Manganese Encephalopathy Caused by Homemade Methcathinone (Ephedrone) Prevalence in Poland
Abstract
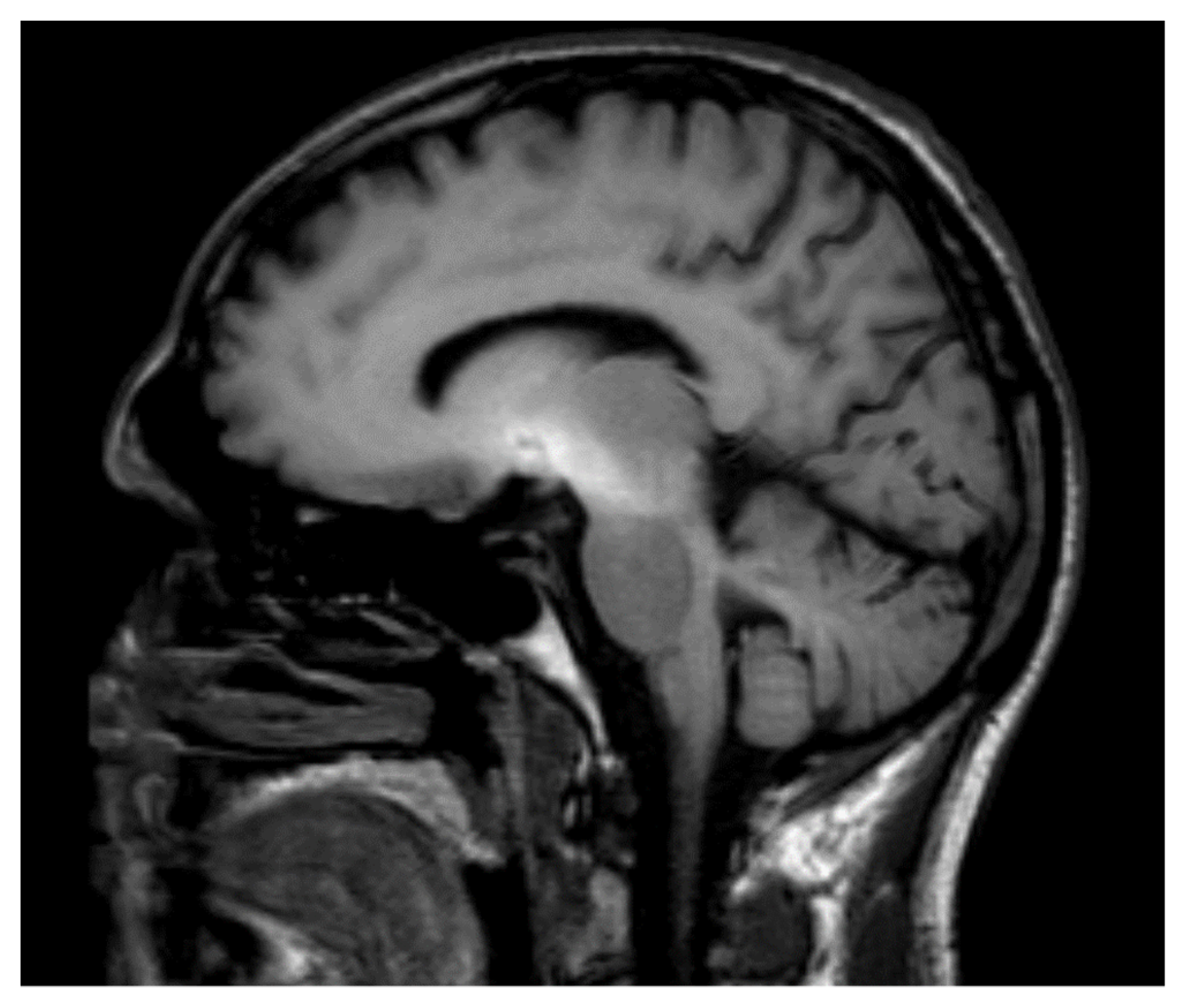
:1. Introduction
- Body posture
- sometimes leading to backward falls,
- Propulsion and/or retropulsion,
- Characteristic dystonic disturbances in the facial muscles
- a forced smile grimace (reminiscent of "risus sardonicus"),
- Dysarthria (speech significantly reduced to anarthria in advanced cases, monotonous speech) [8],
- Extrapyramidal symptoms, such as:
- muscle stiffness,
- slowdown,
- impaired movement,
- impaired facial expressions,
- difficulties in precise movements, mainly of the hands,
- micrograph (writing in smaller and smaller letters),
- Pseudobulbar symptoms: inadequate, forced laughter or crying,
2. Materials and Methods
3. Results
3.1. Questionnaire
3.2. Intravenous Methcathinone Use and Manganese Encephalopathy Symptoms
3.3. Professional Knowledge of Manganese Encephalopathy
- 13 centers (18%) assessed their staff’s knowledge of manganese encephalopathy as none;
- 11 of them (15.3%) declared the need for training;
- 2 did not see such a need due to the currently “non-medical nature” of their center;
- 38 centers (52.8%) declared minimal knowledge in this area;
- 21 centers (29.2%) declared sufficient competence in dealing with people with suspected manganese encephalopathy;
- of which 15 (20.1%) considered that they could still be further trained.
4. Discussion
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. The Questionnaire on the Prevalence of Manganese Encephalopathy among Patients of Addiction Treatment Centers
- Center:
- Inpatient.
- Detoxifying.
- Rehabilitation.
- Other (what kind? …).
- Outpatient.
- Inpatient and outpatient.
- Other (what kind? …).
- In the last six years approx. … patients addicted to substances (other than alcohol) were treated in our center (please provide the number of patients)
- Of them, about … (please provide the number of patients) used intravenous ephedrone preparations from medicines containing pseudoephedrine (Sudafed, Acatar, etc.) - prepared with potassium permanganate.
