6) |
| Overweight (BMI 25–29.9 kg/m2) | 123 (20.2) |
| Obese (BMI > 30.0 kg/m2) | 60 (9.9) |
| Education | |
| Tertiary education (universities) | 130 (21.4) |
| Tertiary technical education | 98 (16.1) |
| Post secondary non-tertiary education | 76 (12.5) |
| High school | 279 (45.9) |
| Lower secondary education school | 25 (4.1) |
| Physical activity level * | |
| Low activity | 473 (77.8) |
| Moderate activity | 101 (16.6) |
| High activity | 34 (5.6) |
| Smoking during pregnancy | |
| Occasional or daily smokers | 91 (15.0) |
| Non-smokers | 517 (85.0) |
| EAR/AI | P1 | P5 | P10 | P15 | P20 | P25 | P30 | P35 | P40 | P45 | P50 | P55 | P60 | P65 | P70 | P75 | P80 | P85 | P90 | P95 | P99 | “Inadequate” population * | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | 6 | 30 | 61 | 91 | 122 | 152 | 182 | 213 | 243 | 274 | 304 | 334 | 365 | 395 | 426 | 456 | 486 | 517 | 547 | 578 | 602 | ||||
| RDA | |||||||||||||||||||||||||
| Phosphorus (mg/day) | 580 | 700 | 982 | 1121 | 1194 | 1248 | 1292 | 1333 | 1369 | 1402 | 1426 | 1467 | 1496 | 1526 | 1553 | 1583 | 1630 | 1669 | 1719 | 1777 | 1845 | 1947 | 2145 | <1% | |
| Carbohydrate (g/day) | 135 | 175 | 145 | 170 | 182 | 188 | 195 | 199 | 204 | 209 | 214 | 219 | 225 | 229 | 233 | 238 | 244 | 249 | 256 | 268 | 280 | 300 | 321 | <1% | |
| Vitamin B12 (μg/day) | 2.2 | 2.6 | 2.2 | 2.9 | 3.3 | 3.7 | 3.8 | 4.0 | 4.2 | 4.4 | 4.6 | 4.7 | 4.8 | 5.0 | 5.2 | 5.3 | 5.4 | 5.6 | 5.8 | 6.2 | 6.5 | 6.8 | 8.1 | <1% | |
| Copper (μg/day) | 800 | 1000 | 794 | 934 | 1007 | 1052 | 1097 | 1137 | 1172 | 1217 | 1254 | 1289 | 1327 | 1375 | 1419 | 1475 | 1528 | 1584 | 1642 | 1706 | 1781 | 1931 | 2196 | 1–5% | |
| Selenium (μg/day) | 49 | 60 | 44 | 51 | 56 | 59 | 61 | 63 | 66 | 67 | 69 | 70 | 72 | 73 | 75 | 77 | 79 | 81 | 83 | 87 | 90 | 98 | 114 | 1–5% | |
| Protein (g/kg/day) | 0.88 | 1.1 | 0.75 | 0.93 | 1.02 | 1.07 | 1.14 | 1.18 | 1.24 | 1.27 | 1.31 | 1.36 | 1.39 | 1.42 | 1.46 | 1.52 | 1.55 | 1.60 | 1.66 | 1.70 | 1.76 | 1.89 | 2.11 | 1–5% | |
