Dietary Interventions with or without Omega-3 Supplementation for the Management of Rheumatoid Arthritis: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Terms and Strategy
2.2. Eligibility Criteria
2.3. Study Selection Process
2.4. Data Extraction
2.5. Quality Assessment
2.6. Data Analysis
3. Results
3.1. Study Selection
3.2. Study and Participant’s Characteristics
3.3. Intervention Characteristics
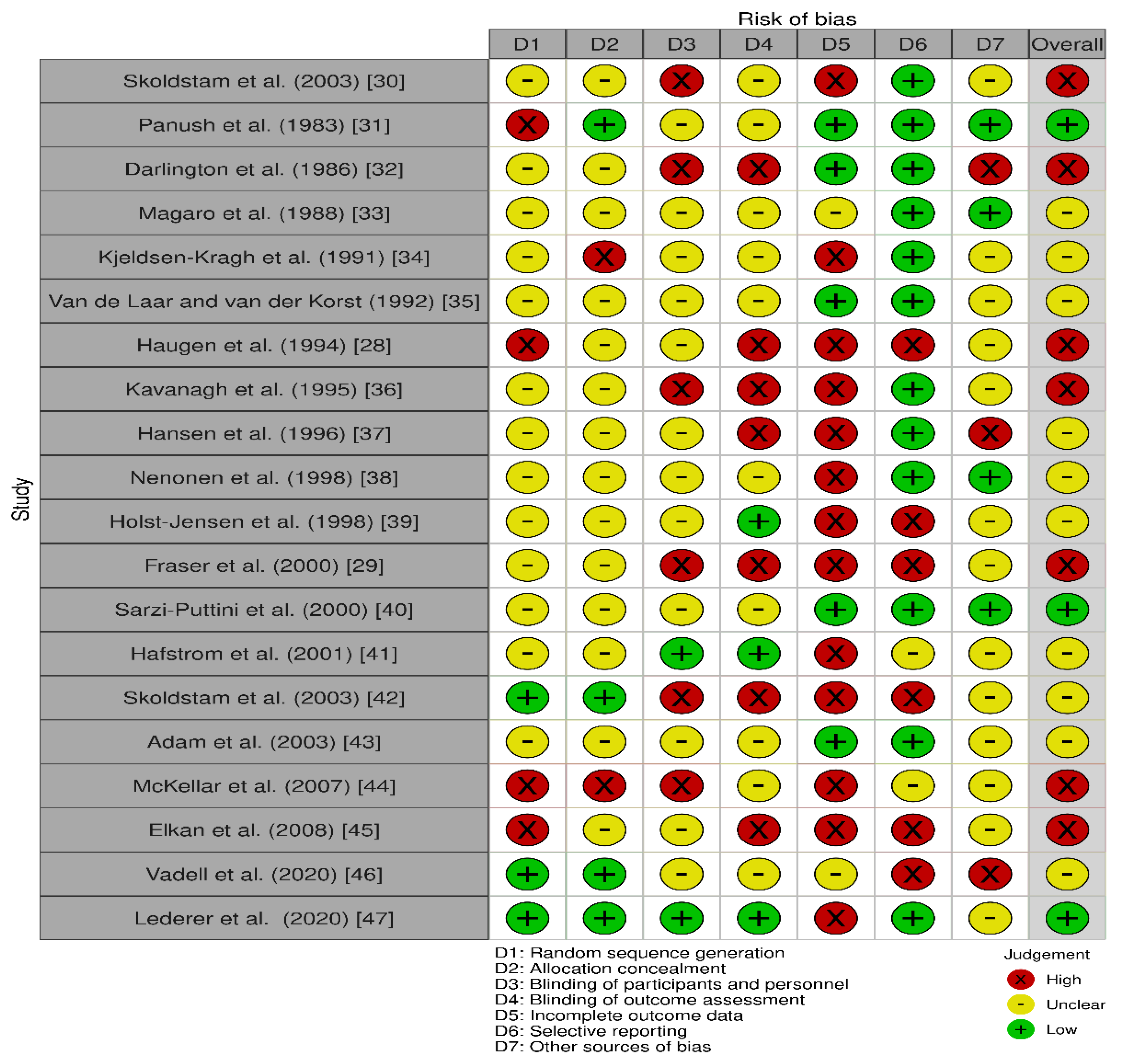
3.4. Risk of Bias within Studies
3.5. Outcome Measures
3.5.1. Inflammatory Markers
Erythrocyte Sedimentation Rate
C-Reactive Protein
Tumor Necrosis Factor-α
Platelet Count
Leukotriene 4
Interleukin-6 and Interleukin-10
Dehydroepiandrosterone Sulfate
Oxidized Low-Density Lipoprotein (OxLDL)
Immunoglobulins
3.5.2. Clinical/Functional Measures
Pain
Early Morning Stiffness (EMS)
Grip Strength
Ritchie’s Index
Disease Activity Score
Number of Tender and Swollen Joints
Global Assessment
3.5.3. Patient Questionnaires
3.5.4. Medications
3.5.5. Radiographs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines. Guidelines for the management of rheumatoid arthritis. Arthritis Rheum. 2002, 46, 328–346. [Google Scholar] [CrossRef]
- Pattison, D.J.; Symmons, D.P.; Young, A. Does diet have a role in the aetiology of rheumatoid arthritis? Proc. Nutr. Soc. 2004, 63, 137–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bullock, J.; Rizvi, S.A.; Saleh, A.M.; Ahmed, S.S.; Do, D.P.; Ansari, R.A.; Ahmed, J. Rheumatoid arthritis: A brief overview of the treatment. Med. Princ. Pr. 2018, 27, 501–507. [Google Scholar] [CrossRef]
- Michaud, K.; Wolfe, F. Comorbidities in rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2007, 21, 885–906. [Google Scholar] [CrossRef]
- Romano, S.; Salustri, E.; Ruscitti, P.; Carubbi, F.; Penco, M.; Giacomelli, R. Cardiovascular and metabolic comorbidities in rheumatoid arthritis. Curr. Rheumatol. Rep. 2018, 20, 81. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekara, S.; Shobha, V.; Dharmanand, B.; Jois, R.; Kumar, S.; Mahendranath, K.; Haridas, V.; Prasad, S.; Singh, Y.; Daware, M.; et al. Comorbidities and related factors in rheumatoid arthritis patients of south India—Karnataka Rheumatoid Arthritis Comorbidity (KRAC) study. Reumatismo 2017, 69, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Listing, J.; Gerhold, K.; Zink, A. The risk of infections associated with rheumatoid arthritis, with its comorbidity and treatment. Rheumatology 2012, 52, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Bijlsma, J.W.J.; Boers, M.; Saag, K.G.; Furst, E.D. Glucocorticoids in the treatment of early and late RA. Ann. Rheum. Dis. 2003, 62, 1033–1037. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.J.-A.; Kavanaugh, A. Pharmacological treatment of established rheumatoid arthritis. Best Pr. Res. Clin. Rheumatol. 2003, 17, 811–829. [Google Scholar] [CrossRef]
- Choy, E. Understanding the dynamics: Pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology 2012, 51, v3–v11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastronuzzi, T.; Grattagliano, I. Nutrition as a health determinant in elderly patients. Curr. Med. Chem. 2019, 26, 3652–3661. [Google Scholar] [CrossRef]
- Skoczyńska, M.; Świerkot, J. The role of diet in rheumatoid arthritis. Reumatologia 2018, 56, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.; Sjöblom, H.; Gjertsson, I.; Ulven, S.M.; Lindqvist, H.M.; Bärebring, L. Do interventions with diet or dietary supplements reduce the disease activity score in rheumatoid arthritis? A systematic review of randomized controlled trials. Nutrients 2020, 12, 2991. [Google Scholar] [CrossRef] [PubMed]
- Philippou, E.; Petersson, S.D.; Rodomar, C.; Nikiphorou, E. Rheumatoid arthritis and dietary interventions: Systematic review of clinical trials. Nutr. Rev. 2020, 79, 410–428. [Google Scholar] [CrossRef] [PubMed]
- Hagen, K.B.; Byfuglien, M.G.; Falzon, L.; Olsen, S.U.; Smedslund, G. Dietary interventions for rheumatoid arthritis. Cochrane Database Syst. Rev. 2009, 21, CD006400. [Google Scholar] [CrossRef] [PubMed]