- In the last six years we had approx. … (please provide the number) patients whose clinical picture was very similar to the description of manganese encephalopathy that was given in the cover letter of this questionnaire and the review papers.
- In the last six years we had approx. … (please provide the number) patients who developed single (up to 3) symptoms listed in the clinical description as above.
- About … (please provide the number) of patients had had above mentioned symptoms also before 2010′.
- The management of patients with suspected manganese encephalopathy (please mark appropriate):
- The suspicions were quickly verified by neurologist who performed MRI of thebrain, as well as measured blood manganese levels.
- The patients were referred to neurologists who did not verify the suspicion of themanganese encephalopathy or did not provide recommendation for the treatment.
- Patients were referred to other medical centers.
- We were not aware of the possibility of any further diagnosis of patients.
- We were unable to refer patients for further examinations due to:
- lack of patients’ insurance.
- refusal from other medical centers.
- other (please specify)…
- The phenomenon of using ephedrone and the manganese preparations (please mark appropriate):
- Lasts for many years, also before 2010′ and has not undergone major changes.
- Intensified after the introduction of the legal changes in 2010.
- The legal changes introduced in 2010 had reduced the use of this preparation.
- It’s hard to say.
- What is the level of knowledge about the manganese encephalopathy in medical staff of your center?
- None and they do not need training.
- None and they do need training.
- Minimal but and they do not need training.
- Minimal and they do need training.
- Sufficient and they do not need training.
- Sufficient, but training can increase their competence.
- Excellent and they do not need training.
- Attached information about manganese encephalopathy:
- Significantly and sufficiently increased the alertness of the staff to this disorder.
- Did not change our treatment policy because the staff is familiar with clinical symptoms of manganese encephalopathy.
- Is unnecessary.