| Riboflavin (mg/day) | 1.2 | 1.4 | 1.0 | 1.2 | 1.4 | 1.5 | 1.6 | 1.7 | 1.7 | 1.8 | 1.9 | 1.9 | 2.0 | 2.1 | 2.2 | 2.2 | 2.3 | 2.4 | 2.5 | 2.6 | 2.7 | 2.9 | 3.2 | 1–5% | |
| Thiamin (mg/day) | 1.2 | 1.4 | 1.0 | 1.2 | 1.2 | 1.3 | 1.4 | 1.4 | 1.5 | 1.5 | 1.6 | 1.6 | 1.6 | 1.7 | 1.7 | 1.8 | 1.8 | 1.9 | 2.0 | 2.0 | 2.1 | 2.2 | 2.5 | 1–5% | |
| Niacin (mg/day) | 14 | 18 | 12 | 13 | 14 | 15 | 15 | 16 | 16 | 16 | 17 | 17 | 18 | 18 | 18 | 19 | 19 | 20 | 20 | 21 | 22 | 24 | 27 | 5–10% | |
| Zinc (mg/day) | 9.5 | 11 | 8.0 | 9.0 | 9.5 | 9.9 | 10.1 | 10.4 | 10.7 | 10.9 | 11.1 | 11.2 | 11.4 | 11.7 | 11.9 | 12.2 | 12.4 | 12.7 | 13.0 | 13.5 | 13.9 | 14.7 | 15.6 | 5–10% | |
| Vitamin C (mg/day) | 70 | 85 | 32 | 51 | 60 | 72 | 81 | 88 | 94 | 102 | 109 | 116 | 126 | 137 | 144 | 152 | 164 | 174 | 182 | 194 | 218 | 246 | 306 | 10–15% | |
| Vitamin B6 (mg/day) | 1.6 | 1.9 | 1.2 | 1.4 | 1.4 | 1.5 | 1.5 | 1.6 | 1.6 | 1.6 | 1.7 | 1.7 | 1.8 | 1.8 | 1.9 | 1.9 | 1.9 | 2.0 | 2.0 | 2.1 | 2.2 | 2.4 | 2.6 | 20–25% | |
| Magnesium (mg/day) | 300 | 360 | 197 | 227 | 243 | 252 | 260 | 266 | 271 | 278 | 287 | 293 | 299 | 307 | 315 | 323 | 331 | 344 | 354 | 365 | 381 | 405 | 456 | 50–55% | |
| Vitamin A (μg/day) | 550 | 770 | 240 | 307 | 343 | 381 | 403 | 423 | 444 | 465 | 481 | 492 | 507 | 531 | 552 | 571 | 591 | 615 | 637 | 667 | 720 | 776 | 922 | 55–60% | |
| Folate (μg/day) | 520 | 600 | 169 | 207 | 229 | 241 | 255 | 265 | 277 | 285 | 292 | 303 | 311 | 322 | 333 | 341 | 350 | 358 | 370 | 388 | 413 | 448 | 519 | >99% | |
| Iron (mg/day) | 22 | 7 | 8 | 9 | 9 | 10 | 10 | 10 | 10 | 10 | 11 | 11 | 11 | 12 | 12 | 12 | 13 | 13 | 14 | 14 | 16 | 17 | >99% | ||
| Sodium (g/day) | 1.5 ** | 1.4 | 1.6 | 1.7 | 1.8 | 1.9 | 2.0 | 2.0 | 2.1 | 2.1 | 2.2 | 2.2 | 2.3 | 2.3 | 2.4 | 2.4 | 2.5 | 2.6 | 2.6 | 2.8 | 3.0 | 3.4 | 1–5% | ||
| Potassium (g/day) | 2.9 ** | 2.1 | 2.4 | 2.5 | 2.6 | 2.7 | 2.8 | 2.9 | 2.9 | 3.0 | 3.0 | 3.1 | 3.2 | 3.2 | 3.3 | 3.4 | 3.5 | 3.6 | 3.7 | 3.8 | 4.1 | 4.7 | 25–30% | ||
| Calcium (mg/day) | 1000 ** | 448 | 597 | 693 | 753 | 803 | 839 | 874 | 913 | 935 | 970 | 998 | 1026 | 1060 | 1101 | 1141 | 1175 | 1225 | 1270 | 1351 | 1484 | 1662 | 50–55% | ||
