Vitamin D Intake in Slovenian Adolescents, Adults, and the Elderly Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Dietary Assessment Methods
2.2.1. General Questionnaire and Anthropometric Measurements
2.2.2. 24-h Dietary Recall
2.2.3. Food Propensity Questionnaire
2.3. Assessment of Nutrient Intake
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Autier, P.; Boniol, M.; Pizot, C.; Mullie, P. Vitamin D status and ill health: A systematic review. Lancet Diabetes Endocrinol. 2014, 2, 76–89. [Google Scholar] [CrossRef]
- Autier, P.; Mullie, P.; Macacu, A.; Dragomir, M.; Boniol, M.; Coppens, K.; Pizot, C.; Boniol, M. Effect of vitamin D supplementation on non-skeletal disorders: A systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017, 5, 986–1004. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D status: Measurement, interpretation, and clinical application. Ann. Epidemiol. 2009, 19, 73–78. [Google Scholar] [CrossRef] [Green Version]
- Zittermann, A. Vitamin D in preventive medicine: Are we ignoring the evidence? Br. J. Nutr. 2003, 89, 552–572. [Google Scholar] [CrossRef]
- Zittermann, A.; Pilz, S.; Hoffmann, H.; Marz, W. Vitamin D and airway infections: A European perspective. Eur. J. Med. Res. 2016, 21, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kimlin, M.G. Geographic location and vitamin D synthesis. Mol. Aspects Med. 2008, 29, 453–461. [Google Scholar] [CrossRef]
- O’Neill, C.M.; Kazantzidis, A.; Ryan, M.J.; Barber, N.; Sempos, C.T.; Durazo-Arvizu, R.A.; Jorde, R.; Grimnes, G.; Eiriksdottir, G.; Gudnason, V.; et al. Seasonal Changes in Vitamin D-Effective UVB Availability in Europe and Associations with Population Serum 25-Hydroxyvitamin, D. Nutrients 2016, 8, 533. [Google Scholar] [CrossRef] [Green Version]
- Ovsenik Jeglič, T. Climate of Slovenia 1971–2000—Sunshine Duration; The Environmental Agency of the Republic of Slovenia: Ljubljana, Slovenia, 2006.
- Hribar, M.; Hristov, H.; Gregorič, M.; Blaznik, U.; Zaletel, K.; Oblak, A.; Osredkar, J.; Kušar, A.; Žmitek, K.; Rogelj, I.; et al. Nutrihealth study: Seasonal variation in vitamin D status among the Slovenian adult and elderly population. Nutrients 2020, 12, 1838. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization/International Research Agency for Cancer (WHO/IARC). Vitamin D and Cancer; WHO: Geneva, Switzerland, 2008; p. 210. Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Working-Group-Reports/Vitamin-D-And-Cancer-2008 (accessed on 10 May 2021).
- Kiely, M.; Black, L.J. Dietary strategies to maintain adequacy of circulating 25-hydroxyvitamin D concentrations. Scand. J. Clin. Lab. Investig. Suppl. 2012, 243, 14–23. [Google Scholar]
- Roseland, J.M.; Phillips, K.M.; Patterson, K.Y.; Pehrsson, P.R.; Taylor, C.L. Vitamin D in Foods: An Evolution of Knowledge. In Vitamin D, 4th ed.; Feldman, D., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 41–77. [Google Scholar]
- Whiting, S.J.; Calvo, M.S. Vitamin D Fortification and Supplementation Policies to Correct Vitamin D Insufficiency/Deficiency Globally. In Vitamin D, 4th ed.; Feldman, D., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 91–108. [Google Scholar]
- Cheney, M.C. Canadian experience with food fortification. Public Health Rev. 2000, 28, 171–177. [Google Scholar] [PubMed]
- Pilz, S.; Marz, W.; Cashman, K.D.; Kiely, M.E.; Whiting, S.J.; Holick, M.F.; Grant, W.B.; Pludowski, P.; Hiligsmann, M.; Trummer, C.; et al. Rationale and Plan for Vitamin D Food Fortification: A Review and Guidance Paper. Front. Endocrinol. 2018, 9, 373. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, Á.; Walton, J.; Flynn, A. The impact of voluntary food fortification on micronutrient intakes and status in European countries: A review. Proc. Nutr. Soc. 2013, 72, 433–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Commission (EC). Commission Regulation (EC) No 1170/2009 of 30 November 2009 amending Directive 2002/46/EC of the European Parliament and of Council and Regulation (EC) No 1925/2006 of the European Parliament and of the Council as regards the lists of vitamin and minerals and their forms that can be added to foods, including food supplements. Off. J. Eur. Union 2009, L314/36, 336–342. [Google Scholar]
- European Commission (EC). Regulation (EC) No 1925/2006 of the European Parliament and of the Council of 20 December 2006 on the addition of vitamins and minerals and of certain other substances to foods. Off. J. Eur. Union 2006, L404, 426–438. [Google Scholar]