References
- Chang, Y.; Kim, Y.; Woo, S.T.; Song, H.J.; Ki, S.H.; Lee, H.; Kwon, Y.J.; Ahn, J.H.; Park, S.J.; Chung, I.S.; et al. High signal intensity on magnetic resonance imaging is a better predictor of neurobehavioral performances than blood manganese in asymptomatic welders. NeuroToxicology 2009, 30, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Riviera-Mancia, S.; Rios, C.; Montes, S. Manganese accumulation in the CNS and associated pathologies. Biometals 2011, 24, 811–825. [Google Scholar] [CrossRef] [PubMed]
- Zimny, A.; Zińska, L.; Bladowska, J.; Neska-Matuszewska, M.; Sąsiadek, M. Intracranial lesions with high signal intensity on T1-weighted MR images—Review of pathologies. Pol. J. Radiol. 2013, 78, 36–46. [Google Scholar]
- Poniatowska, R.; Lusawa, M.; Skierczyńska, A.; Makowicz, G.; Habrat, B.; Sienkiewicz-Jarosz, H. MRI brain findings in ephedrine encephalopathy associated with manganese abuse: Single-center perspective. Pol. J. Radiol. 2014, 79, 150–155. [Google Scholar] [PubMed] [Green Version]
- Habrat, B.; Baran-Furga, H.; Sienkiewicz-Jarosz, H.; Anand, J.; Poniatowska, R. Encefalopatie spowodowane dożylnym używaniem preparatów zawierających nadmanganian potasu stosowany jako reagent w produkcji metkatynonu (efedronu) z leków zawierających pseudoefedrynę. Przegl. Lek. 2013, 70, 613–616. [Google Scholar] [PubMed]
- Sikk, K. Manganese-ephedrone intoxication—Pathogenesis of neurological damage and clinical symptomatology. Ph.D. Dissertation Medicinae Universitatis Taruensis 206, University of Tartu Press, Tartu, Estonia, 2013. [Google Scholar]
- Stepens, A.; Groma, V.; Skuja, S.; Platkajis, A.; Aldins, P.; Ekšteina, I.; Martinsone, I.; Bricis, R.; Donaghy, M. The outcome of the movement disorder in methcathinone abusers: Clinical, MRI and manganesemia changes and neuropathogy. Eur. J. Neurol. 2014, 21, 199–205. [Google Scholar] [CrossRef]
- Rusz, J.; Megrelishvili, M.; Bonnet, C.; Okujava, M.; Brožová, H.; Khatiashvili, I.; Sekhniashvili, M.; Janelidze, M.; Tolosa, E.; Růžička, E. A distinct variant of mixed dysarthria reflects parkinsonism and dystonia due to ephedrone abuse. J. Neural Transm. 2014, 121, 655–664. [Google Scholar] [CrossRef]
- Huang, C.C.; Chu, N.S.; Lu, C.S.; Calne, D.B. Cock gait in manganese intoxication. J. Mov. Disord. 1997, 12, 807–808. [Google Scholar] [CrossRef]
- Janssens, J.; Vandenberghe, W. Dystonic drop foot gait in a patient manganism. Neurology 2010, 75, 835. [Google Scholar] [CrossRef] [Green Version]
- Koksal, A.; Keskinkilic, C.; Sozmen, M.V.; Dirican, A.C.; Aysal, F.; Altunkayanak, Y.; Baybas, S. Evaluation of cognitive characteristic of patients developing manifestation of parkinsonism secondary to long-term ephedrone use. Eur. Neurol. 2014, 71, 208–212. [Google Scholar] [CrossRef]
- Zoni, S.; Albini, E.; Lucchini, R. Neuropsychological testing for the assessment of manganese neurotoxicity: A review and a proposal. Am. J. Ind. Med. 2007, 50, 812–816. [Google Scholar] [CrossRef]
- OSHA. OSHA Annotated Table Z-From. 2017. Available online: https://www.osha.gov/dsg/annotated-pels/tablez-1.html (accessed on 4 April 2018).