| Fiber (g/day) | 28 ** | 12 | 15 | 17 | 18 | 18 | 19 | 20 | 21 | 21 | 22 | 22 | 23 | 24 | 25 | 26 | 26 | 28 | 29 | 30 | 33 | 39 | 75–80% |
| (99%) Bootstrap CI | ||||||
|---|---|---|---|---|---|---|
| Mean Value | SD Value | |||||
| Mean | SD | LL | UL | LL | UL | |
| Protein (g/kg/day) | 1.39 | 0.30 | 1.37 | 1.42 | 0.28 | 0.30 |
| Carbohydrate (g/day) | 226.76 | 38.13 | 223.57 | 230.72 | 36.14 | 39.87 |
| Thiamin (mg/day) | 1.67 | 0.33 | 1.64 | 1.69 | 0.32 | 0.35 |
| Riboflavin (mg/day) | 2.02 | 0.50 | 1.98 | 2.04 | 0.47 | 0.53 |
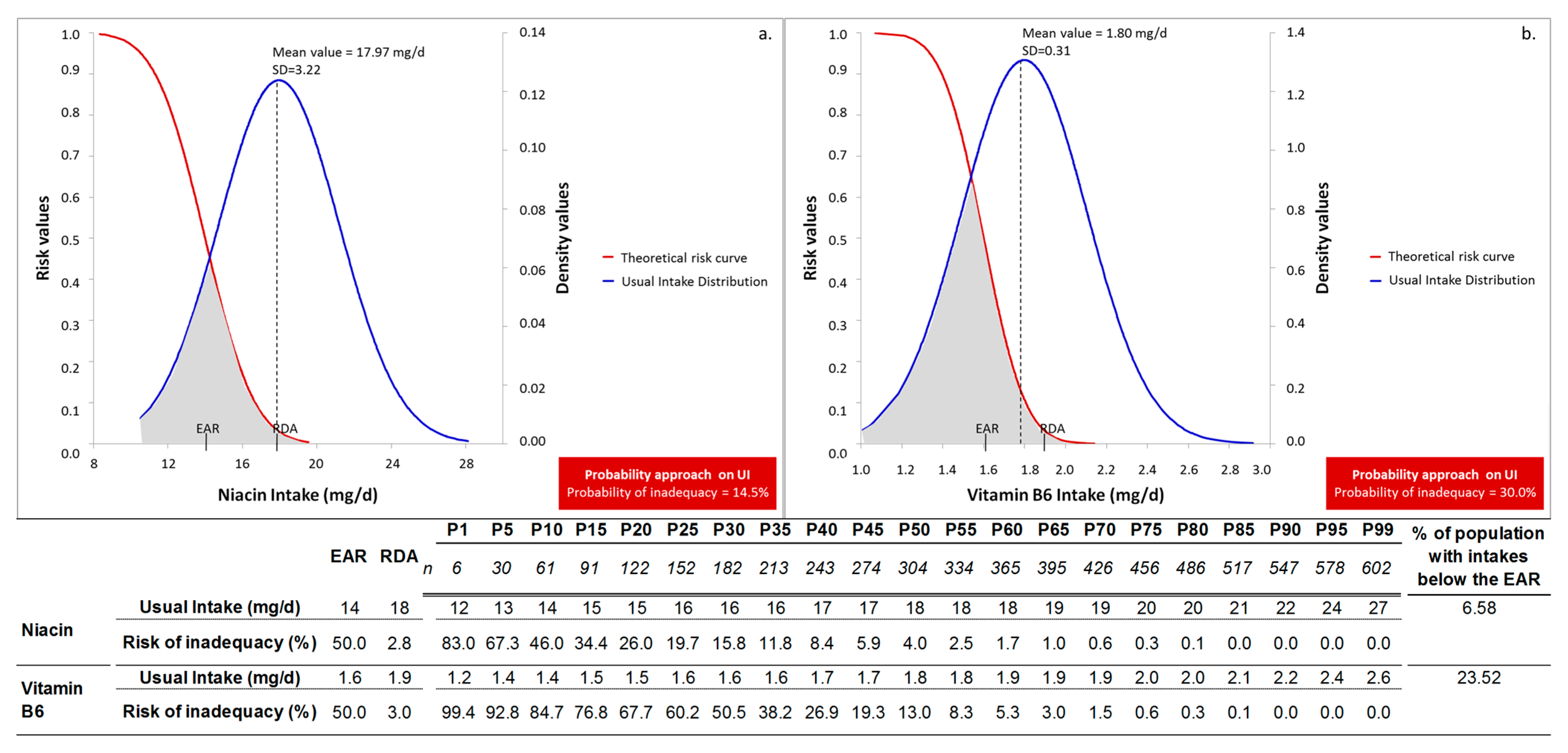
| Niacin (mg/day) | 17.97 | 3.22 | 17.67 | 18.26 | 3.08 | 3.41 |
| Vitamin B6 (mg/day) | 1.80 | 0.31 | 1.77 | 1.82 | 0.28 | 0.33 |
| Folate (μg/day) | 316.79 | 73.72 | 309.55 | 322.93 | 69.01 | 79.64 |
| Vitamin B12 (μg/day) | 4.88 | 1.24 | 4.80 | 4.98 | 1.14 | 1.33 |
| Vitamin C (mg/day) | 134.05 | 61.11 | 129.94 | 138.64 | 58.15 | 68.64 |
| Vitamin A (μg/day) | 524.31 | 143.87 | 512.24 | 535.25 | 134.37 | 152.92 |
| Vitamin E (mg/day) * | 1.05 | 0.10 | 1.04 | 1.06 | 0.09 | 0.10 |
| Phosphorus (mg/day) | 1508.01 | 248.73 | 1493.07 | 1523.85 | 235.50 | 260.52 |
| Magnesium (mg/day) | 306.35 | 55.07 | 300.97 | 310.78 | 52.07 | 58.55 |
| Zinc (mg/day) | 11.60 | 1.71 | 11.45 | 11.75 | 1.60 | 1.80 |
| Copper (μg/day) | 1374.14 | 312.48 | 1349.93 | 1397.06 | 290.72 | 330.37 |
| Selenium (μg/day) | 72.80 | 13.91 | 71.65 | 74.15 | 13.15 | 14.64 |
| Mean Probability of Inadequacy (%) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| On UI (LL–UL of BCa CI) | On Simulated Data | ||||||||||||||||||||
| Combinations of Lowest and Highest CI Limits of Mean/SD Values of UI | Random Combinations within the CI Limits of Mean/SD Values of UI | ||||||||||||||||||||
| Mean/SD of UI | LL of Mean/LL SD of UI | LL of Mean/UL SD of UI | UL of Mean/LL SD of UI | UL of Mean/UL SD of UI | 1 * | 2 * | 3 * | 4 * | 5 * | ||||||||||||
| a | b | a | b | a | b | a | b | a | b | a | b | a | b | a | b | a | b | a | b | ||
