Clinical Implications of Malnutrition in the Management of Patients with Pancreatic Cancer: Introducing the Concept of the Nutritional Oncology Board
Abstract
:1. Introduction
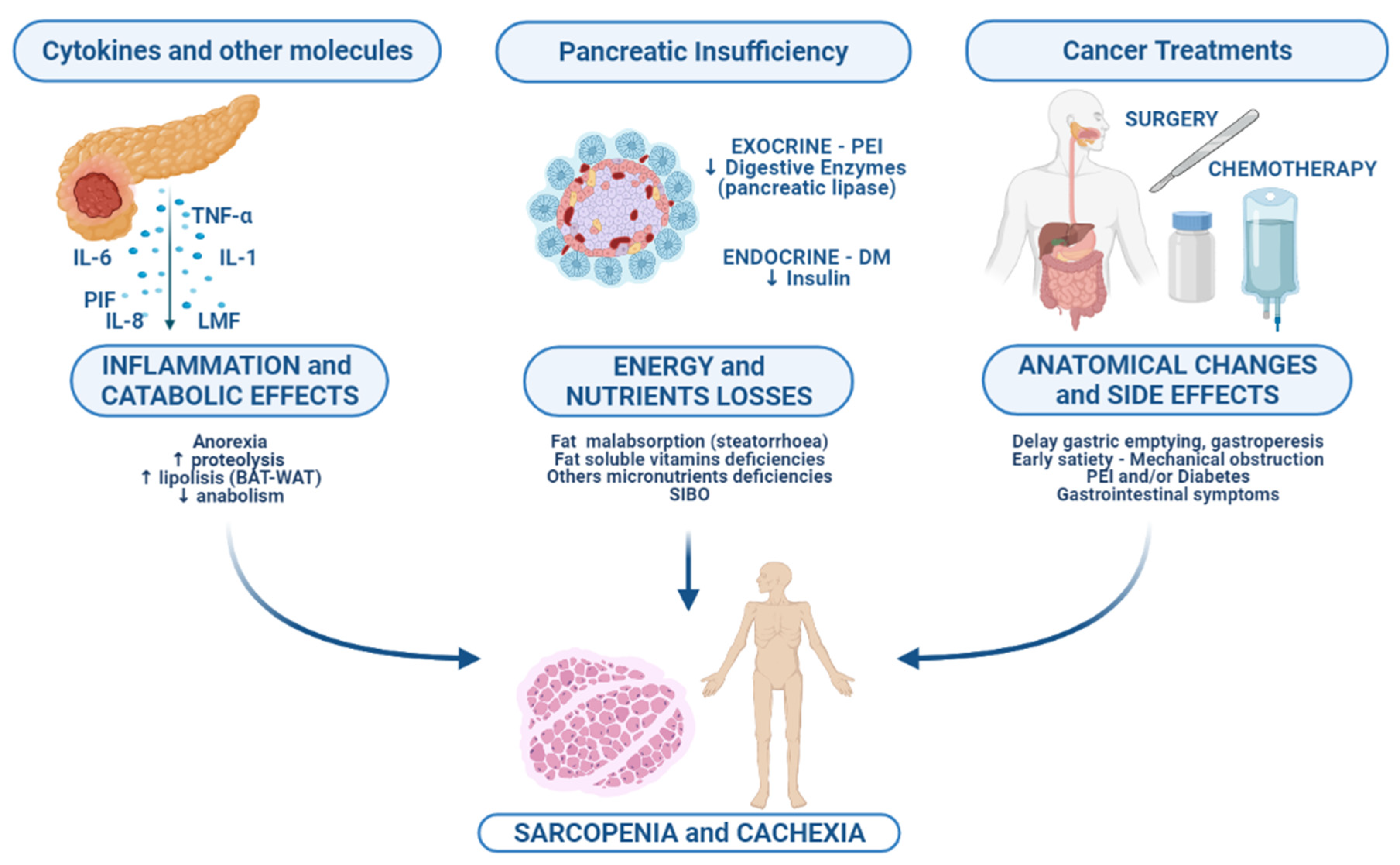
2. Biological Basis of Malnutrition
2.1. Inflammation and Its Catabolic Consequences
2.2. Pancreatic Exocrine Insufficiency and Treatment
2.3. Cancer Anatomic Changes
3. Impact of Malnutrition on Clinical Outcome
3.1. Neoadjuvant and Preoperatory Setting
3.2. Surgical Setting
3.3. Metastatic Setting
Author Contributions
Funding
Conflicts of Interest
References
- AIRTUM Working Group. I Numeri del Cancro in Italia 2019. Pancreas Esocrino. pp. 169–172. Available online: https://www.aiom.it/wp-content/uploads/2019/09/2019_Numeri_Cancro-operatori-web.pdf (accessed on 4 October 2021).