- Sriram, K.; Lin, G.X.; Jefferson, A.M.; Stone, S.; Afshari, A.; Keane, M.J.; McKinney, W.; Jackson, M.; Chen, B.T.; Schwegler-Berry, D.; et al. Modifying welding parameters can reduce the neurotoxic potential of manganese-containing welding fumes. Toxicology 2015, 128, 166–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.D.; Huang, C.C.; Hwang, Y.H.; Chang, J.R.; Lin, J.M.; Chen, J.S. Manganese induced parkinsonism: And outbreak due to an unrepaired ventilation control system in a ferromanganese smelter. Br. J. Ind. Med. 1989, 46, 856–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodier, J. Manganese poisoning in Maroccan miners. Br. J. Ind. Med. 1955, 12, 21–35. [Google Scholar] [PubMed] [Green Version]
- Sińczuk-Walczak, H.; Jakubowski, M.; Matczak, W. Neurological and neurophysiological examinations of workers occupationally exposed to manganese. Int. J. Occup. Environ. Health 2001, 14, 329–337. [Google Scholar]
- Forton, D.M.; Patel, N.; Prince, M.; Oatrige, A.; Hamilton, G.; Goldblatt, J.; Allsop, J.M.; Hajnal, J.V.; Thomas, H.C.; Bassendine, M.; et al. Fatigue and primary biliary cirrhosis: Association of globus pallidus magnetization transfer ratio measurements with fatigue severity and blood manganese levels. Gut 2004, 53, 587–592. [Google Scholar] [CrossRef]
- Klos, K.G.; Ahlskog, J.E.; Kumar, N.; Cambern, S.; Butz, J.; Burrit, M.; Felaey, R.D.; Cowl, C.T.; Parisi, J.E.; Josephs, K.A. Brain metal concentrations in chronic liver failure patients with pallidal T1 MRI hyperintensity. Neurology 2006, 67, 1984–1989. [Google Scholar] [CrossRef]
- Herrero Hernandez, E.H.; Discalzi, G.; Dassi, P.; Jarre, P.; Pira, E. Manganese intoxication: The cause of and inexplicable epileptic syndrome in a 3-year-old child. NeuroToxicology 2003, 24, 633–639. [Google Scholar] [CrossRef]
- Chalela, J.A.; Bonillha, L.; Neyens, R.; Hays, A. Manganese encephalopathy: An under-recognized condition in the intensive care unit. Neurocrit. Care 2011, 14, 456–458. [Google Scholar] [CrossRef]
- Lin, C.C.; Chen, Y.C.; Su, F.C.; Lin, C.M.; Liao, H.F.; Hwang, Y.H.; Hsieh, W.S.; Jeng, S.F.; Su, Y.N.; Chen, P.C. In utero exposure to environmental lead and manganese and neurodevelopment at 2 year of age. Environ. Res. 2013, 123, 52–57. [Google Scholar] [CrossRef]
- Zoni, A.; Lucchini, R.G. Manganese exposure: Cognitive, motor and behavioral effects on children. Curr. Opin. Pediatr. 2013, 25, 255–260. [Google Scholar] [CrossRef] [Green Version]
- Oulhote, Y.; Mergler, D.; Barbeau, B.; Bellinger, D.C.; Bouffard, T.; Brodeur, M.E.; Saint-Amour, D.; Legrand, M.; Sauve, S.; Bouchard, M.E. Neurobehavioral function in school-age children exposed to manganese in drinking water. Environ. Health Perspect. 2014, 122, 1343–1350. [Google Scholar] [CrossRef]
- Grandjean, P.; Landrigan, P.J. Developmental neurotoxicity of industrial chemicals. Lancet 2006, 16, 2167–2178. [Google Scholar] [CrossRef]
- Pihl, R.O.; Parkes, M. Hair element content in learning disabled children. Science 1977, 14, 204–206. [Google Scholar] [CrossRef] [PubMed]