- EFSA Panel on Nutrition; Novel Foods; Food Allergens; Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; et al. Safety of extended uses of UV-treated baker’s yeast as a Novel Food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06602. [Google Scholar]
- Žmitek, K.; Krušič, S.; Pravst, I. An Approach to Investigate Content-Related Quality of Nutraceuticals Used by Slovenian Consumers: A Case Study with Folate and Vitamin D Supplements. Foods 2021, 10, 845. [Google Scholar] [CrossRef]
- Battelino, T. Zapisnik 56. redne seje RSK za pediatrijo. Slov. Pediatrija 2010, 17, 241–243. [Google Scholar]
- van Schoor, N.; Lips, P. Global Overview of Vitamin D Status. Endocrinol. Metab. Clin. 2017, 46, 845–870. [Google Scholar] [CrossRef] [PubMed]
- Lips, P.; Cashman, K.D.; Lamberg-Allardt, C.; Bischoff-Ferrari, H.A.; Obermayer-Pietsch, B.; Bianchi, M.L.; Stepan, J.; Fuleihan, G.E.-H.; Bouillon, R. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: A position statement of the European Calcified Tissue Society. Eur. J. Endocrinol. 2019, 180, 23–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization (WHO). Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Calcium and Vitamin D. The National Academies Collection: Reports funded by National Institutes of Health. In Dietary Reference Intakes for Calcium and Vitamin D; Ross, A.C., Taylor, C.L., Yaktine, A.L., Del Valle, H.B., Eds.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.; Naska, A.; Neuhauser-Berthold, M.; et al. Dietary reference values for vitamin D. EFSA J. 2016, 14, e04547. [Google Scholar]
- Spiro, A.; Buttriss, J.L. Vitamin D: An overview of vitamin D status and intake in Europe. Nutr. Bull. 2014, 39, 322–350. [Google Scholar] [CrossRef] [Green Version]
- Bouillon, R. Comparative analysis of nutritional guidelines for vitamin D. Nat. Rev. Endocrinol. 2017, 13, 466–479. [Google Scholar] [CrossRef]
- German Nutrition, S. New reference values for vitamin D. Ann. Nutr. Metab. 2012, 60, 241–246. [Google Scholar] [CrossRef]
- European Commission (EC). Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers, amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004 Text with EEA relevance. Off. J. Eur. Union 2011, L 304/18, 18–63. [Google Scholar]
- Mensink, G.B.; Fletcher, R.; Gurinovic, M.; Huybrechts, I.; Lafay, L.; Serra-Majem, L.; Szponar, L.; Tetens, I.; Verkaik-Kloosterman, J.; Baka, A.; et al. Mapping low intake of micronutrients across Europe. Br. J. Nutr. 2013, 110, 755–773. [Google Scholar] [CrossRef] [Green Version]
- Novaković, R.; Cavelaars, A.E.; Bekkering, G.E.; Roman-Viñas, B.; Ngo, J.; Gurinović, M.; Glibetić, M.; Nikolić, M.; Golesorkhi, M.; Medina, M.W.; et al. Micronutrient intake and status in Central and Eastern Europe compared with other European countries, results from the EURRECA network. Public Health Nutr. 2013, 16, 824–840. [Google Scholar] [CrossRef] [Green Version]
- Jenab, M.; Salvini, S.; van Gils, C.H.; Brustad, M.; Shakya-Shrestha, S.; Buijsse, B.; Verhagen, H.; Touvier, M.; Biessy, C.; Wallström, P.; et al. Dietary intakes of retinol, beta-carotene, vitamin D and vitamin E in the European Prospective Investigation into Cancer and Nutrition cohort. Eur. J. Clin. Nutr. 2009, 63, S150–S178. [Google Scholar] [CrossRef]
- Roman Viñas, B.; Ribas Barba, L.; Ngo, J.; Gurinovic, M.; Novakovic, R.; Cavelaars, A.; de Groot, L.C.; van’t Veer, P.; Matthys, C.; Serra Majem, L. Projected prevalence of inadequate nutrient intakes in Europe. Ann. Nutr. Metab. 2011, 59, 84–95. [Google Scholar] [CrossRef]
- Freisling, H.; Fahey, M.T.; Moskal, A.; Ocke, M.C.; Ferrari, P.; Jenab, M.; Norat, T.; Naska, A.; Welch, A.A.; Navarro, C.; et al. Region-specific nutrient intake patterns exhibit a geographical gradient within and between European countries. J. Nutr. 2010, 140, 1280–1286. [Google Scholar] [CrossRef] [Green Version]
- Rippin, H.L.; Hutchinson, J.; Jewell, J.; Breda, J.J.; Cade, J.E. Adult Nutrient Intakes from Current National Dietary Surveys of European Populations. Nutrients 2017, 9, 1288. [Google Scholar] [CrossRef] [Green Version]
- Elmadfa, I.; Meyer, A.; Nowak, V.; Hasenegger, V.; Putz, P.; Verstraeten, R.; Remaut-DeWinter, A.M.; Kolsteren, P.; Dostálová, J.; Dlouhý, P.; et al. European Nutrition and Health Report 2009. Forum Nutr. 2009, 62, 12–13. [Google Scholar]