- Tumas, J.; Tumiene, B.; Jurkeviciene, J.; Jasiunas, E.; Sileikis, A. Nutritional and Immune Impairments and Their Effects on Outcomes in Early Pancreatic Cancer Patients Undergoing Pancreatoduodenectomy. Clin. Nutr. 2020, 39, 3385–3394. [Google Scholar] [CrossRef]
- Kamarajah, S.K.; Bundred, J. Comments on: Sarcopenia and Sarcopenic Obesity are Significantly Associated with Poorer Overall Survival in Patients with Pancreatic Cancer: Systematic Review and Meta-Analysis. Int. J. Surg. 2019, 66, 99–100. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.; Denecke, A.; Klapdor, S.; Klapdor, R. Parenteral Nutrition Support for Patients with Pancreatic Cancer—Improvement of the Nutritional Status and the Therapeutic Outcome. Anticancer Res. 2012, 32, 2111–2118. [Google Scholar] [PubMed]
- Latenstein, A.E.J.; Dijksterhuis, W.P.M.; Mackay, T.M.; Beijer, S.; Van Eijck, C.H.J.; De Hingh, I.H.J.T.; Molenaar, I.Q.; Van Oijen, M.G.H.; Van Santvoort, H.C.; De Van Der Schueren, M.A.E.; et al. Cachexia, Dietetic Consultation, and Survival in Patients with Pancreatic and Periampullary Cancer: A Multicenter Cohort Study. Cancer Med. 2020, 9, 9385–9395. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and Classification of Cancer Cachexia: An International Consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Sobotka, L. Basics in Clinical Nutrition; Publishing House Galén: Prague, Czech Republic, 2004; pp. 31–37. [Google Scholar]
- Soeters, P.B.; Reijven, P.L.; Schueren, M.A.V.B.-D.V.D.; Schols, J.M.; Halfens, R.J.; Meijers, J.M.; van Gemert, W.G. A Rational Approach to Nutritional Assessment. Clin. Nutr. 2008, 27, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Punyanitya, M.; Wang, Z.; Gallagher, D.; St.-Onge, M.-P.; Albu, J.; Heymsfield, S.B.; Heshka, S. Total Body Skeletal Muscle and Adipose Tissue Volumes: Estimation from a Single Abdominal Cross-Sectional Image. J. Appl. Physiol. 2004, 97, 2333–2338. [Google Scholar] [CrossRef] [Green Version]
- Hilmi, M.; Jouinot, A.; Burns, R.; Pigneur, F.; Mounier, R.; Gondin, J.; Neuzillet, C.; Goldwasser, F. Body Composition and Sarcopenia: The Next-Generation of Personalized Oncology and Pharmacology? Pharmacol. Ther. 2019, 196, 135–159. [Google Scholar] [CrossRef]
- Porporato, P.E. Understanding Cachexia as a Cancer Metabolism Syndrome. Oncogenesis 2016, 5, e200. [Google Scholar] [CrossRef] [Green Version]
- Petruzzelli, M.; Wagner, E.F. Mechanisms of Metabolic Dysfunction in Cancer-Associated Cachexia. Genes Dev. 2016, 30, 489–501. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.R.; Yaffee, P.M.; Jamil, L.H.; Lo, S.K.; Nissen, N.; Pandol, S.J.; Tuli, R.; Hendifar, A.E. Pancreatic Cancer Cachexia: A Review of Mechanisms and Therapeutics. Front. Physiol. 2014, 5, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoyagi, T.; Terracina, K.P.; Raza, A.; Matsubara, H.; Takabe, K. Cancer Cachexia, Mechanism and Treatment. World J. Gastrointest. Oncol. 2015, 7, 17–29. [Google Scholar] [CrossRef]
- Gilliland, T.M.; Villafane-Ferriol, N.; Shah, K.P.; Shah, R.M.; Cao, H.S.T.; Massarweh, N.N.; Silberfein, E.J.; Choi, E.A.; Hsu, C.; McElhany, A.L.; et al. Nutritional and Metabolic Derangements in Pancreatic Cancer and Pancreatic Resection. Nutrients 2017, 9, 243. [Google Scholar] [CrossRef] [PubMed]
- Martignoni, M.E.; Kunze, P.; Hildebrandt, W.; Künzli, B.; Berberat, P.; Giese, T.; Klöters, O.; Hammer, J.; Büchler, M.W.; Giese, N.A.; et al. Role of Mononuclear Cells and Inflammatory Cytokines in Pancreatic Cancer-Related Cachexia. Clin. Cancer Res. 2005, 11, 5802–5808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Riordain, M.G.; Falconer, J.S.; Maingay, J.; Fearon, K.C.; Ross, J.A. Peripheral Blood Cells from Weight-Losing Cancer Patients Control the Hepatic Acute Phase Response by a Primarily Interleukin-6 Dependent Mechanism. Int. J. Oncol. 1999, 15, 823–827. [Google Scholar] [CrossRef]