- Abdissa, D. Purposeful review to identify the benefits, mechanism of action and practical considerations of omega-3 pol-yunsaturated fatty acid supplementation for the management of diabetes mellitus. Nutr. Diet. Suppl. 2021, 13, 53. [Google Scholar] [CrossRef]
- Venter, C.; Eyerich, S.; Sarin, T.; Klatt, K.C. Nutrition and the immune system: A complicated tango. Nutrients 2020, 12, 818. [Google Scholar] [CrossRef] [Green Version]
- Calder, P. Omega-3 (n-3) polyunsaturated fatty acids and inflammation: From membrane to nucleus and from bench to bed-side. Proc. Nutr. Soc. 2020, 79, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Kostoglou-Athanassiou, I.; Athanassiou, L.; Athanassiou, P. The effect of omega-3 fatty acids on rheumatoid arthritis. Mediterr. J. Rheumatol. 2020, 31, 190–194. [Google Scholar] [CrossRef]
- Lanchais, K.; Capel, F.; Tournadre, A. Could omega 3 fatty acids preserve muscle health in rheumatoid arthritis? Nutrients 2020, 12, 223. [Google Scholar] [CrossRef] [Green Version]
- Gioxari, A.; Kaliora, A.C.; Marantidou, F.; Panagiotakos, D.P. Intake of ω-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: A systematic review and meta-analysis. Nutrition 2018, 45, 114–124. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzee, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffman, T.C.; Mulrow, C.D.; Shamseer, L.; Tezlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnett, F.C.; Edworthy, S.M.; Bloch, D.A.; McShane, D.J.; Fries, J.F.; Cooper, N.S.; Healey, L.A.; Kaplan, S.R.; Liang, M.H.; Luthra, H.S.; et al. The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988, 31, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Kay, J.; Upchurch, K.S. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology 2012, 51, vi5–vi9. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.C.; Savović, J.; Page, M.; Elbers, R.G.; Blencowe, N.; Boutron, I.; Cates, C.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, M.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [Green Version]
- Haugen, A.M.; Kjeldsen-Kragh, J.; Førre, O. A pilot study of the effect of an elemental diet in the management of rheumatoid arthritis. Clin. Exp. Rheumatol. 1994, 12, 275–279. [Google Scholar]
- Fraser, A.D.; Thoen, J.; Djøseland, O.; Førre, O.; Kjeldsen-Kragh, J. Serum levels of interleukin-6 and dehydroepiandrosterone sulphate in response to either fasting or a ketogenic diet in rheumatoid arthritis patients. Clin. Exp. Rheumatol. 2000, 18, 357–362. [Google Scholar]
- Skoldstam, L.; Larsson, L.; Lindström, F.D. Effects of fasting and lactovegetarian diet on rheumatoid arthritis. Scand. J. Rheumatol. 1979, 8, 249–255. [Google Scholar] [CrossRef]
- Panush, R.S.; Carter, R.L.; Katz, P.; Kowsari, B.; Longley, S.; Finnie, S. Diet therapy for rheumatoid arthritis. Off. J. Am. Coll. Rheumatol. 1983, 26, 462–471. [Google Scholar] [CrossRef]
- Darlington, L.; Ramsey, N.; Mansfield, J. Placebo-controlled, blind study of dietary manipulation therapy in rheumatoid arthritis. Lancet 1986, 327, 236–238. [Google Scholar] [CrossRef]
- Magaro, M.; Altomonte, L.; Zoli, A.; Mirone, L.; De Sole, P.; Di Mario, G.; Lippa, S.; Oradei, A. Influence of diet with different lipid composition on neutrophil chemiluminescence and disease activity in patients with rheumatoid arthritis. Ann. Rheum. Dis. 1988, 47, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen-Kragh, J.; Borchgrevink, C.; Laerum, E.; Haugen, M.; Eek, M.; Førre, O.; Mowinkel, P.; Hovi, K. Controlled trial of fasting and one-year vegetarian diet in rheumatoid arthritis. Lancet 1991, 338, 899–902. [Google Scholar] [CrossRef]
- Van De Laar, A.M.; Van Der Korst, J.K. Food intolerance in rheumatoid arthritis I. A double blind, controlled trial of the clinical effects of elimination of milk allergens and azo dyes. Ann. Rheum. Dis. 1992, 51, 298–302. [Google Scholar] [CrossRef] [Green Version]