- Policnik, R.; Pokorn, D.; Kulnik, D.; Micetic-Turk, D.; Hlastan-Ribic, C. Energy and nutrient intake among pre-school children in central slovenia. Acta Aliment. 2013, 42, 291–300. [Google Scholar] [CrossRef]
- Zdešar Kotnik, K. Smiselnost Uporabe Vitaminskih in Mineralnih Prehranskih Dopolnil Pri Mladostnikih = Advisability of Vitamin and Mineral Dietary Supplement Use Among Adolescents. Ph.D. Thesis, Univerza v Ljubljani, Biotehniška Fakulteta, Ljubljana, Slovenia, 2019. [Google Scholar]
- Fidler Mis, N.; Kobe, H.; Štimec, M. Dietary intake of macro-and micronutrients in Slovenian adolescents: Comparison with reference values. Ann. Nutr. Metab. 2012, 61, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Gregorič, M. Ocena Prehranskega Vnosa Pri Mladostnikih Z Vidika Varovanja Zdravja = Assessment of Dietary Intake Among Adolescents from Health Protection Aspect. Ph.D. Thesis, Univerza v Ljubljani, Biotehniška Fakulteta, Ljubljana, Slovenia, 2015. [Google Scholar]
- Koch, V.; Gregorič, M. Prehranska kakovost zajtrka slovenskih srednješolcev = Nutritional quality of breakfast eaten by secondary school students in Slovenia. Zdr. Varst. 2009, 48, 131–142. [Google Scholar]
- Jeretina, B. Dejavniki Vpliva Na Pojav Prezgodnje Menopavze = Risk Factors for The Onset of Premature Menopause. Ph.D. Thesis, Univerza v Ljubljani, Zdravstvena Fakulteta, Ljubljana, Slovenia, 2019. [Google Scholar]
- Juvan, S. Kakovost vegetarijanske prehrane med študenti = Quality of Vegetarian Nutrition between Students. Ph.D. Thesis, Univerza v Ljubljani, Biotehniška Fakulteta, Ljubljana, Slovenia, 1997. [Google Scholar]
- Soltirovska Salamon, A.; Benedik, E.; Bratanič, B.; Velkavrh, M.; Rogelj, I.; Fidler Mis, N.; Bogovič Matijašić, B.; Paro-Panjan, D. Vitamin D status and its determinants in healthy Slovenian pregnant women. Ann. Nutr. Metab. 2015, 67, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Urh, N.; Babnik, K.; Rebec, D.; Poklar Vatovec, T. Ocena prehranskega stanja starejših v socialnovarstvenem zavodu = Assessment of the nutritional status of the elderly in a residential home. Obz. Zdrav. Nege. 2017, 51, 207–218. [Google Scholar] [CrossRef] [Green Version]
- Gregorič, M.; Kotnik, K.Z.; Pigac, I.; Blenkuš, M.G. A Web-Based 24-H Dietary Recall Could Be a Valid Tool for the Indicative Assessment of Dietary Intake in Older Adults Living in Slovenia. Nutrients 2019, 11, 2234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lichthammer, A.; Nagy, B.; Orbán, C.; Tóth, T.; Csajbók, R.; Molnár, S.; Tátrai-Németh, K.; Bálint, M.V. A comparative study of eating habits, calcium and Vitamin D intakes in the population of Central-Eastern European countries. New Med. 2015, 19, 66–70. [Google Scholar]
- European Food Safety Authority. Guidance on the EU Menu methodology. EFSA J. 2014, 12, 3944. [Google Scholar]
- Gregorič, M.; Blaznik, U.; Delfar, N.; Zaletel, M.; Lavtar, D.; Koroušić Seljak, B.; Golja, P.; Zdešar Kotnik, K.; Pravst, I.; Fidler Mis, N.; et al. Slovenian national food consumption survey in adolescents, adults and elderly. EFSA Supporting Publ. 1729, 16, en-1729. [Google Scholar] [CrossRef] [Green Version]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vede, T. Izdelava in validacija slikovnega gradiva za določanje vnosa živil [Design and Validation of Food Picture Book]. Ph.D. Thesis, University of Ljubljana, Biotechnical Faculty, Ljubljana, Slovenia, 2016. [Google Scholar]
- Dodd, K.W.; Guenther, P.M.; Freedman, L.S.; Subar, A.F.; Kipnis, V.; Midthune, D.; Tooze, J.A.; Krebs-Smith, S.M. Statistical methods for estimating usual intake of nutrients and foods: A review of the theory. J. Am. Diet. Assoc. 2006, 106, 1640–1650. [Google Scholar] [CrossRef]
- Korošec, M.; Golob, T.; Bertoncelj, J.; Stibilj, V.; Koroušić Seljak, B. The Slovenian food composition database. Food Chem. 2013, 140, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Harttig, U.; Haubrock, J.; Knüppel, S.; Boeing, H. The MSM program: Web-based statistics package for estimating usual dietary intake using the Multiple Source Method. Eur. J. Clin. Nutr. 2011, 65, S87–S91. [Google Scholar] [CrossRef] [Green Version]
- Nutrition Unit of the National Institute for Health and Welfare. Fineli—The National Food Composition Database. Available online: https://fineli.fi/fineli/en/index (accessed on 10 May 2020).