- Guttridge, D.C.; Mayo, M.W.; Madrid, L.V.; Wang, C.-Y.; Baldwin, A.S., Jr. NF-kappa B-Induced Loss of MyoD Messenger RNA: Possible Role in Muscle Decay and Cachexia. Science 2000, 289, 2363–2366. [Google Scholar] [CrossRef] [Green Version]
- Yakovenko, A.; Cameron, M.; Trevino, J.G. Molecular Therapeutic Strategies Targeting Pancreatic Cancer Induced Cachexia. World J. Gastrointest. Surg. 2018, 10, 95–106. [Google Scholar] [CrossRef]
- Judge, S.M.; Nosacka, R.L.; Delitto, D.; Gerber, M.H.; Cameron, M.E.; Trevino, J.G.; Judge, A.R. Skeletal Muscle Fibrosis in Pancreatic Cancer Patients with Respect to Survival. JNCI Cancer Spectr. 2018, 2, 43. [Google Scholar] [CrossRef]
- Tisdale, M.J. Wasting in Cancer. J. Nutr. 1999, 129, 243S–246S. [Google Scholar] [CrossRef] [Green Version]
- Dev, R.; Bruera, E.; Dalal, S. Insulin Resistance and Body Composition in Cancer Patients. Ann. Oncol. 2018, 29, ii18–ii26. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, W.; Garcia-Prieto, C.; Huang, P. The Warburg Effect and Its Cancer Therapeutic Implications. J. Bioenerg. Biomembr. 2007, 39, 267–274. [Google Scholar] [CrossRef]
- Dunn, A.J. Effects of Cytokines and Infections on Brain Neurochemistry. Clin. Neurosci. Res. 2006, 6, 52–68. [Google Scholar] [CrossRef] [Green Version]
- Collins, P.; Bing, C.; McCulloch, P.B.; Williams, G.M. Muscle UCP-3 mRNA Levels are Elevated in Weight Loss Associated with Gastrointestinal Adenocarcinoma in Humans. Br. J. Cancer 2002, 86, 372–375. [Google Scholar] [CrossRef]
- Kandarian, S.C.; Nosacka, R.L.; Delitto, A.E.; Judge, A.R.; Judge, S.; Ganey, J.D.; Moreira, M.J.; Jackman, R.W. Tumour-Derived Leukaemia Inhibitory Factor is a Major Driver of Cancer Cachexia and Morbidity in C26 Tumour-Bearing Mice. J. Cachexia Sarcopenia Muscle 2018, 9, 1109–1120. [Google Scholar] [CrossRef]
- Sanders, P.M.; Tisdale, M.J. Role of Lipid-Mobilising Factor (LMF) in Protecting Tumour Cells from Oxidative Damage. Br. J. Cancer 2004, 90, 1274–1278. [Google Scholar] [CrossRef] [Green Version]
- Lorite, M.J.; Thompson, M.G.; Drake, J.L.; Carling, G.; Tisdale, M.J. Mechanism of Muscle Protein Degradation Induced by a Cancer Cachectic Factor. Br. J. Cancer 1998, 78, 850–856. [Google Scholar] [CrossRef]
- Whitehouse, A.S.; Tisdale, M.J. Increased Expression of the Ubiquitin—Proteasome Pathway in Murine Myotubes by Proteolysis-Inducing Factor (PIF) is Associated with Activation of the Transcription Factor NF-κB. Br. J. Cancer 2003, 89, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Eley, H.L.; Russell, S.T.; Baxter, J.H.; Mukerji, P.; Tisdale, M.J. Signaling Pathways Initiated by β-hydroxy-β-methylbutyrate to Attenuate the Depression of Protein Synthesis in Skeletal Muscle in Response to Cachectic Stimuli. Am. J. Physiol. Metab. 2007, 293, E923–E931. [Google Scholar] [CrossRef] [PubMed]
- Roubenoff, R. Excess Baggage: Sarcopenia, Obesity, and Cancer Outcomes. Lancet Oncol. 2008, 9, 605–607. [Google Scholar] [CrossRef]
- Sandini, M.; Bernasconi, D.P.; Fior, D.; Molinelli, M.; Ippolito, D.; Nespoli, L.; Caccialanza, R.; Gianotti, L. A High Visceral Adipose Tissue-to-Skeletal Muscle Ratio as a Determinant of Major Complications after Pancreatoduodenectomy for Cancer. Nutrients 2016, 32, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Nishigori, T.; Tsunoda, S.; Okabe, H.; Tanaka, E.; Hisamori, S.; Hosogi, H.; Shinohara, H.; Sakai, Y. Impact of Sarcopenic Obesity on Surgical Site Infection after Laparoscopic Total Gastrectomy. Ann. Surg. Oncol. 2016, 23, 524–531. [Google Scholar] [CrossRef]
- Pecorelli, N.; Carrara, G.; DE Cobelli, F.; Cristel, G.; Damascelli, A.; Balzano, G.; Beretta, L.; Braga, M. Effect of Sarcopenia and Visceral Obesity on Mortality and Pancreatic Fistula Following Pancreatic Cancer Surgery. Br. J. Surg. 2016, 103, 434–442. [Google Scholar] [CrossRef]