- Kavanagh, R.; Workman, E.; Nash, P.; Smith, M.; Hazleman, B.; Hunter, J. The effects of elemental diet and subsequent food reintroduction on rheumatoid arthritis. Rheumatology 1995, 34, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Hansen, G.V.O.; Nielsen, L.; Kluger, E.; Thysen, M.; Emmertsen, H.; Stengaard-Pedersen, K.; Hansen, E.L.; Unger, B.; Andersen, P.W. Nutritional status of danish rheumatoid arthritis patients and effects of a diet adjusted in energy intake, fish-meal, and antioxidants. Scand. J. Rheumatol. 1996, 25, 325–333. [Google Scholar] [CrossRef]
- Nenonen, M.T.; Helve, T.A.; Rauma, A.L.; Hanninen, O.O. Uncooked, lactobacilli-rich, vegan food and rheumatoid arthritis. Rheumatology 1998, 37, 274–281. [Google Scholar] [CrossRef] [Green Version]
- Holst-Jensen, E.S.; Pfeiffer-Jensen, M.; Monsrud, M.; Tarp, U.; Buus, A.; Hessov, I.; Thorling, E.; Stengaard-Pedersen, K. Treatment of rheumatoid arthritis with a peptide diet: A randomized, controlled trial. Scand. J. Rheumatol. 1998, 27, 329–336. [Google Scholar] [CrossRef]
- Sarzi-Puttini, P.; Comi, D.; Boccassini, L.; Muzzupappa, S.; Turiel, M.; Panni, B.; Salvaggio, A. Diet therapy for rheumatoid arthritis. A controlled double-blind study of two different dietary regimens. Scand. J. Rheumatol. 2000, 29, 302–307. [Google Scholar] [PubMed]
- Hafstrom, I.; Ringertz, B.; Spångberg, A.; Von Zweigbergk, L.; Brannemark, S.; Nylander, I.; Rönnelid, J.; Laasonen, L.; Klareskog, L. A vegan diet free of gluten improves the signs and symptoms of rheumatoid arthritis: The effects on arthritis correlate with a reduction in antibodies to food antigens. Rheumatology 2001, 40, 1175–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skoldstam, L.; Hagfors, L.; Johansson, G. An experimental study of a Mediterranean diet intervention for patients with rheumatoid arthritis. Ann. Rheum. Dis. 2003, 62, 208–214. [Google Scholar] [CrossRef]
- Adam, O.; Beringer, C.; Kless, T.; Lemmen, C.; Adam, A.; Wiseman, M.; Adam, P.; Klimmek, R.; Forth, W. Anti-inflammatory effects of a low arachidonic acid diet and fish oil in patients with rheumatoid arthritis. Rheumatol. Int. 2003, 23, 27–36. [Google Scholar] [CrossRef]
- McKellar, G.; Morrison, E.; McEntegart, A.; Hampson, R.; Tierney, A.; Mackle, G.; Scoular, J.; Scott, J.; Capell, A.H. A pilot study of a Mediterranean-type diet intervention in female patients with rheumatoid arthritis living in areas of social deprivation in Glasgow. Ann. Rheum. Dis. 2007, 66, 1239–1243. [Google Scholar] [CrossRef] [Green Version]
- Elkan, A.-C.; Sjöberg, B.; Kolsrud, B.; Ringertz, B.; Hafström, I.; Frostegård, J. Gluten-free vegan diet induces decreased LDL and oxidized LDL levels and raised atheroprotective natural antibodies against phosphorylcholine in patients with rheumatoid arthritis: A randomized study. Arthritis Res. Ther. 2008, 10, R34. [Google Scholar] [CrossRef] [Green Version]
- Vadell, A.K.; Bärebring, L.; Hulander, E.; Gjertsson, I.; Lindqvist, H.M.; Winkvist, A. Anti-inflammatory diet in rheumatoid arthritis (ADIRA)—A randomized, controlled crossover trial indicating effects on disease activity. Am. J. Clin. Nutr. 2020, 111, 1203–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lederer, A.K.; Maul-Pavicic, A.; Hannibal, L.; Hettich, M.; Steinborn, C.; Gründemann, C.; Zimmerman-Klemd, A.M.; Muller, A.; Sehnert, B.; Salzer, U.; et al. Vegan diet reduces neutrophils, monocytes and platelets related to branched-chain amino acids–A randomized, controlled trial. Clin. Nutr. 2020, 39, 3241–3250. [Google Scholar] [CrossRef] [PubMed]
- Berglin, E.; Dahlqvist, S.R. Comparison of the 1987 ACR and 2010 ACR/EULAR classification criteria for rheumatoid arthritis in clinical practice: A prospective cohort study. Scand. J. Rheumatol. 2013, 42, 362–368. [Google Scholar] [CrossRef]
- Harrison, B.J.; Symmons, D.; Barrett, E.M.; Silman, A.J. The performance of the 1987 ARA classification criteria for rheumatoid arthritis in a population based cohort of patients with early inflammatory polyarthritis. American Rheumatism Association. J. Rheumatol. 1998, 25, 2324–2330. [Google Scholar]
- McKenna, F.; Wright, V. Pain and rheumatoid arthritis. Ann. Rheum. Dis. 1985, 44, 805. [Google Scholar] [CrossRef] [Green Version]