- Roe, M.; Pinchen, H.; Church, S.; Finglas, P. McCance and Widdowson’s The Composition of Foods Seventh Summary Edition and updated Composition of Foods Integrated Dataset. Nutr. Bull. 2015, 40, 36–39. [Google Scholar] [CrossRef]
- Bodner-Montville, J.; Ahuja, J.K.; Ingwersen, L.A.; Haggerty, E.S.; Enns, C.W.; Perloff, B.P. USDA food and nutrient database for dietary studies: Released on the web. J. Food Compos. 2006, 19, S100–S107. [Google Scholar] [CrossRef]
- Department of Health. Regulations Amending Certain Regulations Made Under the Food and Drugs Act (Nutrition Symbols, Other Labelling Provisions Partially Hydrogenated Oils and Vitamin D.). In Part I; No. 6; Canada Gazette: Ottawa, ON, Canada, 2018; Volume 152, p. 256. [Google Scholar]
- Zupanič, N.; Hristov, H.; Gregorič, M.; Blaznik, U.; Delfar, N.; Koroušić Seljak, B.; Ding, E.L.; Fidler Mis, N.; Pravst, I. Total and Free Sugars Consumption in a Slovenian Population Representative Sample. Nutrients 2020, 12, 1729. [Google Scholar] [CrossRef] [PubMed]
- Haubrock, J.; Nöthlings, U.; Volatier, J.-L.; Dekkers, A.; Ocké, M.; Harttig, U.; Illner, A.-K.; Knüppel, S.; Andersen, L.F.; Boeing, H.; et al. Estimating Usual Food Intake Distributions by Using the Multiple Source Method in the EPIC-Potsdam Calibration Study. J. Nutr. 2011, 141, 914–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolenikov, S. Calibrating Survey Data using Iterative Proportional Fitting (Raking). Stata J. 2014, 14, 22–59. [Google Scholar] [CrossRef] [Green Version]
- Koenig, J.; Elmadfa, I. Status of calcium and vitamin D of different population groups in Austria. Int. J. Vitam. Nutr. Res. 2000, 70, 214–220. [Google Scholar] [CrossRef]
- Kudlacek, S.; Schneider, B.; Peterlik, M.; Leb, G.; Klaushofer, K.; Weber, K.; Woloszczuk, W.; Willvonseder, R. Assessment of vitamin D and calcium status in healthy adult Austrians. Eur. J. Clin. Invest. 2003, 33, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Kamycheva, E.; Joakimsen, R.M.; Jorde, R. Intakes of Calcium and Vitamin D Predict Body Mass Index in the Population of Northern Norway. J. Nutr. 2003, 133, 102–106. [Google Scholar] [CrossRef]
- de Mestral, C.; Marques-Vidal, P.; Gaspoz, J.-M.; Theler, J.-M.; Guessous, I. Independent association between socioeconomic indicators and macro- and micro-nutrient intake in Switzerland. PLoS ONE 2017, 12, e0174578. [Google Scholar] [CrossRef]
- Galobardes, B.; Morabia, A.; Bernstein, M.S. Diet and socioeconomic position: Does the use of different indicators matter? Int. J. Epidemiol. 2001, 30, 334–340. [Google Scholar] [CrossRef] [Green Version]
- Rossum, C.T.M.v.; Fransen, H.P.; Verkaik-Kloosterman, J.; Buurma-Rethans, E.J.M.; Ocké, M.C. Dutch National Food Consumption Survey 2007–2010: Diet of Children and Adults Aged 7 to 69 Years; National Institute for Public Health and the Environment: Utrecht, The Netherlands, 2011. Available online: https://www.rivm.nl/bibliotheek/rapporten/350050006.pdf (accessed on 14 May 2021).