| Phosphorus | 0.0 (0–0) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Carbohydrate | 1.1 (0.7–1.5) | 1.4 | 2.0 | 1.7 | 2.3 | 2.1 | 2.5 | 1.0 | 1.7 | 1.9 | 2.3 | 1.6 | 2.0 | 1.7 | 1.9 | 2.4 | 2.5 | 1.7 | 1.9 | 1.7 | 2.4 |
| Vitamin B12 | 1.2 (0.7–1.8) | 2.7 | 3.6 | 1.4 | 2.4 | 2.7 | 3.3 | 0.9 | 1.1 | 1.5 | 2.0 | 1.5 | 2.1 | 1.9 | 1.8 | 0.8 | 1.2 | 2.6 | 3.4 | 2.3 | 2.9 |
| Copper | 2.2 (1.6–2.8) | 5.6 | 6.3 | 3.2 | 3.6 | 5.4 | 6.8 | 2.9 | 3.6 | 4.4 | 4.6 | 2.8 | 3.4 | 3.8 | 4.5 | 4.0 | 4.6 | 4.4 | 5.6 | 6.7 | 7.7 |
| Selenium | 4.3 (3.2–5.4) | 5.1 | 6.3 | 4.8 | 5.6 | 8.6 | 9.7 | 3.4 | 4.1 | 5.3 | 6.0 | 5.7 | 6.7 | 4.1 | 4.9 | 4.4 | 4.9 | 4.9 | 5.9 | 5.6 | 6.9 |
| Protein | 4.5 (3.4–5.6) | 4.0 | 5.3 | 4.2 | 4.9 | 5.2 | 5.8 | 3.9 | 4.7 | 4.7 | 5.6 | 4.6 | 5.2 | 4.3 | 5.7 | 4.1 | 5.6 | 4.9 | 5.7 | 4.1 | 5.0 |
| Rivoflavin | 5.0 (3.7–6.5) | 5.1 | 6.9 | 5.1 | 5.3 | 9.3 | 9.4 | 3.9 | 5.7 | 6.1 | 7.5 | 4.3 | 5.7 | 7.2 | 8.4 | 5.5 | 6.1 | 5.0 | 5.9 | 4.5 | 6.0 |
| Thiamin | 8.7 (7.1–10.5) | 8.9 | 9.7 | 8.8 | 10.4 | 12.5 | 14.3 | 6.3 | 7.2 | 8.0 | 8.4 | 9.9 | 11.1 | 8.6 | 9.6 | 8.1 | 8.5 | 9.5 | 10.7 | 10.1 | 11.0 |
| Zinc | 14.0 (12.1–15.7) | 13.0 | 13.4 | 14.1 | 15.3 | 18.8 | 19.8 | 11.5 | 13.0 | 13.1 | 13.8 | 15.3 | 16.2 | 13.1 | 15.0 | 15.8 | 17.3 | 13.2 | 13.5 | 16.0 | 17.1 |
| Vitamin C | 14.2 (11.5–17) | 16.9 | 16.6 | 14.3 | 15.8 | 17.6 | 18.5 | 11.1 | 13.1 | 17.0 | 17.6 | 13.0 | 14.1 | 12.5 | 13.9 | 15.3 | 18.0 | 16.8 | 18.0 | 15.4 | 17.9 |
| Niacin | 14.5 (12.8–16) | 16.2 | 17.2 | 16.4 | 18.0 | 20.6 | 21.7 | 11.4 | 13.2 | 12.6 | 14.1 | 16.0 | 17.4 | 13.9 | 14.7 | 16.2 | 16.5 | 16.7 | 17.2 | 13.5 | 14.0 |
| Vitamin B6 | 30.0 (27.3–32.4) | 27.1 | 27.8 | 30.2 | 31.6 | 33.1 | 33.2 | 26.4 | 27.7 | 26.6 | 27.8 | 30.5 | 32.3 | 30.0 | 30.4 | 26.3 | 27.6 | 26.2 | 26.5 | 30.0 | 29.9 |
| Magnesium | 48.9 (45.8–51.8) | 43.7 | 44.8 | 47.9 | 47.2 | 46.4 | 47.2 | 42.2 | 42.1 | 43.4 | 43.6 | 44.3 | 44.6 | 51.1 | 51.2 | 42.8 | 43.7 | 45.0 | 45.9 | 50.6 | 50.2 |
| Vitamin A | 57.2 (54.8–59.7) | 56.4 | 56.3 | 57.9 | 57.2 | 59.0 | 58.7 | 54.5 | 54.5 | 53.7 | 53.9 | 55.6 | 54.6 | 53.8 | 58.7 | 52.7 | 53.4 | 54.4 | 54.1 | 55.0 | 54.7 |