- Tan, B.H.; Birdsell, L.A.; Martin, L.; Baracos, V.E.; Fearon, K.C. Sarcopenia in an Overweight or Obese Patient Is an Adverse Prognostic Factor in Pancreatic Cancer. Clin. Cancer Res. 2009, 15, 6973–6979. [Google Scholar] [CrossRef] [Green Version]
- Dalal, S.; Hui, D.; Bidaut, L.; Lem, K.; Del Fabbro, E.; Crane, C.; Reyes-Gibby, C.C.; Bedi, D.; Bruera, E. Relationships Among Body Mass Index, Longitudinal Body Composition Alterations, and Survival in Patients with Locally Advanced Pancreatic Cancer Receiving Chemoradiation: A Pilot Study. J. Pain Symptom Manag. 2012, 44, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Rollins, K.; Tewari, N.; Ackner, A.; Awwad, A.; Madhusudan, S.; Macdonald, I.; Fearon, K.C.; Lobo, D.N. The Impact of Sarcopenia And Myosteatosis on Outcomes of Unresectable Pancreatic Cancer or Distal Cholangiocarcinoma. Clin. Nutr. 2016, 35, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and Clinical Implications of Sarcopenic Obesity in Patients with Solid Tumours of The Respiratory and Gastrointestinal Tracts: A Population-Based Study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Vujasinovic, M.; Valente, R.; Del Chiaro, M.; Permert, J.; Löhr, J.-M. Pancreatic Exocrine Insufficiency in Pancreatic Cancer. Nutrients 2017, 9, 183. [Google Scholar] [CrossRef] [PubMed]
- Lindkvist, B. Diagnosis and Treatment of Pancreatic Exocrine Insufficiency. World J. Gastroenterol. 2013, 19, 7258–7266. [Google Scholar] [CrossRef] [PubMed]
- De La Iglesia, D.; Avci, B.; Kiriukova, M.; Panic, N.; Bozhychko, M.; Sandru, V.; De-Madaria, E.; Capurso, G. Pancreatic Exocrine Insufficiency and Pancreatic Enzyme Replacement Therapy in Patients with Advanced Pancreatic Cancer: A Systematic Review and Meta-Analysis. United Eur. Gastroenterol. J. 2020, 8, 1115–1125. [Google Scholar] [CrossRef]
- Azer, S.; Sankararaman, S. Steatorrhea; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Halloran, C.; Cox, T.; Chauhan, S.; Raraty, M.G.; Sutton, R.; Neoptolemos, J.; Ghaneh, P. Partial Pancreatic Resection for Pancreatic Malignancy Is Associated with Sustained Pancreatic Exocrine Failure and Reduced Quality of Life: A Prospective Study. Pancreatology 2011, 11, 535–545. [Google Scholar] [CrossRef]
- Partelli, S.; Frulloni, L.; Minniti, C.; Bassi, C.; Barugola, G.; D’Onofrio, M.; Crippa, S.; Falconi, M. Faecal Elastase-1 is an Independent Predictor of Survival in Advanced Pancreatic Cancer. Dig. Liver Dis. 2012, 44, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Sikkens, E.C.; Cahen, D.L.; de Wit, J.; Looman, C.W.; van Eijck, C.; Bruno, M.J. A Prospective Assessment of the Natural Course of the Exocrine Pancreatic Function in Patients with a Pancreatic Head Tumor. J. Clin. Gastroenterol. 2014, 48, e43–e46. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.M.; Joo, J.; Kim, S.Y.; Park, S.-J.; Han, S.-S.; Kim, T.H.; Koh, Y.H.; Chung, S.H.; Kim, Y.-H.; Moon, H.; et al. Efficacy of Pancreatic Exocrine Replacement Therapy for Patients with Unresectable Pancreatic Cancer in a Randomized Trial. Pancreatology 2016, 16, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Hirano, K.; Isayama, H.; Nakai, Y.; Saito, K.; Umefune, G.; Akiyama, D.; Watanabe, T.; Takagi, K.; Hamada, T.; et al. The Role of Pancreatic Enzyme Replacement Therapy in Unresectable Pancreatic Cancer. Pancreas 2017, 46, 341–346. [Google Scholar] [CrossRef]
- Saito, T.; Nakai, Y.; Isayama, H.; Hirano, K.; Ishigaki, K.; Hakuta, R.; Takeda, T.; Saito, K.; Umefune, G.; Akiyama, D.; et al. A Multicenter Open-Label Randomized Controlled Trial of Pancreatic Enzyme Replacement Therapy in Unresectable Pancreatic Cancer. Pancreas 2018, 47, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.M.; Newcomer, A.D.; Moertel, C.G.; Go, V.L.W.; DiMagno, E.P. Assessment of Weight Loss, Food Intake, Fat Metabolism, Malabsorption, and Treatment of Pancreatic Insufficiency in Pancreatic Cancer. Cancer 1983, 52, 346–352. [Google Scholar] [CrossRef]