- WORLDOMETERS. Coronavirus in Slovenia. Available online: https://www.worldometers.info/coronavirus/country/slovenia/ (accessed on 23 September 2021).
- IOS. The Eastern European & Central Asian Regional Audit Epidemiology, Costs & Burden of Osteoporosis in 2010. Available online: https://www.osteoporosis.foundation/sites/iofbonehealth/files/2019-06/2010_Eastern_European_Central_Asian_Audit_English.pdf (accessed on 23 September 2021).
- Borgström, F.; Karlsson, L.; Ortsäter, G.; Norton, N.; Halbout, P.; Cooper, C.; Lorentzon, M.; McCloskey, E.V.; Harvey, N.C.; Javaid, M.K.; et al. Fragility fractures in Europe: Burden, management and opportunities. Arch. Osteoporos. 2020, 15, 1–21. [Google Scholar] [CrossRef] [Green Version]
- European Commission (EC). Community Register on the Addition of Vitamins and Minerals and of Certain other Substances to Foods. Available online: https://ec.europa.eu/food/system/files/2021-01/labelling_nutrition-vitamins_minerals-comm_reg_en.pdf (accessed on 23 September 2021).
- Jääskeläinen, T.; Itkonen, S.T.; Lundqvist, A.; Erkkola, M.; Koskela, T.; Lakkala, K.; Dowling, K.G.; Hull, G.L.; Kröger, H.; Karppinen, J.; et al. The positive impact of general vitamin D food fortification policy on vitamin D status in a representative adult Finnish population: Evidence from an 11-y follow-up based on standardized 25-hydroxyvitamin D data. Am. J. Clin. Nutr 2017, 105, 1512–1520. [Google Scholar] [CrossRef] [Green Version]
- Calvo, M.S.; Whiting, S.J. Vitamin D Fortification in North America: Current Status and Future Considerations. In Handbook of Food Fortification and Health; Humana Press: Totowa, NJ, USA, 2013; pp. 259–275. [Google Scholar]
- Itkonen, S.T.; Erkkola, M.; Lamberg-Allardt, C.J.E. Vitamin D Fortification of Fluid Milk Products and Their Contribution to Vitamin D Intake and Vitamin D Status in Observational Studies-A Review. Nutrients 2018, 10, 1054. [Google Scholar] [CrossRef] [Green Version]
- Black, L.J.; Seamans, K.M.; Cashman, K.D.; Kiely, M. An updated systematic review and meta-analysis of the efficacy of vitamin D food fortification. J. Nutr. 2012, 142, 1102–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, J.; Sandmann, A.; Ignatius, A.; Amling, M.; Barvencik, F. New perspectives on vitamin D food fortification based on a modeling of 25(OH)D concentrations. Nutr. J. 2013, 12, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Mahony, L.; Stepien, M.; Gibney, M.J.; Nugent, A.P.; Brennan, L. The potential role of vitamin D enhanced foods in improving vitamin D status. Nutrients 2011, 3, 1023–1041. [Google Scholar] [CrossRef] [Green Version]