- Collipp, P.; Chen, S.; Maitinsky, S. Manganese in Infant Formulas and Learning Disability. Ann. Nutr. Metab. 1983, 27, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, M.; Laforest, F.; Vandelac, L.; Bellinger, D.; Mergler, D. Hair manganese and hyperactive behaviors: Pilot study of school-age children exposed through tap water. Environ. Health Perspect. 2007, 115, 122–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, K.; Factor-Litvak, P.; Wasserman, G.A.; Liu, X.; Ahmed, E.; Parvez, F.; Slavkovich, V.; Levy, D.; Mey, J.; van Geen, A.; et al. Manganese exposure from drinking water and children’s classroom behavior in Bangladesh. Environ. Health Perspect. 2011, 119, 1501–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schullehner, J.; Thygesen, M.; Kristiansen, S.M.; Hansen, B.; Pedersen, C.B.; Dalsgaard, S. Exposure to Manganese in Drinking Water during Childhood and Association with Attention-Deficit Hyperactivity Disorder: A Nationwide Cohort Study. Environ. Health Perspect. 2020, 128, 97004. [Google Scholar] [CrossRef]
- Saleh, S.A.K.; Adly, H.M.; Abdelkhaliq, A.A.; Nassir, A.M. Serum Levels of Selenium, Zinc, Copper, Manganese, and Iron in Prostate Cancer Patients. Curr. Urol. 2020, 14, 44–49. [Google Scholar] [CrossRef]
- Schmidt, T.; Dalubaeva, D. Neurological complications of ephedrine drug abuse (ephedrine encephalopathy). In Anniversary Collection: Diagnostic and Treatment of Neurological Diseases; Medicine: Moscow, Russia, 1990; pp. 183–186. [Google Scholar]
- Fedorova, N.; Amosova, N.; Ismailova, T. Clinical features of motor disturbances at toxic encephalopathy provoked by using of substitute psychoactive substances. J. Mov. Disord. 2007, 22, S109. [Google Scholar]
- Levin, O.S.; Datieva, V.K. The use of biperiden (Akineton) in patients with ephedron encephalopathy. Zhournal Nevrol. I Psikhiatrii Im SS Korsakova 2013, 113, 33–37. [Google Scholar]
- Levin, O.S. “Ephedron” encephalopathy. Zhournal Nevrol. I Psikhiatrii Im SS Korsakova 2005, 105, 12–20. [Google Scholar]
- Khatiashvili, I.; Akhvlediani, K.; Megrelishvili, M.; Janelidze, M.; Lobjanidze, N. Movement disorder caused by injections of manganese containing compounds. J. Mov. Disord. 2007, 22, S110. [Google Scholar]
- Stepens, A.; Logina, I.; Viesturs, L.; Aldins, P.; Ekšteina, I.; Platkajis, A.; Martinsone, I.; Terauds, E.; Rozentale, B.; Donaghy, M. A parkinsonian syndrome in methcathinone user and the role of manganese. N. Engl. J. Med. 2008, 358, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Stepens, A.; Stagg, C.J.; Platkäjis, A.; Boudrias, M.H.; Johansen-Berg, H.; Donaghy, M. White matter abnormalities in methcathinone abusers with an extrapyramidal syndrome. Brain 2010, 133, 3676. [Google Scholar] [CrossRef] [PubMed]
- Aquilionius, S.; Sikk, K.; Taba, P.; Bergquist, J.; Nyholm, D.; Zjablov, G.; Asser, T.; Haldre, S. The Sudafed story—Manganism, ephedrine or both. J. Mov. Disord. 2006, 21, S373. [Google Scholar]