- van Nierop, J.E.W.; Lochtenberg-Potjes, C.M.; Wierdsma, N.J.; Scheffer, H.J.; Kazemier, G.; Ottens-Oussoren, K.; Meijerink, M.R.; de van der Schueren, M.A.E. Assessment of Nutritional Status, Digestion and Absorption, and Quality of Life in Patients with Locally Advanced Pancreatic Cancer. Gastroenterol. Res. Pract. 2017, 2017, 6193765. [Google Scholar] [CrossRef] [Green Version]
- Pezzilli, R.; Caccialanza, R.; Capurso, G.; Brunetti, O.; Milella, M.; Falconi, M. Pancreatic Enzyme Replacement Therapy in Pancreatic Cancer. Cancers 2020, 12, 275. [Google Scholar] [CrossRef] [Green Version]
- Petzel, M.Q.B.; Hoffman, L. Nutrition Implications for Long-Term Survivors of Pancreatic Cancer Surgery. Nutr. Clin. Pract. 2017, 32, 588–598. [Google Scholar] [CrossRef]
- Memba, R.; Duggan, S.N.; Ni Chonchubhair, H.M.; Griffin, O.M.; Bashir, Y.; O’Connor, D.B.; Murphy, A.; McMahon, J.; Volcov, Y.; Ryan, B.; et al. The Potential Role of Gut Microbiota in Pancreatic Disease: A Systematic Review. Pancreatology 2017, 17, 867–874. [Google Scholar] [CrossRef]
- Rickels, M.R.; Norris, A.W.; Hull, R.L. A Tale of Two Pancreases: Exocrine Pathology and Endocrine Dysfunction. Diabetologia 2020, 63, 2030–2039. [Google Scholar] [CrossRef]
- Chung, K.M.; Singh, J.; Lawres, L.; Dorans, K.J.; Garcia, C.; Burkhardt, D.; Robbins, R.; Bhutkar, A.; Cardone, R.; Zhao, X.; et al. Endocrine-Exocrine Signaling Drives Obesity-Associated Pancreatic Ductal Adenocarcinoma. Cell 2020, 181, 832–847. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Oldfield, L.; Ney, A.; Hart, P.A.; Keane, M.G.; Pandol, S.J.; Li, D.; Greenhalf, W.; Jeon, C.Y.; Koay, E.J.; et al. Early Detection of Pancreatic Cancer. Lancet Gastroenterol. Hepatol. 2020, 5, 698–710. [Google Scholar] [CrossRef]
- Kang, J.S.; Jang, J.-Y.; Kang, M.J.; Kim, E.; Jung, W.; Chang, J.; Shin, Y.; Han, Y.; Kim, S.-W. Endocrine Function Impairment after Distal Pancreatectomy: Incidence and Related Factors. World J. Surg. 2016, 40, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, S.; Matsumoto, I.; Toyama, H.; Shinzeki, M.; Ajiki, T.; Fukumoto, T.; Ku, Y. Pancreatic Volumetric Assessment as a Predictor of New-Onset Diabetes Following Distal Pancreatectomy. J. Gastrointest. Surg. 2012, 16, 2212–2219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rault, A.; SaCunha, A.; Klopfenstein, D.; Larroudé, D.; Epoy, F.N.D.; Collet, D.; Masson, B. Pancreaticojejunal Anastomosis is Preferable to Pancreaticogastrostomy after Pancreaticoduodenectomy for Longterm Outcomes of Pancreatic Exocrine Function. J. Am. Coll. Surg. 2005, 201, 239–244. [Google Scholar] [CrossRef]
- Park, J.W.; Jang, J.; Kim, E.; Kang, M.J.; Kwon, W.; Chang, Y.R.; Han, I.W.; Kim, S. Effects of Pancreatectomy on Nutritional State, Pancreatic Function and Quality of Life. Br. J. Surg. 2013, 100, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, R.A.; Gerber, S.M.; Tholey, R.M.; Lamb, K.M.; Somasundaram, A.; McIntyre, C.A.; Fradkin, E.C.; Ashok, A.P.; Felte, R.F.; Mehta, J.M.; et al. Incidence and Severity of Pancreatogenic Diabetes after Pancreatic Resection. J. Gastrointest. Surg. 2015, 19, 217–225. [Google Scholar] [CrossRef]
- Maitra, A.; Sharma, A.; Brand, R.E.; Eeden, S.K.V.D.; Fisher, W.E.; Hart, P.A.; Hughes, S.J.; Mather, K.J.; Pandol, S.J.; Park, W.G.; et al. A Prospective Study to Establish a New-Onset Diabetes Cohort. Pancreas 2018, 47, 1244–1248. [Google Scholar] [CrossRef]
- Dominguez-Munoz, J.E. Pancreatic Enzyme Therapy for Pancreatic Exocrine Insufficiency. Curr. Gastroenterol. Rep. 2007, 9, 116–122. [Google Scholar] [CrossRef]
- Forsmark, C.E.; Tang, G.; Xu, H.; Tuft, M.; Hughes, S.J.; Yadav, D. The Use of Pancreatic Enzyme Replacement Therapy in Patients with a Diagnosis of Chronic Pancreatitis and Pancreatic Cancer in the US is Infrequent and Inconsistent. Aliment. Pharmacol. Ther. 2020, 51, 958–967. [Google Scholar] [CrossRef]