- Whiting, S.J.; Langlois, K.A.; Vatanparast, H.; Greene-Finestone, L.S. The vitamin D status of Canadians relative to the 2011 Dietary Reference Intakes: An examination in children and adults with and without supplement use. Am. J. Clin. Nutr. 2011, 94, 128–135. [Google Scholar] [CrossRef] [Green Version]
- Janssen, M.; Chang, B.P.I.; Hristov, H.; Pravst, I.; Profeta, A.; Millard, J. Changes in Food Consumption During the COVID-19 Pandemic: Analysis of Consumer Survey Data From the First Lockdown Period in Denmark, Germany, and Slovenia. Front. Nutr. 2021, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Žmitek, K.; Hribar, M.; Lavriša, Ž.; Hristov, H.; Kušar, A.; Pravst, I. Socio-Demographic and Knowledge-Related Determinants of Vitamin D Supplementation in the Context of the COVID-19 Pandemic: Assessment of an Educational Intervention. Front. Nutr. 2021, 8, 290. [Google Scholar] [CrossRef]

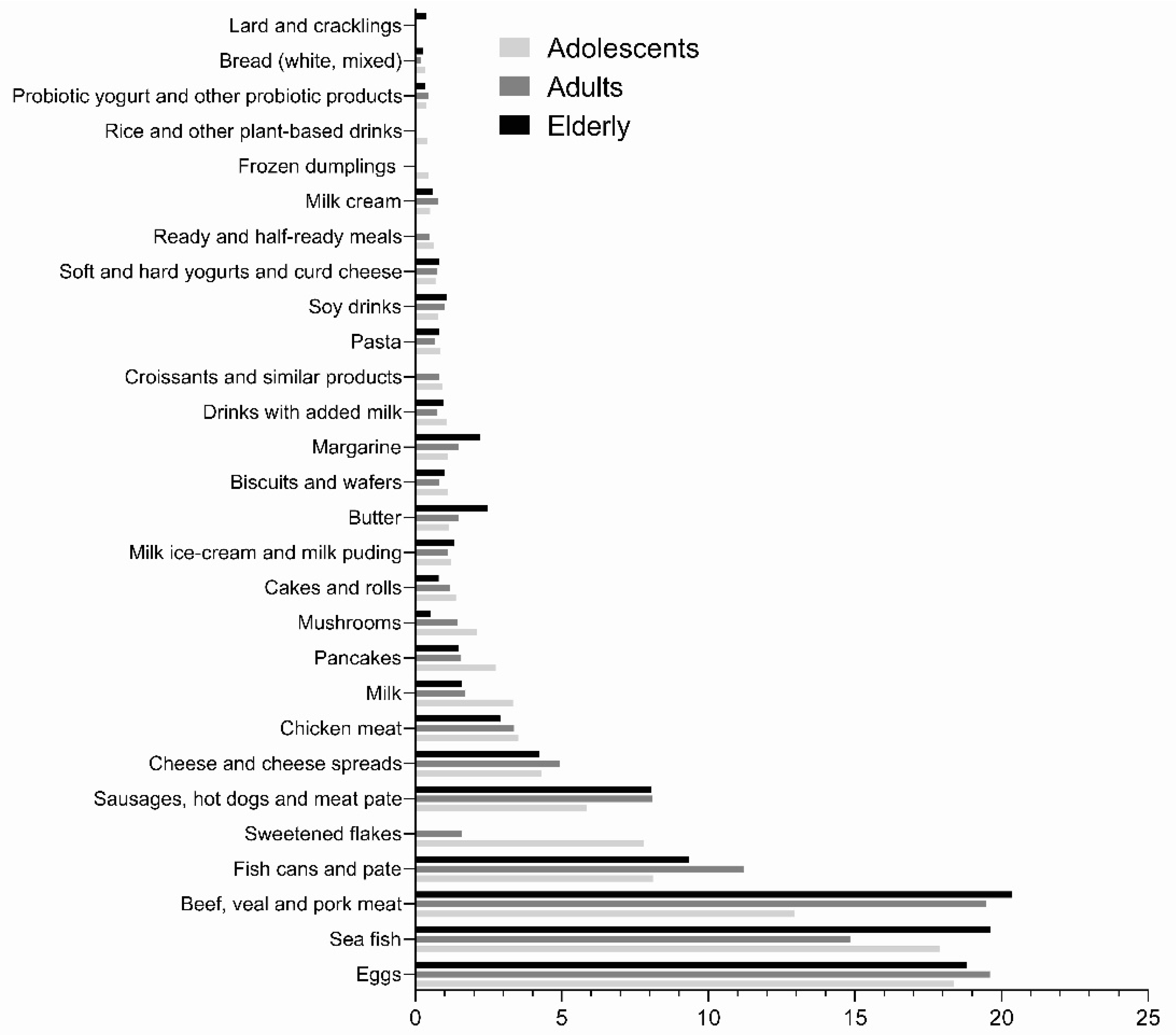

| Age Cohorts | ||||
|---|---|---|---|---|
| Adolescents | Adults | Elderly | ||
| (10–17 Years) | (18–64 Years) | (65–74 Years) | ||
| n = 468 | n = 364 | n = 416 | ||
| Age; years—mean (SD) | 13.4 (2.37) | 43.6 (13.81) | 68.7 (2.7) | |
| Place of living—n (%) | Rural | 270 (57.7) | 202 (55.5) | 229 (55.1) |
| Intermediate | 76 (16.2) | 56 (15.4) | 71 (17.1) | |
| Urban | 122 (26.1) | 106 (29.1) | 116 (27.9) | |
| Sex—n (%) | Male | 238 (50.9) | 173 (47.5) | 213 (51.2) |
| Female | 230 (49.1) | 191 (52.5) | 203 (48.8) | |
| Education—n (%) | No university degree | n.a. | 249 (68.4) | 342 (82.2) |
| University degree | n.a. | 115 (31.6) | 74 (17.8) | |
| Family monthly net income—n (%) | Below average | n.a. | 118 (38.4) | 269 (71.5) |
| Above average | n.a. | 189 (61.6) | 107 (28.5) | |
| BMI—mean (SD) | 21.0 (4.2) | 26.7 (5.2) | 28.4 (5.0) | |
| n (%) | Normal | 301 (64.6) | 148 (40.7) | 108 (26.0) |
| Overweight and obese | 167 (35.7) | 216 (59.3) | 308 (74.0) | |
| IPAQ—n (%) | Low intensity | 108 (23.3) | 127 (35.3) | 137 (33.4) |
| Moderate | 141 (30.5) | 108 (30.0) | 133 (32.4) | |
| High intensity | 214 (46.2) | 125 (34.7) | 140 (34.2) | |
| Employment status—n (%) | Employed | n.a. | 226 (62.1) | n.a. |
| Unemployed | n.a. | 42 (11.5) | n.a. | |
| Student | n.a. | 32 (8.8) | n.a. | |
| Retired | n.a. | 64 (17.6) | n.a. | |
| Use of dietary supplements—n (%) | Vitamin D | 17 (3.63) | 22 (6.04) | 20 (4.81) |
| Multivitamin | 72 (15.4) | 52 (14.3) | 11 (2.64) | |
| Vitamin D and/or multivitamin supplements | 80 (17.1) | 63 (17.3) | 29 (6.97) | |
| Adolescents (10–17) | Adults (18–64) | Elderly (65–74) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All | Male | Female | All | Male | Female | All | Male | Female | |