| a | b | a | b | a | b | a | b | a | b | a | b | a | b | a | b | a | b | a | b | ||
| Vitamin E ¥ | 61.8 (59.4–64.4) | 59.8 | 59.0 | 63.8 | 63.4 | 62.0 | 61.7 | 59.7 | 60.2 | 54.9 | 54.4 | 55.3 | 55.2 | 63.1 | 62.4 | 59.5 | 58.7 | 61.4 | 61.2 | 58.4 | 58.0 |
| Folate | 98.0 (97.2–98.8) | 99.2 | 99.0 | 99.1 | 99.0 | 98.3 | 97.9 | 98.4 | 98.0 | 98.2 | 97.6 | 99.4 | 99.1 | 98.8 | 98.3 | 99.2 | 98.9 | 98.6 | 98.3 | 99.0 | 98.8 |
| EAR/AI | % of Population with Intakes below the EAR/AI (LL-UL of BCa CI) | |
|---|---|---|
| Phosphorus (mg/day) | 580 | 0.00 (JPY) |
| Carbohydrate (g/day) | 135 | 0.16 (0.0–0.7) |
| Vitamin B12 (μg/day) | 2.2 | 0.82 (0.3–1.7) |
| Copper (μg/day) | 800 | 0.99 (0.3–2.0) |
| Selenium (μg/day) | 49 | 2.96 (1.2–4.7) |
| Protein (g/kg/day) | 0.88 | 3.13 (1.6–5.5) |
| Riboflavin (mg/day) | 1.2 | 3.13 (1.7–5.6) |
| Thiamin (mg/day) | 1.2 | 4.28 (2.8–6.2) |
| Niacin (mg/day) | 14 | 6.58 (4.8–9.8) |
| Zinc (mg/day) | 9.5 | 9.54 (6.6–15.0) |
| Vitamin C (mg/day) | 70 | 13.70 (10.9–19.4) |
| Vitamin B6 (mg/day) | 1.6 | 23.52 (19.8–28.9) |
| Magnesium (mg/day) | 300 | 50.00 (45.3–62.9) |
| Vitamin A (μg/day) | 550 | 59.54 (55.4–69.6) |
| Folate (μg/day) | 520 | 99.18 (98.2–99.9) |
| Iron (mg/day) | 22 | 100.00 (JPY) |
| Sodium (g/day) | 1.5 * | 1.32 (0.5–2.4) |
| Potassium (g/day) | 2.9 * | 29.93 (23.3–36.7) |
| Calcium (mg/day) | 1000 * | 50.16 (47.1–61.2) |
| Fiber (g/day) | 28 * | 78.95 (76.6–81.9) |
| % of Population with Intakes below the EAR/AI | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Country | Record of Usual Intake | Extraction of Nutrient Values | Thiamin | Niacin | Riboflavin | Vitamin B6 | Vitamin B12 | Folate | Vitamin C | Vitamin A | Fe | Mg | Ca | P | Cu | Zn |
| [47] ◊ | Spain | FFQ | USDA † | 99.6 | 14.4 | 4.6 | 67.9 | 0.0 | |||||||||
| [49] ◊ | Greece | 3DRs | Food processor Software | 87.2 | 97.9 | 83.0 | 55.3 | ||||||||||
| [54] | USA | 3DRs | Nutrition Data System for Research software | 66 | 89 | 28 | |||||||||||