- Bruce, B.; Fries, J.F. The Health Assessment Questionnaire (HAQ). Clin. Exp. Rheumatol. 2005, 23, S14–S18. [Google Scholar]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Couderc, M.; Pereira, B.; Schaeverbeke, T.; Thomas, T.; Chapurlat, R.; Gaudin, P.; Morel, J.; Dougados, M.; Soubrier, M. GlutenSpA trial: Protocol for a randomised double-blind placebo-controlled trial of the impact of a gluten-free diet on quality of life in patients with axial spondyloarthritis. BMJ 2020, 10, e038715. [Google Scholar]
- Haghighatdoost, F.; Bellissimo, N.; de Zepetnek, J.T.; Rouhani, M.H. Association of vegetarian diet with inflammatory biomarkers: A systematic review and meta-analysis of observational studies. Public Health Nutr. 2017, 20, 2713–2721. [Google Scholar] [CrossRef] [PubMed]
- Rennie, K.L.; Hughes, J.; Lang, R.; Jebb, S.A. Nutritional management of rheumatoid arthritis: A review of the evidence. J. Hum. Nutr. Diet. 2003, 16, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Badsha, H. Role of Diet in influencing rheumatoid arthritis disease activity. Open Rheumatol. J. 2018, 12, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Conner, E.M.; Grisham, M.B. Inflammation, free radicals, and antioxidants. Nutrition 1996, 12, 274–277. [Google Scholar] [CrossRef]
- Veselinovic, M.; Barudzic, N.; Vuletic, M.; Zivkovic, V.; Tomic-Lucic, A.; Djuric, D.; Jakovljevic, V. Oxidative stress in rheumatoid arthritis patients: Relationship to diseases activity. Mol. Cell. Biochem. 2014, 391, 225–232. [Google Scholar] [CrossRef]
- Martínez, J.A.; Etxeberría, U.; Galar, A.; Milagro, F. Role of dietary polyphenols and inflammatory processes on disease progression mediated by the gut microbiota. Rejuvenation Res. 2013, 16, 435–437. [Google Scholar] [CrossRef]
- Martini, D. Health benefits of Mediterranean diet. Nutrients 2019, 11, 1802. [Google Scholar] [CrossRef] [Green Version]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [Green Version]
- Estruch, R. Anti-inflammatory effects of the Mediterranean diet: The experience of the PREDIMED study. Proc. Nutr. Soc. 2010, 69, 333–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strzelecka, M.; Bzowska, M.; Kozieł, J.; Szuba, B.; Dubiel, O.; Núńez, D.R.; Heinrich, M.; Bereta, J. Anti-inflammatory effects of extracts from some traditional Mediterranean diet plants. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2005, 56, 139–156. [Google Scholar]
- Oliviero, F.; Spinella, P.; Fiocco, U.; Ramonda, R.; Sfriso, P.; Punzi, L. How the Mediterranean diet and some of its components modulate inflammatory pathways in arthritis. Swiss Med. Wkly. 2015, 145, w14190. [Google Scholar] [CrossRef] [PubMed]
- Infante, M.; Ricordi, C.; Padilla, N.; Alvarez, A.; Linetsky, E.; Lanzoni, G.; Mattina, A.; Bertuzzi, F.; Fabbri, A.; Baidal, D.; et al. The role of vitamin D and omega-3 pufas in islet transplantation. Nutrients 2019, 11, 2937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapoor, B.; Kapoor, D.; Gautam, S.; Singh, R.; Bhardwaj, S. Dietary polyunsaturated fatty acids (PUFAs): Uses and potential health benefits. Curr. Nutr. Rep. 2021, 10, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Simonetto, M.; Infante, M.; Sacco, R.L.; Rundek, T.; Della-Morte, D. A novel anti-inflammatory role of omega-3 PUFAs in prevention and treatment of atherosclerosis and vascular cognitive impairment and dementia. Nutrients 2019, 11, 2279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadaki, A.; Nolen-Doerr, E.; Mantzoros, C.S. The effect of the Mediterranean diet on metabolic health: A systematic review and meta-analysis of controlled trials in adults. Nutrients 2020, 12, 3342. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Mediterranean dietary pattern, inflammation and endothelial function: A systematic review and meta-analysis of intervention trials. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Morales-Ivorra, I.; Romera-Baures, M.; Roman-Viñas, B.; Serra-Majem, L. Osteoarthritis and the Mediterranean diet: A systematic review. Nutrients 2018, 10, 1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forsyth, C.; Kouvari, M.; D’Cunha, N.M.; Georgousopoulou, E.N.; Panagiotakos, D.B.; Mellor, D.D.; Kellett, J.; Naumovski, N. The effects of the Mediterranean diet on rheumatoid arthritis prevention and treatment: A systematic review of human prospective studies. Rheumatol. Int. 2017, 38, 737–747. [Google Scholar] [CrossRef]