- Sikk, K.; Taba, P.; Haldre, S.; Bergquist, J.; Nyholm, D.; Askmark, H.; Danfors, T.; Sorensen, J.; Thurfjell, L.; Raininko, R.; et al. Clinical, neuroimaging and neurophysiological features in addicts with manganese-ephedrone exposure. Acta Neurol. Scand. 2010, 121, 237–243. [Google Scholar] [CrossRef]
- Meral, H.; Kutukcu, Y.; Atmaca, B.; Ozer, F.; Hamamcioglu, K. Parkinsonism caused by chronic usage of intravenous potassium permanganate. Neurologist 2007, 13, 99–102. [Google Scholar] [CrossRef]
- Koksal, A.; Baybas, S.; Sozmen, V.; Koksal, N.S.; Altunkayanak, Y.; Dirican, A.; Mutluay, B.; Kucukoglu, H.; Keskinkilic, C. Chronic manganese toxicity due to substance abuse in Turkish patients. Neurol. India 2012, 60, 224–227. [Google Scholar]
- De Bie, R.M.; Gladstome, R.M.; Strafella, A.P.; Ko, J.H.; Lang, A.E. Manganese-induced parkinsonism associated with methcathinone (ephedrone) abuse. Arch. Neurol. 2007, 64, 886–887. [Google Scholar] [CrossRef] [Green Version]
- Colosimo, C.; Guidi, M. Parkinsonism due to ephedrone neurotoxicity: A case report. Eur. J. Neurol. 2009, 16, e114–e116. [Google Scholar] [CrossRef]
- Iqbal, M.; Monaghan, T.; Redmont, J. Manganese toxicity with ephedrone abuse manifesting as parkinsonism: A case report. J. Med. Case Rep. 2012, 6, 52. [Google Scholar] [CrossRef] [Green Version]
- Habrat, B.; Anand, J.S.; Sienkiewicz-Jarosz, H.; Kałwa, A.; Baran-Furga, H. Subacute encephalopathy caused by manganese compounds used for ephedrone production. Addictologia Hung. 2012, 11, 53–54. [Google Scholar]
- Fudalej, S.; Kołodziejczyk, I.; Gajda, T.; Majkowska-Zwolińska, B.; Wojnar, M. Manganese-induced parkinsonism among ephedrone users and drug policy in Poland. J. Addict. Med. 2013, 7, 302–303. [Google Scholar] [CrossRef] [PubMed]
- Golasik, M.; Wodowski, G.; Gomółka, E.; Herman, M.; Piekoszewski, W. Urine as material for evaluation of exposure to manganese in methcathinone users. J. Trace Elem. Med. Biol. 2014, 28, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Dolgan, A.; Budrewicz, S.; Koszewicz, M.; Bladowska, J.; Slotwinski, K.; Zagrajek, M.; Koziorowska-Gawron, E.; Podemski, R. Acute hyperkinetic syndrome due to ephedrine abuse. J. Addict. Med. 2015, 9, 244–245. [Google Scholar] [CrossRef] [PubMed]
- Chintalova-Dallas, R.; Case, P.; Kitsenko, N.; Lazzarini, Z.; Boltushka, A. A homemade amphetamine type stimulant and HIV risk in Odessa, Ukraine. Int. J. Drug Policy 2009, 20, 347–351. [Google Scholar] [CrossRef] [Green Version]
- Dorman, D.C.; Struve, M.F.; Wong, B.A.; Dye, J.A.; Robertson, I.D. Correlation of brain magnetic resonance imaging changes with pallidal manganese concentrations in rhesus monkeys following subchronic manganese inhalation. Toxicol. Sci. 2006, 92, 219–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asser, A.; Raki, M.; Jurmaa, J.; Krispin, V.; Muldmaa, M.; Ratsep, H.; Poldsepp, P.; Mannisto, P.; Koks, S.; Bergstrom, K.; et al. Methcathinone (ephedrone) and manganese both reduce D2-receptor function: An animal SPECT study. J. Mov. Disord. 2012, 27, S430. [Google Scholar]