| Sample Size, n (%) | 468 (100) | 238 (50.85) | 230 (49.15) | 364 (100) | 173 (47.53) | 191 (52.47) | 416 (100) | 213 (51.20) | 203 (48.80) |
| Vitamin D intake | |||||||||
| Mean [µg/day] (95% CI) | 2.73 (2.56–2.91) | 3.02 (2.83–3.22) | 2.42 (2.14–2.70) | 2.85 (2.69–3.00) | 3.39 (3.17–3.62) | 2.30 (2.14–2.44) | 2.45 (2.34–2.57) | 2.60 (2.42–2.78) | 2.32 (2.16–2.48) |
| Std.Err. | 0.09 | 0.10 | 0.14 | 0.08 | 0.12 | 0.08 | 0.06 | 0.09 | 0.08 |
| Median [µg/day] | 2.33 | 2.70 | 1.95 | 2.66 | 3.01 | 2.04 | 2.21 | 2.30 | 2.13 |
| Mean [µg/1000 kcal per day] (95% CI) | 1.24 (1.15–1.33) | 1.23 (1.12–1.35) | 1.25 (1.11–1.40) | 1.34 (1.27–1.41) | 1.47 (1.37–1.57) | 1.21 (1.13–1.29) | 1.20 (1.13–1.27) | 1.20 (1.10–1.30) | 1.20 (1.10–1.29) |
| Prevalence of very low vitamin D intake (%) | |||||||||
| <2.5 [µg/day] | 55.0 (47.4–62.4) | 38.8 (30.0–48.5) | 72.6 (64.6–79.3) | 45.8 (40.1–51.6) | 21.0 (15.2–28.2) | 71.0 (63.5–77.5) | 61.0 (51.7–69.6) | 58.2 (43.6–71.5) | 63.5 (50.6–74.7) |
| Variable | Adolescents (10–17 Years Old) | Adults (18–64 Years Old) | Elderly (65–74 Years Old) | ||||
|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | ||
| Sex | Male | 3.01 (2.82–3.20) | 2.95 (2.72–3.17) | 3.35 (3.16–3.53) | 3.22 (3.03–3.42) | 2.67 (2.51–2.83) | 2.55 (2.37–2.73) |
| Female | 2.44 (2.18–2.70) | 2.52 (2.29–2.76) | 2.31 (2.15–2.46) | 2.46 (2.28–2.64) | 2.35 (2.16–2.54) | 2.49 (2.30–2.68) | |
| Place of living | Rural | 2.64 (2.46–2.80) | 2.63 (2.42–2.84) | 2.88 (2.71–3.05) | 2.86 (2.68–3.03) | 2.57 (2.41–2.73) | 2.56 (2.39–2.73) |
| Intermediate | 2.87 (2.24-3.50) | 2.85 (2.46-3.24) | 2.66 (2.25–3.07) | 2.68 (2.36–3.00) | 2.38 (2.06–2.71) | 2.50 (2.20–2.81) | |
| Urban | 2.86 (2.54–3.17) | 2.90 (2.60–3.21) | 2.73 (2.52–2.94) | 2.81 (2.59–3.04) | 2.48 (2.24–2.72) | 2.44 (2.20–2.69) | |
| Education | No university degree | n.a. | n.a. | 2.74 (2.59–2.88) | 2.76 (2.61–2.92) | 2.43 (2.31–2.55) | 2.45 (2.31–2.60) |
| University degree | 2.94 (2.69–3.19) | 2.92 (2.69–3.16) | 2.88 (2.47–3.29) | 2.85 (2.52–3.17) | |||
| Family net income | Below average (≤EUR 1300) | n.a. | n.a. | 2.63 (2.40–2.85) | 2.71 (2.50–2.93) | 2.44 (2.30–2.60) | 2.51 (2.33–2.64) |
| Above average (>EUR 1300) | 2.92 (2.74–3.10) | 2.89 (2.71–3.05) | 2.68 (2.42–2.94) | 2.56 (2.31–2.81) | |||
| BMI | Normal | 2.95 (2.72–3.16) | 2.94 (2.75–3.14) | 2.72 (2.52–2.92) | 2.81 (2.60–3.01) | 2.51 (2.28–2.73) | 2.57 (2.31–2.83) |
| Overweight and obese | 2.34 (2.13–2.56) | 2.35 (2.10–2.62) | 2.86 (2.69–3.02) | 2.82 (2.66–2.98) | 2.52 (2.37–2.66) | 2.50 (2.36–2.65) | |