- Mori, T.; Beilin, L.J. Omega-3 fatty acids and inflammation. Curr. Atheroscler. Rep. 2004, 6, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, S.K.; Frits, M.; Cui, J.; Zhang, Z.Z.; Mahmoud, T.; Iannaccone, C.; Lin, T.-C.; Yoshida, K.; Weinblatt, M.E.; Shadick, N.A.; et al. Diet and rheumatoid arthritis symptoms: Survey results from a rheumatoid arthritis registry. Arthritis Rheum. 2017, 69, 1920–1925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panush, R.S. Does food cause or cure arthritis? Rheum. Dis. Clin. N. Am. 1991, 17, 259–272. [Google Scholar] [CrossRef]
- Alwarith, J.; Kahleova, H.; Rembert, E.; Yonas, W.; Dort, S.; Calcagno, M.; Burgess, N.; Crosby, L.; Barnard, N.D. Nutrition interventions in rheumatoid arthritis: The potential use of plant-based diets. A review. Front. Nutr. 2019, 6, 141. [Google Scholar] [CrossRef] [PubMed]
- Itzlinger, A.; Branchi, F.; Elli, L.; Schumann, M. Gluten-free diet in celiac disease—Forever and for all? Nutrients 2018, 10, 1796. [Google Scholar] [CrossRef] [Green Version]
- De Punder, K.; Pruimboom, L. The dietary intake of wheat and other cereal grains and their role in inflammation. Nutrients 2013, 5, 771–787. [Google Scholar] [CrossRef] [Green Version]
- Tugwell, P.; Boers, M.; Brooks, P.; Simon, L.; Strand, V.; Idzerda, L. OMERACT: An international initiative to improve outcome measurement in rheumatology. Trials 2007, 8, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malm, K.; Bremander, A.; Arvidsson, B.; Andersson, M.L.E.; Bergman, S.; Larsson, I. The influence of lifestyle habits on quality of life in patients with established rheumatoid arthritis—A constant balancing between ideality and reality. Int. J. Qual. Stud. Health Well-Being 2016, 11, 30534. [Google Scholar] [CrossRef]
- Kataria, S.; Ravindran, V. Digital health: A new dimension in rheumatology patient care. Rheumatol. Int. 2018, 38, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Gold, N.; Yau, A.; Rigby, B.; Dyke, C.; Remfry, E.A.; Chadborn, T. Effectiveness of digital interventions for reducing behavioral risks of cardiovascular disease in nonclinical adult populations: Systematic review of reviews. J. Med. Internet Res. 2021, 23, e19688. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.T.; Allman-Farinelli, M.; Chen, J.; Partridge, S.R.; Collins, C.; Rollo, M.; Haslam, R.; Diversi, T.; Campbell, K.L. Dietitians Australia position statement on telehealth. Nutr. Diet. 2020, 77, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Michie, S.; Prestwich, A. Are interventions theory-based? Development of a theory coding scheme. Health Psychol. 2010, 29, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen-Kragh, J. Mediterranean diet intervention in rheumatoid arthritis. Ann. Rheum. Dis. 2003, 62, 193–195. [Google Scholar] [CrossRef] [Green Version]

| PICOS | Inclusion/Exclusion Criteria |
|---|---|
| Population | Inclusion: Adults with a definite diagnosis of RA according to a specified diagnostic criterion Exclusion: All animal and pediatric studies, or studies involving women who are pregnant/breastfeeding |
| Intervention | Inclusion:
|
| Comparator | Inclusion:
|
| Outcomes | Inclusion: Studies that reported the effect of diet with or without omega-3 supplementation on aspects of RA management, i.e., symptom control, clinical and biochemical measures, disease activity Exclusion: Studies that investigated the effect of a dietary intervention on the risk of developing RA were not included. |
| Study design | Inclusion: The current systematic review included randomized and non-randomized controlled trials. Publications were eligible if they were published in peer-reviewed scientific journals and were written in English language. Exclusion: Reviews, cohort studies, cross-sectional studies, case–control studies, conference abstracts, editorials, letters, and reviews. |