- Zdenkowski, N.; Radvan, G.; Pugliese, L.; Charlton, J.; Oldmeadow, C.; Fraser, A.; Bonaventura, A. Treatment of Pancreatic Insufficiency Using Pancreatic Extract in Patients with Advanced Pancreatic Cancer: A Pilot Study (PICNIC). Support. Care Cancer 2017, 25, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.; Bannister, C.; Schrem, H. Enzyme Replacement Improves Survival among Patients with Pancreatic Cancer: Results of a Population Based Study. Pancreatology 2019, 19, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Muñoz, J.E.; Nieto-Garcia, L.; López-Díaz, J.; Larińo-Noia, J.; Abdulkader, I.; Iglesias-Garcia, J. Impact of the Treatment of Pancreatic Exocrine Insufficiency on Survival of Patients with Unresectable Pancreatic Cancer: A Retrospective Analysis. BMC Cancer 2018, 18, 534. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, T.; Delafontaine, P. Mechanisms of Cachexia in Chronic Disease States. Am. J. Med. Sci. 2015, 350, 250–256. [Google Scholar] [CrossRef] [Green Version]
- Martin-Perez, E.; Domínguez-Muñoz, J.E.; Botella-Romero, F.; Cerezo, L.; Teresa, F.M.; Serrano, T.; Vera, R. Multidisciplinary Consensus Statement on the Clinical Management of Patients with Pancreatic Cancer. Clin. Transl. Oncol. 2020, 22, 1963–1975. [Google Scholar] [CrossRef] [Green Version]
- Sandini, M.; Patino, M.; Ferrone, C.R.; Alvarez-Pérez, C.A.; Honselmann, K.C.; Paiella, S.; Catania, M.; Riva, L.; Tedesco, G.; Casolino, R.; et al. Association Between Changes in Body Composition and Neoadjuvant Treatment for Pancreatic Cancer. JAMA Surg. 2018, 153, 809–815. [Google Scholar] [CrossRef]
- Naumann, P.; Eberlein, J.; Farnia, B.; Hackert, T.; Debus, J.; Combs, S.E. Continued Weight Loss and Sarcopenia Predict Poor Outcomes in Locally Advanced Pancreatic Cancer Treated with Chemoradiation. Cancers 2019, 11, 709. [Google Scholar] [CrossRef] [Green Version]
- Cooper, A.; Slack, R.; Fogelman, D.; Holmes, H.M.; Petzel, M.; Parker, N.; Balachandran, A.; Garg, N.; Ngo-Huang, A.; Varadhachary, G.; et al. Characterization of Anthropometric Changes that Occur During Neoadjuvant Therapy for Potentially Resectable Pancreatic Cancer. Ann. Surg. Oncol. 2015, 22, 2416–2423. [Google Scholar] [CrossRef]
- Griffin, O.M.; Duggan, S.N.; Ryan, R.; McDermott, R.; Geoghegan, J.; Conlon, K.C. Characterising the Impact of Body Composition Change During Neoadjuvant Chemotherapy for Pancreatic Cancer. Pancreatology 2019, 19, 850–857. [Google Scholar] [CrossRef]
- Probst, P.; Haller, S.; Bruckner, T.; Ulrich, A.; Strobel, O.; Hackert, T.; Diener, M.K.; Büchler, M.W.; Knebel, P. Prospective Trial to Evaluate the Prognostic Value of Different Nutritional Assessment Scores in Pancreatic Surgery (NURIMAS Pancreas). Br. J. Surg. 2017, 104, 1053–1062. [Google Scholar] [CrossRef]
- Cao, Q.; Xiong, Y.; Zhong, Z.; Ye, Q. Computed Tomography-Assessed Sarcopenia Indexes Predict Major Complications Following Surgery for Hepatopancreatobiliary Malignancy: A Meta-Analysis. Ann. Nutr. Metab. 2019, 74, 24–34. [Google Scholar] [CrossRef]
- Ratnayake, B.; Loveday, B.P.; Shrikhande, S.V.; Windsor, J.A.; Pandanaboyana, S. Impact of Preoperative Sarcopenia on Postoperative Outcomes Following Pancreatic Resection: A Systematic Review and Meta-Analysis. Pancreatology 2018, 18, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Trestini, I.; Paiella, S.; Sandini, M.; Sperduti, I.; Elio, G.; Pollini, T.; Melisi, D.; Auriemma, A.; Soldà, C.; Bonaiuto, C.; et al. Prognostic Impact of Preoperative Nutritional Risk in Patients Who Undergo Surgery for Pancreatic Adenocarcinoma. Ann. Surg. Oncol. 2020, 27, 5325–5334. [Google Scholar] [CrossRef]