| IPAQ | Low intensity | 2.77 (2.55–2.99) | 2.72 (2.40–3.05) | 2.79 (2.60–2.99) | 2.78 (2.56–2.99) | 2.56 (2.33–2.78) | 2.54 (2.32–2.76) |
| Moderate | 2.64 (2.27–3.02) | 2.71 (2.42–3.00) | 2.74 (2.48–2.98) | 2.82 (2.60–3.05) | 2.30 (2.14–2.46) | 2.35 (2.13–2.57) | |
| High intensity | 2.79 (2.56–3.01) | 2.77 (2.53–2.99) | 2.89 (2.65–3.12 | 2.85 (2.63–3.06) | 2.69 (2.45–2.93) | 2.67 (2.45–2.89) | |
| Employment | Employed | n.a. | n.a. | 2.91 (2.75–3.08) | 2.81 (2.64–2.98) | n.a. | n.a. |
| Unemployed | 2.51 (2.14–2.87) | 2.85 (2.46–3.23) | |||||
| Student | 2.91 (2.53–3.29) | 2.96 (2.47–3.44) | |||||
| Retired | 2.54 (2.23–2.86) | 2.76 (2.44–3.07) | |||||
| Adolescents (10–17) | Adults (18–64) | Elderly (65–74) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All | Male | Female | All | Male | Female | All | Male | Female | |
| Unadjusted mean vitamin D intake—μg/day (SD) | |||||||||
| Regular diet | 2.73 (1.77) | 3.01 (2.44) | 2.44 (2.00) | 2.80 (1.24) | 3.35 (1.23) | 2.31 (1.03) | 2.51 (1.26) | 2.67 (1.16) | 2.35 (1.35) |
| Projected fortified diet * | 4.82 (2.51) | 5.46 (2.29) | 4.16 (2.56) | 4.01 (1.88) | 4.53 (1.90) | 3.56 (1.73) | 3.58 (1.79) | 3.62 (1.83) | 3.54 (1.75) |
| Median vitamin D intake—μg/day | |||||||||
| Regular diet | 2.25 | 2.66 | 1.95 | 2.59 | 3.02 | 2.02 | 2.26 | 2.38 | 2.01 |
| Projected increase with fortification * | 2.01 | 4.10 | 3.15 | 1.10 | 1.04 | 1.23 | 1.01 | 0.87 | 1.25 |
| Projected fortified diet * | 4.26 | 6.76 | 5.1 | 3.69 | 4.06 | 3.25 | 3.27 | 3.25 | 3.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hribar, M.; Hristov, H.; Lavriša, Ž.; Koroušić Seljak, B.; Gregorič, M.; Blaznik, U.; Žmitek, K.; Pravst, I. Vitamin D Intake in Slovenian Adolescents, Adults, and the Elderly Population. Nutrients 2021, 13, 3528. https://doi.org/10.3390/nu13103528
Hribar M, Hristov H, Lavriša Ž, Koroušić Seljak B, Gregorič M, Blaznik U, Žmitek K, Pravst I. Vitamin D Intake in Slovenian Adolescents, Adults, and the Elderly Population. Nutrients. 2021; 13(10):3528. https://doi.org/10.3390/nu13103528
Chicago/Turabian StyleHribar, Maša, Hristo Hristov, Živa Lavriša, Barbara Koroušić Seljak, Matej Gregorič, Urška Blaznik, Katja Žmitek, and Igor Pravst. 2021. "Vitamin D Intake in Slovenian Adolescents, Adults, and the Elderly Population" Nutrients 13, no. 10: 3528. https://doi.org/10.3390/nu13103528
APA StyleHribar, M., Hristov, H., Lavriša, Ž., Koroušić Seljak, B., Gregorič, M., Blaznik, U., Žmitek, K., & Pravst, I. (2021). Vitamin D Intake in Slovenian Adolescents, Adults, and the Elderly Population. Nutrients, 13(10), 3528. https://doi.org/10.3390/nu13103528