| Author (Year) | Country | Study Design | Participants’ Characteristics | Intervention | Comparator | Duration | Primary Outcome Measures | Results (Post- Intervention Changes) | |
|---|---|---|---|---|---|---|---|---|---|
| Within Group | Between Groups | ||||||||
| Skoldstam et al. (1979) [30] | Sweden | RCT | n = 26 Mean age: 53 yrs Sex: 73% f | 7–10 days fasting followed by 9-week lactovegetarian diet | Habitual diet | 10 weeks | Pain, EMS, dose of NSAIDs | NSD | |
| Panush et al. (1983) [31] | US | RCT | n = 33 Mean age: 55 yrs Sex: 34.6% f | Diet free of additives, preservatives, fruit, red meat, herbs, and dairy products | Placebo diet | 10 weeks | EMS, number of tender and swollen joints, grip strength, patient and examiner assessment, walk time, ESR, RF, Hct/C3/C4 | NSD | |
| Darlington et al. (1986) [32] | UK | RCT | n = 45 Mean age: not reported Sex: 89% f | Elimination diet Week 1: tolerated foods followed by reintroduction of foods that are unlikely to cause intolerance followed by habitual diet | Habitual diet | 6 weeks | Pain, EMS, grip strength, number of painful joints | NSD | Inadequate reporting |
| Magaro et al. (1988) [33] | Italy | RCT | n = 12 Mean age: Group A: 37 yrs Group B: 36 yrs Sex: 100% f | Group B: Diet high in PUFAs (P:S ratio 5:0) + fish oil supplement (1.6 g EPA/d and 1.1 g DHA/d) | Group A: Diet high in saturated fatty acids (P:S ratio 1:33) | 4 weeks | DAS28, neutrophil chemiluminescence, Ritchie’s index, EMS, grip strength | Significant improvements in Group B: Ritchie’s inde X(17.2 (3.38) to 10.6 (3.48)); (p < 001), EMS (33 (7.34) to 22 (8.45)) mins; (p < 001); Grip strength (116 (13–26) to 136 (12–88)) mmHg; (p < 001) | Significant differences in: Ritchie’s index (Group B: 10.6 (3.48) vs. Group A: 21–4 (3.2); (p < 0.005) EMS (Group B: 22 (8.45) vs. Group A:36 (10.17) minutes; (p < 0.01) Grip strength (Group B:136 (12–88) vs. Group A: 104 (21–58) mmHg; (p < 0.01) |
| Kjeldsen-Kragh et al. (1991) [34] | Norway | RCT | n = 53 Mean age: 4.5 years Sex: 85% f | 7–10 days: fasting followed by 3·5 months: gluten-free vegan diet followed by 9 months: vegetarian diet | Habitual diet | 13 months | Grip strength, Ritchie index, EMS, Global assessment, Number of tender and swollen joints, pain HAQ, ESR, CRP, white blood cells/platelet count | Significant improvements in the intervention group for: Grip strength (p < 0.0005), Ritchie Index (p < 0.0004), EMS (p < 0.0002); Number of tender joints (p < 0.0002), Number of swollen joints (p < 0.04), Pain (VAS) (p < 0.0001 for intervention group and p < 0.02 for control), HAQ (p < 0.0001), ESR (p < 0.002), CRP (p < 0.005) White blood cells/platelet count decreased significantly in the intervention group (p < 0.0010) and in the control group (p < 0.006) | Significant improvement in the intervention group as compared with control for: Grip strength (p < 0.02), Ritchie index (p < 0.0004), EMS (p < 0.0001), Global assessment (p < 0.0001), Number of tender joints (p < 0.0001), Number of swollen joints (p < 0.02), pain (p < 0.02), HAQ (p < 0.0001), ESR (p < 0.001), CRP (p < 0.0001) |
| Van de Laar and van der Korst (1992) [35] | Netherlands | RCT | n = 94 Mean age: 58 yrs Sex: 70% f | Allergen free diet | Allergen restricted diet | 12 weeks | EMS, number of tender and swollen joints, Ritchie’s index, grip strength, global assessment, ESR, CRP, walking time | Significant decrease in body weight in the allergen free diet group (p = 0.016) | NSD |
| Haugen et al. (1994) [28] | Norway | NRCT | n = 17 Mean age: 50 yrs Sex: 80% f | Elemental diet (E028) | Soup that included: milk, meat, fish, shellfish, orange, pineapples, tomatoes, peas and flour of wheat and corn | 3 weeks | Ritchie‘s index, number of tender and swollen joints, grip strength, EMS, pain, ESR, CRP, hemoglobin, albumin and erythrocyte count, global assessment | Number of tender joints decreased significantly in the intervention group (p = 0.04) ESR and thrombocyte count improved in the control group (p = 0.03) and (p = 0.02), respectively | NSD |