- Peng, P.; Hyder, O.; Firoozmand, A.; Kneuertz, P.; Schulick, R.D.; Huang, D.; Makary, M.; Hirose, K.; Edil, B.; Choti, M.A.; et al. Impact of Sarcopenia on Outcomes Following Resection of Pancreatic Adenocarcinoma. J. Gastrointest. Surg. 2012, 16, 1478–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okumura, S.; Kaido, T.; Hamaguchi, Y.; Fujimoto, Y.; Masui, T.; Mizumoto, M.; Hammad, A.; Mori, A.; Takaori, K.; Uemoto, S. Impact of Preoperative Quality as Well as Quantity of Skeletal Muscle on Survival after Resection of Pancreatic Cancer. Surgery 2015, 157, 1088–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bundred, J.; Kamarajah, S.K.; Roberts, K.J. Body Composition Assessment and Sarcopenia in Patients with Pancreatic Cancer: A Systematic Review and Meta-Analysis. HPB 2019, 21, 1603–1612. [Google Scholar] [CrossRef]
- Mintziras, I.; Miligkos, M.; Wächter, S.; Manoharan, J.; Maurer, E.; Bartsch, D.K. Sarcopenia and Sarcopenic Obesity are Significantly Associated with Poorer Overall Survival in Patients with Pancreatic Cancer: Systematic Review and Meta-Analysis. Int. J. Surg. 2018, 59, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Klute, K.A.; Brouwer, J.; Jhawer, M.; Sachs, H.; Gangadin, A.; Ocean, A.; Popa, E.; Dai, T.; Wu, G.; Christos, P.; et al. Chemotherapy Dose Intensity Predicted by Baseline Nutrition Assessment in Gastrointestinal Malignancies: A Multicentre Analysis. Eur. J. Cancer 2016, 63, 189–200. [Google Scholar] [CrossRef]
- Basile, D.; Parnofiello, A.; Vitale, M.G.; Cortiula, F.; Gerratana, L.; Fanotto, V.; Lisanti, C.; Pelizzari, G.; Ongaro, E.; Bartoletti, M.; et al. The IMPACT Study: Early Loss of Skeletal Muscle Mass in Advanced Pancreatic Cancer Patients. J. Cachexia Sarcopenia Muscle 2019, 10, 368–377. [Google Scholar] [CrossRef] [Green Version]
- Kurita, Y.; Kobayashi, N.; Tokuhisa, M.; Goto, A.; Kubota, K.; Endo, I.; Nakajima, A.; Ichikawa, Y. Sarcopenia is a Reliable Prognostic Factor in Patients with Advanced Pancreatic Cancer Receiving FOLFIRINOX Chemotherapy. Pancreatology 2019, 19, 127–135. [Google Scholar] [CrossRef]
- Kays, J.K.; Shahda, S.; Stanley, M.; Bell, T.; O’Neill, B.H.; Kohli, M.D.; Couch, M.E.; Koniaris, L.G.; Zimmers, T.A. Three Cachexia Phenotypes and the Impact of Fat-Only Loss on Survival in FOLFIRINOX Therapy for Pancreatic Cancer. J. Cachexia Sarcopenia Muscle 2018, 9, 673–684. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, S.Y.; Chung, M.J.; Park, J.Y.; Bang, S.; Park, S.W.; Song, S.Y. Skeletal Muscle Mass Predicts Poor Prognosis in Patients with Advanced Pancreatic Cancer Undergoing Second-Line FOLFIRINOX Chemotherapy. Nutr. Cancer 2019, 71, 1100–1107. [Google Scholar] [CrossRef]
- Argiles, J.M. Cancer-Associated Malnutrition. Eur. J. Oncol. Nurs. 2005, 9, S39–S50. [Google Scholar] [CrossRef]
- Caccialanza, R.; Lobascio, F.; Brugnatelli, S.; Pedrazzoli, P. Nutritional Support in Pancreatic Cancer. Cancer 2020, 126, 1810–1811. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.C.; Deutz, N.E.; Erickson, N.; Laviano, A.; Lisanti, M.P.; Lobo, D.N.; et al. ESPEN Expert Group Recommendations for Action Against Cancer-Related Malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobert, C.M.; Mott, S.L.; Nepple, K. Malnutrition Diagnosis during Adult Inpatient Hospitalizations: Analysis of a Multi-Institutional Collaborative Database of Academic Medical Centers. J. Acad. Nutr. Diet. 2018, 118, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Caccialanza, R.; De Lorenzo, F.; Pedrazzoli, P. The Integrating Nutritional Therapy in Oncology (INTO) Project: Rationale, Structure and Preliminary Results. ESMO Open 2017, 2, e000221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muscaritoli, M.; Arends, J.; Aapro, M. from Guidelines to Clinical Practice: A Roadmap for Oncologists for Nutrition Therapy for Cancer Patients. Ther. Adv. Med. Oncol. 2019, 11, 1758835919880084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caccialanza, R.; Lobascio, F.; Cereda, E.; Aprile, G.; Farina, G.; Traclò, F.; Borioli, V.; Caraccia, M.; Turri, A.; De Lorenzo, F.; et al. Cancer-Related Malnutrition Management: A Survey Among Italian Oncology Units and Patients’ Associations. Curr. Probl. Cancer 2020, 44, 100554. [Google Scholar] [CrossRef]