- Dorman, D.C.; Struve, M.F.; Clewell, H.J.; Andersen, M.E. Application of pharmacokinetic data to the risk assessment of inhaled manganese. Neurotoxicology 2006, 27, 752–764. [Google Scholar] [CrossRef]
- Burton, N.C.; Guilarte, T.R. Manganese neurotoxicity: Lessons learned from longitudinal studies in nonhuman primates. Environ. Health Perspect. 2009, 117, 325–332. [Google Scholar] [CrossRef]
- Janowska, E.; Chudzikiewicz, E.; Lechowicz, W. Ephedrone—New street drug obtained from Proasthmin. Z Zagadnień Nauk. Sądowych. 1999, 39, 44–53. [Google Scholar]
- Zuba, D. Medicines containing ephedrine and pseudoephedrine as a source of methcathinone. Probl. Forensic Sci. 2007, 71, 323–333. [Google Scholar]
- Habrat, B.; Anand, S.J.; Baran-Furga, H.; Sienkiewicz-Jarosz, H. Neurotoxicity caused by intravenous use of methcathinone produced from pseudoephedrine with the use of potassium permanganate and spirit vinegar. J. Clin. Toxicol. 2012, 50, 343. [Google Scholar]
- Sanotsky, Y.; Lesyk, R.; Fedoryshyn, L.; Komnatska, I.; Matviyenko, Y.; Fahn, S. Manganic encephalopathy due to “ephedrone” abuse. J. Mov. Disord. 2007, 22, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, R.C.; Cunningham, J.K.; Sykes, J.; Kish, S.J. Increased risk of Parkinson’s disease in individuals hospitalized with conditions related to the use of methamphetamine or other amphetamine-type drugs. Drug Alcohol Depend. 2012, 120, 35. [Google Scholar] [CrossRef] [PubMed]
- Curtin, K.; Fleckenstein, A.E.; Robison, R.J.; Crookston, M.J.; Smith, K.R.; Hanson, G.R. Methampetamine/amphetamine abuse and risk of Parkinson’s disease in Utah: A population-based assessment. Drug Alcohol Depend. 2015, 146, 30–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikk, K.; Kõks, S.; Soomets, U.; Schalkwyk, L.C.; Fernandes, C.; Haldre, S.; Aquilonius, S.-M.; Taba, P. Peripheral blood RNA expression profiling in illicit methcathinone users reveals effect on immune system. Front. Gene 2011, 2, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikk, K.; Haldre, S.; Aquilonius, S.M.; Asser, A.; Paris, M.; Roose, Ä.; Petterson, J.; Eriksson, S.L.; Bergquist, J.; Taba, P. Manganese-induced parkinsonism in methcathinone abusers: bio-markers of exposure and follow-up. Eur. J. Neurol. 2013, 20, 915–922. [Google Scholar] [CrossRef]
- Chinchaladze, L.; Khatiashvili, I.; Lobjanidze, N.; Akiashvili, N.; Maisuradze, T.; Megrelishvili, M.; Janelidze, M. Ephedrone encephalopathy: Correlation between clinical course and hyperintensivity of the basal ganglia on the T1-weighted MRI images (Tbilisi, Georgia). In Proceedings of the 16th International Congress of Parkinson’s Disease and Movement Disorders, Dublin, Ireland, 17–21 June 2012. [Google Scholar]
- Giorgishvili, I.; Lobjanidze, N.; Akiashvili, N.; Janelidze, M.; Beridze, M. Manganese-induced parkinsonism and motor neuron damage—Can there be a relationship? Mov. Disord. 2016, 31 (Suppl. 2). [Google Scholar]