| [57] * ◊ | Canada | 3DRs | NFCT | 7.6 | 1.3 | 32.9 | 3.8 | 60.8 | 22.8 | 17.7 | 89.9 | 19.0 | 13.9 | 1.3 | 8.9 | ||
| [58] | Canada | FRs (3d) | Food processor Software † | 4.2 | 0.0 | 0.4 | 24.5 | 1.1 | 66.8 | 7.2 | 9.8 | 95.2 | 15.8 | 10.5 | 0.0 | 16.9 | |
| [35] ◊ | USA | 2DRs | USDA †¤ | 11.5 | 2.8 | 5.0 | 25.4 | 2.4 | 35.8 | 24.7 | 27.7 | 83.8 | 53.3 | 21.2 | 5.4 | 21.5 | |
| Present study (LL–UL of BCa CI) | Greece | FFQ | ** | 4.28 (2.8–6.2) | 6.58 (4.8–9.8) | 3.13 (1.7–5.6) | 23.52 (19.8–28.9) | 0.82 (0.3–2.0) | 99.18 (98.2–99.9) | 13.70 (10.9–19.4) | 59.54 (55.4–69.6) | 100.00 (¥) | 50.00 (45.3–62.9) | 50.16 (47.1–61.2) | 0.00 (¥) | 0.99 (0.3–2.0) | 9.54 (6.6–15.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsakoumaki, F.; Kyrkou, C.; Athanasiadis, A.P.; Menexes, G.; Michaelidou, A.-M. Nutritional Inadequacy: Unraveling the Methodological Challenges for the Application of the Probability Approach or the EAR Cut-Point Method—A Pregnancy Perspective. Nutrients 2021, 13, 3473. https://doi.org/10.3390/nu13103473
Tsakoumaki F, Kyrkou C, Athanasiadis AP, Menexes G, Michaelidou A-M. Nutritional Inadequacy: Unraveling the Methodological Challenges for the Application of the Probability Approach or the EAR Cut-Point Method—A Pregnancy Perspective. Nutrients. 2021; 13(10):3473. https://doi.org/10.3390/nu13103473
Chicago/Turabian StyleTsakoumaki, Foteini, Charikleia Kyrkou, Apostolos P. Athanasiadis, Georgios Menexes, and Alexandra-Maria Michaelidou. 2021. "Nutritional Inadequacy: Unraveling the Methodological Challenges for the Application of the Probability Approach or the EAR Cut-Point Method—A Pregnancy Perspective" Nutrients 13, no. 10: 3473. https://doi.org/10.3390/nu13103473
APA StyleTsakoumaki, F., Kyrkou, C., Athanasiadis, A. P., Menexes, G., & Michaelidou, A.-M. (2021). Nutritional Inadequacy: Unraveling the Methodological Challenges for the Application of the Probability Approach or the EAR Cut-Point Method—A Pregnancy Perspective. Nutrients, 13(10), 3473. https://doi.org/10.3390/nu13103473