| Kavanagh et al. (1995) [36] | UK | RCT | n = 47 Mean age: 45.6 yrs Sex: 78.7% f | E028 followed by reintroduction of food | Habitual diet with E028 | 4 weeks | ESR, CRP, Ritchie’s index, thermographic score, grip strength, functional score | Significant improvements in the intervention group for: Ritchie’s index (12.6 ± 6.8 to 10.4 ± 7.2) (p = 0.006), Grip strength (140.2 ± 96 to 155.9 ± 98.3 mmHg) (p = 0.008) | NSD |
| Hansen et al. (1996) [37] | Denmark | RCT | n = 109 Mean age:57 yrs Sex: 74.6% f | Graastener diet: 20–30% fat, 1.5 g/kg BW protein, 800 g fresh fish per week | Habitual diet | 4 months | Number of tender and swollen joints, pain, HAQ, Global assessment, acute phase reactant, X-ray, EMS | Authors state: ‘Significant improvement in the duration of morning stiffness, number of swollen joints, pain status’ | NSD |
| Nenonen et al. (1998) [38] | Finland | RCT | n = 43 Mean age:53 yrs Sex: 83% f | Uncooked vegan diet | Habitual diet | 3 months | Pain, number of swollen joints, number of tender joints, EMS, HAQ, Ritchie’s index, CRP, ESR | NSD | |
| Holst-Jensen et al. (1998) [39] | Denmark | RCT | n = 30 Mean age: 49.5 yrs Sex: 80% f | Commerical liquid elemental diet (top upTM Standard, Ferrosan Ltd., Denmark) | Habitual diet | 4 months | EMS, HAQ, number of swollen joints, pain, Ritchie’s index, global assessment, ESR | EMS decreased significantly in the control group (3.5 to 2.5 min) (p < 0.05) Ritchie’s inde Xdecreased significantly in the control group (12.5 to 10) (p < 0.05) | Significant reductions in the intervention group as compared with control for: Number of tender joints (7 vs. 9) (p = 0.006), ESR (40 vs. 47 mm/h) (p = 0.018) |
| Fraser et al. (2000) [29] | Norway | NRCT | n = 23 Fasting group: Mean age: 49 yrs, Sex: 90% f Ketogenic group: Mean age:44 yrs, Sex: 92% f | 7-day ketogenic diet | 7-day fast | 1 week | IL-6, DHEAS | IL-6 decreased significantly after fasting for 7 days (35.5 to 22.5 pg/mL) (p < 0.05) DHEAS increased significantly after fasting for 7 days (3.28 to 4.40 mmol/L) (p < 0.01) and after a 7-day ketogenic diet group (2.42 to 3.23 mmol/L) (p < 0.01) | Not reported |
| Sarzi-Puttini et al. (2000) [40] | Italy | RCT | n = 50 Mean age:50 yrs Sex: 78% f | Diet free from: wheat meal, eggs, milk, strawberries and acid fruit, tomato, chocolate, crustacean, dried fruit Lean cuts of red meat allowed | Diet containing common allergenic foods | 24 weeks | EMS, HAQ, number of tender and swollen joints, pain, Ritchie’s index | Number of tender and swollen joints decreased significantly in the intervention group (9.5 ± 4.1 to 7.1 ± 3.2) (p = 0.031) and (6.4 ± 3.1 to 5.1 ± 2.3) (p = 0.002), respectively Ritchie’s inde Xdecreased significantly in the intervention group (13.2 ± 4.4 to 9.2 ± 3.8) (p = 0.002) | Not reported |
| Hafstrom et al. (2001) [41] | Sweden | RCT | n = 66 Mean age: 50 yrs Sex: not reported | Gluten free vegan diet | Well-balanced non-vegan diet | 12 months | IgG, IgA, radiographic progression | IgG anti-gliadin decreased significantly in the vegan diet group (5 to 2) (p = 0.0183) IgA anti-gliadin decreased significantly in the non-vegan diet group (14.5 to 12.5) (p = 0.0201) Modified Larsen score, number of erosions and the joint count improved significantly in both groups | NSD |
| Skoldstam et al. (2003) [42] | Sweden | RCT | n = 56 Mean age: 58.5 yrs Sex: = 82% f | Cretan Mediterranean diet (MD) | Habitual diet (HD) | 12 weeks | DAS 28, HAQ, SF-36, dose of NSAIDs | DAS28 decreased significantly in MD group (4.4 to 3.9) (p < 0.001) HAQ decreased significantly in MD group (0.7 to 0.6) (p = 0.02) Improvement in vitality (+11.3) (p = 0.018) and overall health compared to one year earlier (−0.6) (p = 0.016) in the SF- 36 in MD group | Significant improvements in MD group as compared to control group for: DAS28 (3.9 for MD vs. 4.3 for control) (p = 0.047) HAQ: (0.6 for MD vs. 0.8 for control) (p = 0.012) |