- Bonnet, C.; Rusz, J.; Megrelishvili, M.; Sieger, T.; Matoušková, O.; Okujava, M.; Brožová, H.; Nikolai, T.; Hanuška, J.; Kapianidze, M.; et al. Eye Movements in Ephedrone-Induced Parkinsonism. PLoS ONE 2014, 9, e104784. [Google Scholar] [CrossRef]
- Ismailova, T.; Fedorova, N.; Savchenko, L. The treatment of patients with toxic encephalopathy caused by using surrogate psychoactive manganese-containing compounds. Zh Nevrol Psikhiatr Im S S Korsakova. 2005, 105, 18–21. [Google Scholar]
- Selikhova, M.; Fedoryshyn, L.; Matviyenko, Y.; Komnatska, I.; Kyrylchuk, M.; Krolicki, L.; Friedman, A.; Taylor, A.; Jäger, H.R.; Lees, A.; et al. Parkinsonism and dystonia caused by the illicit use of ephedrine—A longitudinal study. Mov. Disord. 2008, 23, 2224–2231. [Google Scholar] [CrossRef] [PubMed]
- Djamshidian, A.; Sanotsky, Y.; Matviyenko, Y.; O’Sullivan, S.S.; Sharman, S.; Selikhova, M.; Fedoryshyn, L.; Filts, Y.; Bearn, J.; Lees, A.J.; et al. Increased reflection impulsivity in patients with ephedrone-induced Parkinsonism. Addiction 2013, 108, 771–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aschner, M.; Aschner, J.L. Manganese neurotoxicity: Cellular effects and blood-brain barrier transport. Neurosci. Biobehav. Rev. 1991, 15, 333–340. [Google Scholar] [CrossRef]

| Authors | Year | Country | N | M:F | Average Age |
|---|---|---|---|---|---|
| Sikk et al. [61] | 2011 | Estonia | 20 | 17:3 | - |
| Sikk et al. [62] | 2013 | Estonia | 38 | 31:7 | 33 |
| Sikk et al. [62] | 2013 | Estonia | 24 | - | - |
| Khatiashvili et al. [36] | 2007 | Georgia | 10 | - | - |
| Chinchaladze et al. [63] | 2012 | Georgia | 22 | - | - |
| Giorgishvili et al. [64] | 2016 | Georgia | 3 | - | - |
| Bonnet et al. [65] | 2014 | Georgia | 28 | 27:1 | - |
| Rusz et al. [8] | 2014 | Georgia | 28 | - | 39.9 +/−4.9 |
| Stepens et al. [37] | 2010 | Latvia | 10 | 8:2 | 40 (30–55) |
| Stepens et al. [38] | 2011 | Latvia | 23 | - | - |
| Stepens et al. [7] | 2014 | Latvia | 18 | 17:1 | 36.5 (23–47) |
| Golasik et al. [48] | 2014 | Poland | 24 | 17:7 | |
| Levin [35] | 2005 | Russia | 21 | 14:7 | - |
| Ismailova et al. [66] | 2005 | Russia | 65 | 60:5 | - |
| Fedorova et al. [33] | 2007 | Russia | 65 | - | - |
| Levin & Datieva [34] | 2013 | Russia | 35 | - | - |
| Sanotsky et al. [58] | 2007 | Ukraine | 13 | 13:0 | - |
| Selighova et al. [67] | 2008 | Ukraine | 13 | 13:0 | - |
| Djamshidian et al. [68] | 2012 | Ukraine | 15 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habrat, B.; Silczuk, A.; Klimkiewicz, A. Manganese Encephalopathy Caused by Homemade Methcathinone (Ephedrone) Prevalence in Poland. Nutrients 2021, 13, 3496. https://doi.org/10.3390/nu13103496
Habrat B, Silczuk A, Klimkiewicz A. Manganese Encephalopathy Caused by Homemade Methcathinone (Ephedrone) Prevalence in Poland. Nutrients. 2021; 13(10):3496. https://doi.org/10.3390/nu13103496
Chicago/Turabian StyleHabrat, Bogusław, Andrzej Silczuk, and Anna Klimkiewicz. 2021. "Manganese Encephalopathy Caused by Homemade Methcathinone (Ephedrone) Prevalence in Poland" Nutrients 13, no. 10: 3496. https://doi.org/10.3390/nu13103496