- Caccialanza, R.; Pedrazzoli, P.; Cereda, E.; Gavazzi, C.; Pinto, C.; Paccagnella, A.; Beretta, G.D.; Nardi, M.; Laviano, A.; Zagonel, V. Nutritional Support in Cancer Patients: A Position Paper from the Italian Society of Medical Oncology (AIOM) and the Italian Society of Artificial Nutrition and Metabolism (SINPE). J. Cancer 2016, 7, 131–135. [Google Scholar] [CrossRef]
- Thompson, K.L.; Elliott, L.; Fuchs-Tarlovsky, V.; Levin, R.M.; Voss, A.C.; Piemonte, T. Oncology Evidence-Based Nutrition Practice Guideline for Adults. J. Acad. Nutr. Diet. 2017, 117, 297–310. [Google Scholar] [CrossRef]
- Caccialanza, R.; Goldwasser, F.; Marschal, O.; Ottery, F.; Schiefke, I.; Tilleul, P.; Zalcman, G.; Pedrazzoli, P. Unmet Needs in Clinical Nutrition in Oncology: A Multinational Analysis of Real-World Evidence. Ther. Adv. Med. Oncol. 2020, 12, 1758835919899852. [Google Scholar] [CrossRef]
- Rossi, R.; Serra, P.; Suzzi, M.; Guerra, D.; Bilotta, S.; Ricci, M.; Pallotti, M.C.; Ibrahim, T.; Frassineti, G.L.; Zavoiu, V.; et al. The Challenge for Nutritional Care in a Cancer Center: The Need for Integration Between Clinical Nutritionist, Oncologist, and Palliative Care Physician. Curr. Probl. Cancer 2020, 44, 100618. [Google Scholar] [CrossRef] [PubMed]
- Marín Caro, M.M.; Laviano, A.; Pichard, C. Nutritional Intervention and Quality of Life in Adult Oncology Patients. Clin. Nutr. 2007, 26, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Hopkinson, J.B. Psychosocial Impact of Cancer Cachexia. J. Cachexia Sarcopenia Muscle 2014, 5, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Bayer, L.M.; Bauers, C.M.; Kapp, S.R. Psychosocial Aspects of Nutritional Support. Nurs. Clin. N. Am. 1983, 18, 119–128. [Google Scholar] [PubMed]
- Ravasco, P. Nutrition in Cancer Patients. J. Clin. Med. 2019, 8, 1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muscaritoli, M.; Molfino, A.; Gioia, G.; Laviano, A.; Fanelli, F.R. The Parallel Pathway: A Novel Nutritional and Metabolic Approach to Cancer Patients. Intern. Emerg. Med. 2011, 6, 105–112. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rovesti, G.; Valoriani, F.; Rimini, M.; Bardasi, C.; Ballarin, R.; Di Benedetto, F.; Menozzi, R.; Dominici, M.; Spallanzani, A. Clinical Implications of Malnutrition in the Management of Patients with Pancreatic Cancer: Introducing the Concept of the Nutritional Oncology Board. Nutrients 2021, 13, 3522. https://doi.org/10.3390/nu13103522
Rovesti G, Valoriani F, Rimini M, Bardasi C, Ballarin R, Di Benedetto F, Menozzi R, Dominici M, Spallanzani A. Clinical Implications of Malnutrition in the Management of Patients with Pancreatic Cancer: Introducing the Concept of the Nutritional Oncology Board. Nutrients. 2021; 13(10):3522. https://doi.org/10.3390/nu13103522
Chicago/Turabian StyleRovesti, Giulia, Filippo Valoriani, Margherita Rimini, Camilla Bardasi, Roberto Ballarin, Fabrizio Di Benedetto, Renata Menozzi, Massimo Dominici, and Andrea Spallanzani. 2021. "Clinical Implications of Malnutrition in the Management of Patients with Pancreatic Cancer: Introducing the Concept of the Nutritional Oncology Board" Nutrients 13, no. 10: 3522. https://doi.org/10.3390/nu13103522
APA StyleRovesti, G., Valoriani, F., Rimini, M., Bardasi, C., Ballarin, R., Di Benedetto, F., Menozzi, R., Dominici, M., & Spallanzani, A. (2021). Clinical Implications of Malnutrition in the Management of Patients with Pancreatic Cancer: Introducing the Concept of the Nutritional Oncology Board. Nutrients, 13(10), 3522. https://doi.org/10.3390/nu13103522