| Adam et al. (2003) [43] | Germany | RCT Double-blind crossover | n = 68 Mean age: 57.4 ± 12.8 yrs Sex: 93.3% f | Anti-inflammatory diet (AID) Patients in both diet groups were assigned to receive either placebo or fish oil capsules (30 mg/kg body weight) | Western diet (WD) | 6 months | Global assessment, pain, grip strength, EMS, HAQ, Number of tender and swollen joints, blood cells, cytokines, eicosanoids, dose of Corticosteroids, CRP, LBT4, TNF-α | CRP decreased significantly for individuals in both WD and AID groups who are on methotrexate when fish oil was supplemented (2.03 ± 1.8 mg/dL vs. 1.69 ± 1.5 mg/dL) (p < 0.05) Number of tender joints improved significantly in AID group when fish oil was supplemented in months 5,6,7,8 (37% improvement) (p < 0.001) LTB4 decreased significantly in AID group when fish oil was supplemented for 3 months (p = 0.009) Dose of corticosteroid decreased significantly in both WD and AID groups after 3 months of fish oil supplementation (p = 0.027 for WD group, p = 0.022 for AID group) TNF-α decreased significantly in both WD and AID groups when fish oil was supplemented for months 6,7, 8 (p = 0.004) | The number of tender and swollen improved significantly in the AID group as compared to WD group (28% vs. 11%) and (34% vs. 22%) (p < 0.01), respectively Patients’ and physicians’ global assessment of disease activity and patients’ assessments of pain improved significantly more in the AID group as compared to WD group (p < 0.05) |
| McKellar et al. (2007) [44] | Scotland | RCT | n = 130 Mean age: 54 yrs Sex: 100% f | Mediterranean diet (MD) | Healthy diet | 5 months | Number of tender and swollen joints, patient global assessment, pain, EMS, DAS28, HAQ, ESR, CRP, IL-6 | Not reported | Significant improvements in the intervention group as compared with the control group for: patient global assessment (p = 0.002), pain (p = 0.049) and EMS (p = 0.041) |
| Elkan et al. (2008) [45] | Sweden | RCT | n = 58 Vegan group: Mean age: 49.9 yrs, 93.3% f Non-vegan group Mean age:50.8 yrs, 85.6% f | Gluten- free vegan diet | Well-balanced non-vegan diet | 12 months | oxLDL, anti-PCs | OxLDL decreased Significantly in the vegan diet group (54.7 to 48.6) (p = 0.09) | Anti-PC IgM was significantly higher in vegan group (F = 8.0, p = 0.0006) |
| Vadell et al. (2020) [46] | Sweden | RCT | n = 50 Mean age: 61 ± 12 yrs Sex: 77% f | Diet rich in anti-inflammatory foods | Habitual diet | 10 weeks | DAS28-ESR | DAS28-ESR decreased significantly in the intervention group (3.39 to 3.05) (p = 0.012) | NSD |
| Lederer et al. (2020) [47] | Germany | RCT | n = 53 Mean age: 31 yrs Sex: 63% f | Vegan diet (VD) | Meat rich diet | 5 weeks | Sialylated antibodies, percentage of regulatory T-cells, IL-10 | Significant improvement in: Sialylated antibodies in VD (0.8 ± 0.4 to 1.4 ± 1.4) (p = 0.023) and in the meat rich group (0.9 ± 0.5 to 1.6 ± 1.2) (p = 0.010) T-cells in VD group (6.0 ± 1.7% to 7.1 ± 1.9%) (p < 0.001) and in meat rich group (6.3 ± 2.2% to 7.7 ± 2.4%) (p < 0.001) | NSD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raad, T.; Griffin, A.; George, E.S.; Larkin, L.; Fraser, A.; Kennedy, N.; Tierney, A.C. Dietary Interventions with or without Omega-3 Supplementation for the Management of Rheumatoid Arthritis: A Systematic Review. Nutrients 2021, 13, 3506. https://doi.org/10.3390/nu13103506
Raad T, Griffin A, George ES, Larkin L, Fraser A, Kennedy N, Tierney AC. Dietary Interventions with or without Omega-3 Supplementation for the Management of Rheumatoid Arthritis: A Systematic Review. Nutrients. 2021; 13(10):3506. https://doi.org/10.3390/nu13103506
Chicago/Turabian StyleRaad, Tala, Anne Griffin, Elena S. George, Louise Larkin, Alexander Fraser, Norelee Kennedy, and Audrey C. Tierney. 2021. "Dietary Interventions with or without Omega-3 Supplementation for the Management of Rheumatoid Arthritis: A Systematic Review" Nutrients 13, no. 10: 3506. https://doi.org/10.3390/nu13103506
APA StyleRaad, T., Griffin, A., George, E. S., Larkin, L., Fraser, A., Kennedy, N., & Tierney, A. C. (2021). Dietary Interventions with or without Omega-3 Supplementation for the Management of Rheumatoid Arthritis: A Systematic Review. Nutrients, 13(10), 3506. https://doi.org/10.3390/nu13103506







